Abstract
Exocytic vesicles that accumulate in a temperature-sensitive sec6 mutant at a restrictive temperature can be separated into at least two populations with different buoyant densities and unique cargo molecules. Using a sec6 mutant background to isolate vesicles, we have found that vacuolar protein sorting mutants that block an endosome-mediated route to the vacuole, including vps1, pep12, vps4, and a temperature-sensitive clathrin mutant, missort cargo normally transported by dense exocytic vesicles, such as invertase, into light exocytic vesicles, whereas transport of cargo specific to the light exocytic vesicles appears unaffected. Immunoisolation experiments confirm that missorting, rather than a changed property of the normally dense vesicles, is responsible for the altered density gradient fractionation profile. The vps41Δ and apl6Δ mutants, which block transport of only the subset of vacuolar proteins that bypasses endosomes, sort exocytic cargo normally. Furthermore, a vps10Δ sec6 mutant, which lacks the sorting receptor for carboxypeptidase Y (CPY), accumulates both invertase and CPY in dense vesicles. These results suggest that at least one branch of the yeast exocytic pathway transits through endosomes before reaching the cell surface. Consistent with this possibility, we show that immunoisolated clathrin-coated vesicles contain invertase.
Keywords: protein sorting; TGN; endosome; VPS; yeast secretion
Introduction
Exocytic cargo can reach the cell surface by multiple pathways in most, if not all, eukaryotic cells (for reviews see Keller and Simons, 1997; Traub and Kornfeld, 1997). The sorting of cargo into unique vesicle populations makes possible the cargo-specific spatial or temporal regulation of exocytosis, as seen in polarized epithelial cells in which distinct vesicles are targeted to the apical and basolateral surfaces (Mostov et al., 2000) and in cells with both constitutive and regulated exocytic pathways, in which one class of vesicles requires an external stimulus to trigger fusion with the cell surface (Burgess and Kelly, 1987). However, the reasons for sorting cargo into separate exocytic pathways are not always clear. Sorting may take place among cargo directed to the same surface domain of polarized cells; for example, basolaterally targeted membrane and soluble proteins are transported by different vesicle populations in hepatic cells (Saucan and Palade, 1994). Furthermore, apical and basolateral proteins expressed in various fibroblast cell lines are sorted into different vesicles (Yoshimori et al., 1996), and the same sorting signals operate in fibroblasts and polarized cells (Müsch et al., 1996), indicating unique sorting mechanisms and exocytic pathways for different classes of cargoes in both polarized and nonpolarized cells.
Secretory proteins are sorted and packaged into various types of vesicles at the trans-Golgi network (TGN),*and cargo bound for lysosomes is sorted at this point from most proteins destined for the cell surface (Keller and Simons, 1997; Traub and Kornfeld, 1997). Many lysosomal proteins probably first reach early endosomes where they are again sorted into carrier vesicles that transport them to late endosomes (Ludwig et al., 1991; Press et al., 1998), whereas other lysosomal proteins bypass early endosomes and are transported to lysosomes either directly or via late endosomes or some other intermediate compartment (Cowles et al., 1997b; Piper et al., 1997; Stepp et al., 1997; Press et al., 1998). Although it is not known whether the latter route transits late endosomes or is direct to lysosomes, this distinction may not be significant because late endosomes fuse with lysosomes (Luzio et al., 2000), and the two compartments are thought to be in a dynamic equilibrium (Mellman, 1996).
Like the TGN, early endosomes have a highly tubular morphology and are specialized for protein sorting (Mellman, 1996; Lemmon and Traub, 2000; Woodman, 2000). Endocytosed proteins destined for degradation are sorted in early endosomes for transport to late endosomes along with newly synthesized lysosomal proteins, whereas most plasma membrane proteins recycle back to the cell surface. Transport from early endosomes to the plasma membrane has been best characterized for the trafficking of transferrin receptor (Mellman, 1996; Brown et al., 2000) and of synaptic vesicle components (Hannah et al., 1999). However, recycling and transcytotic proteins and membranes are not the only cargo that reach the cell surface from early endosomes; two illustrations of newly synthesized surface proteins passing through early endosomes are the biosynthetic transport of transferrin receptor (Futter et al., 1995) and asialoglycoprotein receptor H1 (Leitinger et al., 1995; Laird and Spiess, 2000). Major histocompatibility complex class II molecules are also transported directly from the TGN to various endosomal compartments where they bind partially degraded endocytosed antigens to present them on the cell surface (Wolf and Ploegh, 1995). Major histocompatibility complex class II–containing compartments were once believed to be specialized organelles with late endosome and lysosome-like properties unique to antigen-presenting cells, but it now appears that they include conventional compartments of perhaps all stages of the endocytic pathway (Kleijmeer et al., 1997). Although biosynthetic transport through endosomal compartments may exist in all cell types, these are most likely relatively minor routes, since time-lapse imaging of green fluorescent protein–tagged cargo molecules in polarized cells revealed only a direct TGN-to-plasma membrane route (Keller et al., 2001).
The complexity of the late secretory pathways has made it difficult to characterize both the many transport routes and the molecular machinery involved in cargo sorting and vesicle formation. Genetic screens in the yeast Saccharomyces cerevisiae have identified a large number of mutants that are blocked at various points along the exocytic, endocytic, and vacuolar/lysosomal pathways, and these mutants have greatly facilitated the identification of components required for membrane and protein trafficking in all eukaryotic cells (for reviews see Stack et al., 1995; Kaiser et al., 1997; Conibear and Stevens, 1998). Secretory cargo proteins appear to share similar requirements in ER-to-Golgi transport and in vesicle targeting and fusion with the cell surface, and the many proteins that mediate these transport steps have been particularly well characterized (Kuehn and Schekman, 1997; Guo et al., 2000). However, relatively few mutants have been found that block transport of exocytic cargo from the Golgi, and in both yeast and mammalian cells much less is known about the machinery responsible for sorting and packaging cargo into the vesicles that transport them to the cell surface.
The purification and analysis of secretory vesicles that accumulate in late (post-Golgi–blocked) exocytic yeast mutants has identified two vesicle populations with different densities and distinct enriched cargo, indicating that exocytic cargo can be transported by at least two routes (Harsay and Bretscher, 1995; David et al., 1998). In the past, defects in yeast exocytosis may have gone undetected because only a single secretory enzyme (usually invertase) was followed. Furthermore, cargo in a blocked pathway may be rerouted and secreted by an alternative unblocked route. Therefore, assaying for the transport of cargo in both exocytic pathways should allow identification of new exocytic mutants and reassessment of mutants judged previously as normal for exocytosis. In the present study, we show that some vacuolar protein sorting (VPS) proteins play an important role in cargo transport in at least one branch of the exocytic pathway, and we present evidence suggesting that this pathway may transit an endosomal compartment before reaching the cell surface.
Results
Invertase is missorted by several VPS mutants that block carboxypeptidase Y (CPY) transport to the vacuole
The sec6-4 mutant was among the first group of conditional yeast secretory mutants isolated in a screen for mutants that are blocked for growth and secretion and accumulate secretory organelles at a restrictive temperature (Novick et al., 1980). Sec6p is part of a protein complex (the “Exocyst”) involved in the polarized fusion of exocytic vesicles with the plasma membrane (TerBush et al., 1996). The mutant grows as well as wild-type cells at 24°C, but growth ceases at 37°C and the cells accumulate abundant 100-nm vesicles. These vesicles can be separated into two populations by isodensity gradient centrifugation (Fig. 1 A; Harsay and Bretscher, 1995). The more abundant, lighter density, vesicles contain the plasma membrane and cell wall proteins Pma1p and Bgl2p, respectively, whereas the denser vesicles contain the periplasmic enzymes invertase and acid phosphatase. Both vesicle populations also contain an exoglucanase activity, which most likely comprises two different enzymes (Harsay and Bretscher, 1995). Wild-type cells contain very few secretory vesicles at steady state, so it is difficult to distinguish vesicles from other organelles in cell fractionation experiments. Therefore, to analyze the effects of various mutations on the two exocytic pathways, we performed all fractionations with cells having a sec6-4 mutant background.
Figure 1.
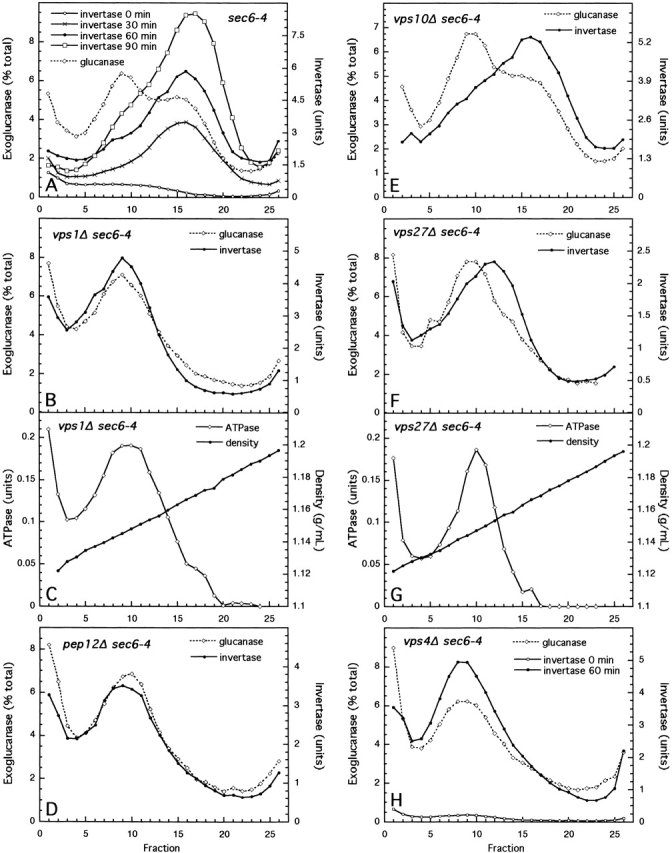
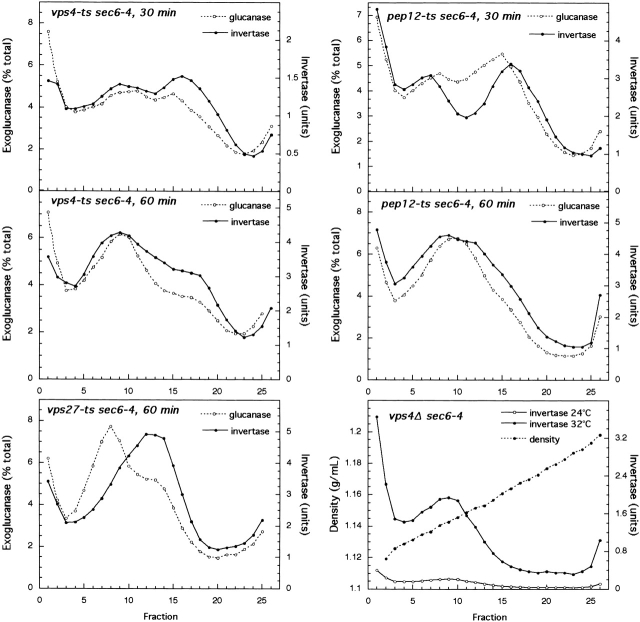
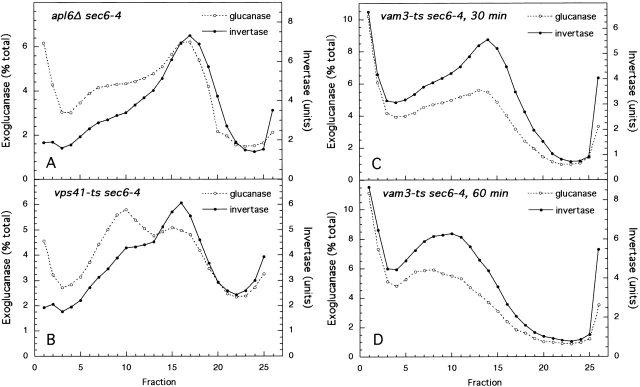
Gradient fractionation of sec6 and vpsΔ sec6 mutants. sec6 (EHY227), vps1Δ sec6 (EHY225), pep12Δ sec6 (EHY232), vps10Δ sec6 (EHY282), vps27Δ sec6 (EHY309), and vps4Δ sec6 (EHY327) cells were grown at 24°C in YPD for 12–14 h and then shifted to 37°C in prewarmed YPD, pH 4.5, for 1 h or for the times indicated. Cells were fractionated as described in Materials and methods. Membrane pellets (100,000 g) were loaded into the bottoms of 15–30% Nycodenz/0.8 M sorbitol linear gradients and floated to equilibrium. Fractions were collected from the top and assayed for enzyme activities. Where two graphs are shown for a strain (vps1Δ sec6 and vps27Δ sec6), activities were obtained from a single gradient. The density profiles were similar for all gradients.
Among the proteins known to be involved in vesicle formation, dynamins have been shown to function in the pinching off of clathrin-coated buds and caveolae at the plasma membrane (for review see McNiven et al., 2000) and in the formation of both constitutive and regulated exocytic vesicles and clathrin-coated vesicles from the TGN (Jones et al., 1998; Kreitzer et al., 2000; Yang et al., 2001). Dynamin homologs have been localized to other secretory organelles, including late endosomes (Nicoziani et al., 2000) and are therefore likely to function in several membrane-trafficking events. One of the yeast dynamin homologs, Vps1p, was identified in a screen for mutants that missort the vacuolar protein carboxypeptidase Y (CPY) to the cell surface (Rothman et al., 1990; Nothwehr et al., 1995). To determine whether Vps1p plays a role in the exocytic pathway, we used gradient fractionation to isolate secretory vesicles accumulated in vps1Δ sec6-4 cells. As shown in Fig. 1, B and C, this mutant accumulated both exoglucanase and invertase only at the density of light vesicles, suggesting that cargo normally transported by dense vesicles was missorted into light secretory vesicles. A similar result was obtained for the pep12Δ sec6-4 and vps4Δ sec6-4 mutants (Fig. 1, D and H). Pep12p, an endosomal t-SNARE (Becherer et al., 1996), is believed to function in the fusion of vesicles transporting vacuolar hydrolases from the Golgi to an endosomal compartment, and Vps4p is required for the formation of vesicles from endosomes (Babst et al., 1997). In the sec6-4 mutant, invertase is not sorted into light vesicles even after longer time shifts at the restrictive temperature (Fig. 1 A), suggesting that invertase in light vesicles in vps sec6-4 mutants is unlikely to represent backup into an upstream organelle.
Like vps4, vps27 blocks exit from an endosomal compartment and traps vacuolar and endocytosed proteins and recycling Golgi proteins in an exaggerated endosome (Piper et al., 1995), a characteristic of defects in 13 VPS genes grouped together as class E (Raymond et al., 1992). However, unlike vps4Δ sec6-4, the vps27Δ sec6-4 mutant did not missort invertase into light-density vesicles; instead, invertase accumulated at an intermediate density peak (Fig. 1, F and G). Because the density of sec6-4 invertase vesicles was consistent between fractionation experiments, this density shift is significant and indicates either altered properties of the invertase exocytic vesicles or the missorting or accumulation of invertase in some other compartment. We favor the latter possibility, as a vps27 mutant but not a vps4 mutant (without a sec6-4 background) has a mild secretory defect (unpublished data).
A vps mutant that lacks the sorting receptor for CPY, vps10Δ (Marcusson et al., 1994), was capable of sorting invertase properly (Fig. 1 E). However, unlike for all other mutants fractionated invertase sorting in the vps10Δ sec6-4 mutant varied between experiments. Lowering the pH of the growth medium to pH 4.5 (from pH 6.5 in standard rich medium reduced missorting, suggesting that a lower pH may be more optimal for proper invertase sorting. However, for all mutants except vps10Δ sec6, lowering the pH of the medium made no difference other than resulting in slightly lower levels of invertase activity. All results shown in Figs. 1 and 2 are from experiments in which cells were shifted into pH 4.5 medium.
Figure 2.
Gradient fractionation of vps-ts sec6-4 mutants. Strains were grown at 24°C in SD with required amino acids for maintaining plasmids (vps4-ts sec6, EHY348; pep12-ts sec6, EHY413) or in YPD (vps27-ts sec6, EHY374; vps4Δ sec6, EHY327). Strains grown in SD were shifted to YPD at 24°C for 2 h before 30- or 60-min shifts to 37°C in prewarmed YPD, pH 4.5. (Bottom right) A single culture of vps4Δ sec6 cells was split in half; one half was maintained at 24°C and the other half shifted to 32°C (semi-permissive for sec6-4) for 1h. Cells were fractionated as for Fig. 1, and gradient fractions were assayed for invertase and exoglucanase activities.
Conditional VPS mutants rapidly missort invertase
Although disruption mutations have the advantage that differences observed between mutants are not due to variations in the degree of loss of function, there is a possibility that the phenotypes are indirect long term effects of the mutations. Therefore, vps mutants with temperature-sensitive alleles were used to analyze invertase sorting shortly after the inactivation of these proteins. A 30-min shift to a restrictive temperature was sufficient to detect accumulated vesicles in the sec6-4 mutant (Fig. 1 A), so vps-ts sec6-4 double mutants were fractionated after 30- and 60-min incubations at a restrictive temperature (Fig. 2). For both vps4 (Babst et al., 1997) and pep12 (Burd et al., 1997) temperature-sensitive mutants, close to half of the accumulated invertase was in light vesicles by 30 min with most of the accumulation occurring in light vesicles after this time point, demonstrating that invertase was rapidly missorted in these mutants. Invertase accumulation in a vps27-ts sec6-4 mutant was in intermediate and high density membranes after a 60-min shift to a restrictive temperature (Fig. 2), indicating a difference between the vps4-ts and vps27-ts class E mutants, as was also observed for the disruption alleles (Fig. 1).
Although a vps4 mutation does not cause a kinetic lag in invertase transport (unpublished data), it is possible that when combined with a complete secretory block caused by the sec6-4 mutation, invertase is backed up into an upstream compartment. We wished to explore this possibility by shifting a vps4Δ sec6-4 strain to a semipermissive temperature at which there is only a small block in invertase transport. As shown in Fig. 2, all invertase was still in light-density vesicles under these conditions. Therefore, it is likely that invertase is transported in light-density vesicles in the vps4Δ mutant without a secretory block, and the invertase-containing membranes that accumulate in vps4 sec6-4 cells represent exocytic vesicles rather than an upstream compartment.
CPY and invertase cofractionate in vps sec6 mutants
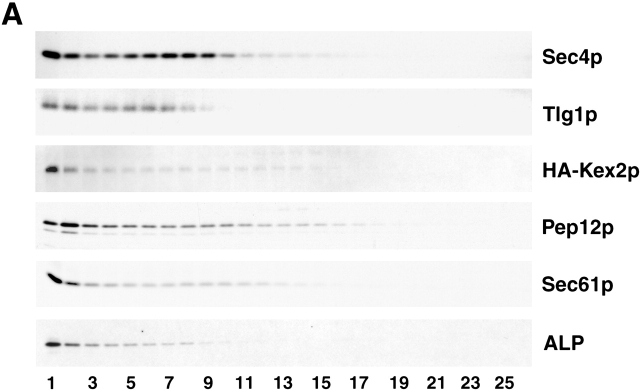
Exocytic vesicles that accumulate in sec6-4 cells did not cofractionate (copeak) with other organelles (Fig. 3 A; Harsay and Bretscher, 1995), although Sec4p on exocytic vesicles was only slightly denser than the early endosome/Golgi syntaxin Tlg1p, so we cannot exclude the possibility that Tlg1p is present on light-density vesicles. The fractionation of the TGN/endosomal marker Kex2p was difficult to assess, since Kex2p is known to become unstable in cells shifted to 37°C (Wilcox et al., 1992), and this instability was exacerbated in sec6-4 cells (Fig. 3 A). In wild-type cells, Kex2p peaks at the top of the gradient and in intermediate-density fractions (Fig. 3 B) that correspond to the density of accumulated invertase in vps27 sec6-4 cells (Figs. 1 and 2). Kex2p is believed to cycle between a late Golgi compartment and endosomes (Cereghino et al., 1995; Bryant and Stevens, 1997), but the identities and relative densities of Golgi and endosomal compartments in density gradients are unclear (Singer-Krüger et al., 1993; Holthuis et al., 1998a). The late endosomal syntaxin, Pep12p, the ER membrane protein, Sec61p, and the vacuolar membrane protein, alkaline phosphatase (ALP), clearly do not copeak with exocytic vesicles (Fig. 3 A). Similar results were obtained for vps sec6 cells (unpublished data; we did not examine Kex2p and Tlg1p in double mutants).
Figure 3.
Missorted CPY peaks with secretory vesicles in vps sec6 mutants but not with vacuolar, ER, or Golgi markers. (A) Western blots of gradient fractions from sec6-4 cells incubated at 37°C for 60 min show that secretory vesicles (Sec4p peak in fraction #8) do not cofractionate with the TGN/early endosome markers Kex2p and Tlg1p or with late endosomes (Pep12p), vacuoles (ALP), or ER (Sec61p). Sec4p and Tlg1p were detected in a single gradient from EHY227 cells; the other proteins were detected for EHY432 (sec6-4 cells with a plasmid expressing HA-tagged Kex2p). Cells were fractionated as in the legend to Fig. 1. (B) Immunoblots of gradient fractions indicate that missorted CPY cofractionates with the light secretory vesicle markers Pma1p and Bgl2p in the vps1Δ sec6-4 (EHY225) and vps4Δ sec6-4 (EHY478) mutants, whereas in vps10Δ sec6-4 (EHY282) CPY is in dense vesicles. In VPS SEC cells (EHY376 shifted to 37°C), Kex2p peaks at a density intermediate between light and dense secretory vesicles (Kex2p is unstable in mutants shifted to restrictive temperature), suggesting that invertase and CPY are not in Kex2p compartments in vps sec6 mutants. Cells were grown and fractionated as in the legend Fig. 1, except that for the vps10Δ sec6-4 and vps4Δ sec6-4 gradients, cells were shifted to 37°C for 40 min rather than 60 min, which greatly reduced the proteolysis of CPY; invertase profiles were similar for the two shift times. Lane numbers correspond with gradient fraction numbers. Unnumbered lanes are CPY processing standards: sec18-1 after a temperature shift contains ER (p1) and vacuole (m) forms, whereas pep4Δ contains the Golgi (p2) form.
We have also examined the fractionation of CPY in sec6 and vps sec6 mutants (Fig. 3 B). Because most of the vacuole was removed by differential centrifugation, only a small amount of CPY was present in a gradient from sec6-4 cells (Fig. 3 B). CPY in this gradient was primarily in the ER-modified p1 form (67 kD) and the mature vacuolar form (61 kD), peaking at the top of the gradient where the vacuolar membrane protein ALP fractionates (Fig. 3 A). The fractionation of the Golgi-processed p2 form (69 kD) was difficult to follow due to its sensitivity to proteolysis and low levels at steady state. However, because vps mutants missort and secrete vacuolar proteins, it was possible to evaluate p2 CPY accumulation in exocytic vesicles in vps sec6-4 mutants. The p2 form of CPY was present in light-density vesicles in the vps1Δ sec6-4 mutant (Fig. 3 B) as expected because this mutant appears to lack dense secretory vesicles. Of special interest was the fractionation of CPY in the vps10Δ sec6-4 mutant, since this mutant accumulated both populations of exocytic vesicles (Fig. 1 E). Under conditions in which invertase was in dense vesicles, p2 CPY likewise peaked with dense vesicles (Fig. 3 B). As mentioned above, invertase sorting varied in this mutant, but in all cases p2 CPY cofractionated with invertase (unpublished data). CPY in the vps4Δ sec6 mutant likewise fractionated at the density of secretory vesicles as indicated by the light vesicle markers Bgl2p and Pma1p (Fig. 3 B). However, in this case a significant portion of CPY was in the processed form. Such processing is unlikely to have occurred in vacuoles or endosomes, which fractionate in lighter fractions (Fig. 3 A); rather, secretory vesicles may contain proteases that are missorted along with CPY. It is not clear why CPY in vps1Δ sec6-4 cells, presumably in the same vesicles, was not likewise processed. However, the p2 CPY in these cells appears to be hypoglycosylated and is perhaps for that reason not efficiently proteolyzed. Invertase was likewise severely hypoglycosylated in vps1Δ sec6-4 cells but only slightly hypoglycosylated in other vps sec6-4 mutants (unpublished data). Graham et al. (1994) have shown that α-1,3 mannosyltransferase is mislocalized in a clathrin mutant, and a similar defect appears likely in vps1Δ cells.
Immunoisolated Pma1p-transporting vesicles contain invertase in vps sec6 mutants
The cofractionation of Pma1p and missorted invertase in vps sec6-4 mutants suggests that the proteins may be packaged into a common carrier. However, it is also possible that invertase is missorted into a different class of vesicles with fractionation properties very similar to that of Pma1p-transporting vesicles. To distinguish between these two possibilities, we immunoisolated Pma1p-transporting vesicles from vps sec6-4 mutants and assessed whether these vesicles contain invertase (Figs. 4 and 5) . Immunoisolations were performed with membranes fractionated on Percoll step gradients. The purpose of gradient fractionation was to separate light and dense secretory vesicles and to remove soluble invertase released from organelles and float vesicles away from proteases that reduce immunoisolation efficiency. A Percoll gradient was used rather than Nycodenz in order to maintain osmotic conditions during the gradient fractionation and immunoisolation procedures. We found that the invertase vesicles are particularly sensitive to osmotic changes. The low viscosity of Percoll also enabled organelles to reach equilibrium density after a 1-h centrifugation so that immunoisolation of fragile vesicles could be performed more quickly. Fig. 4 A shows the Percoll gradient fractionation profile of invertase from sec6-4 and vps1Δ sec6-4 cells. Using two different anti-Pma1p monoclonal antibodies bound to magnetic beads (see Materials and methods), we could isolate close to 60% of the invertase present in vps1 sec6 invertase peak fractions (Fig. 4 B). Very similar results were obtained for the light vesicle cargo protein Bgl2p (Fig. 4 C). The fraction of invertase and Bgl2p not isolated may correspond to leakage from the vesicles or their presence in cofractionating organelles with lower amounts of Pma1p. To demonstrate the specificity of the immunoisolation procedure, we used peptides corresponding to the mapped epitopes of each of the antibodies (Serrano et al., 1993) in competition experiments (Fig. 4, B and C). In each case, the corresponding peptide competed specifically with the monoclonal antibody, with the peptide for antibody #17 being a more effective competitor (most likely due to a closer resemblance to the corresponding sequence in the folded native protein).
Figure 4.
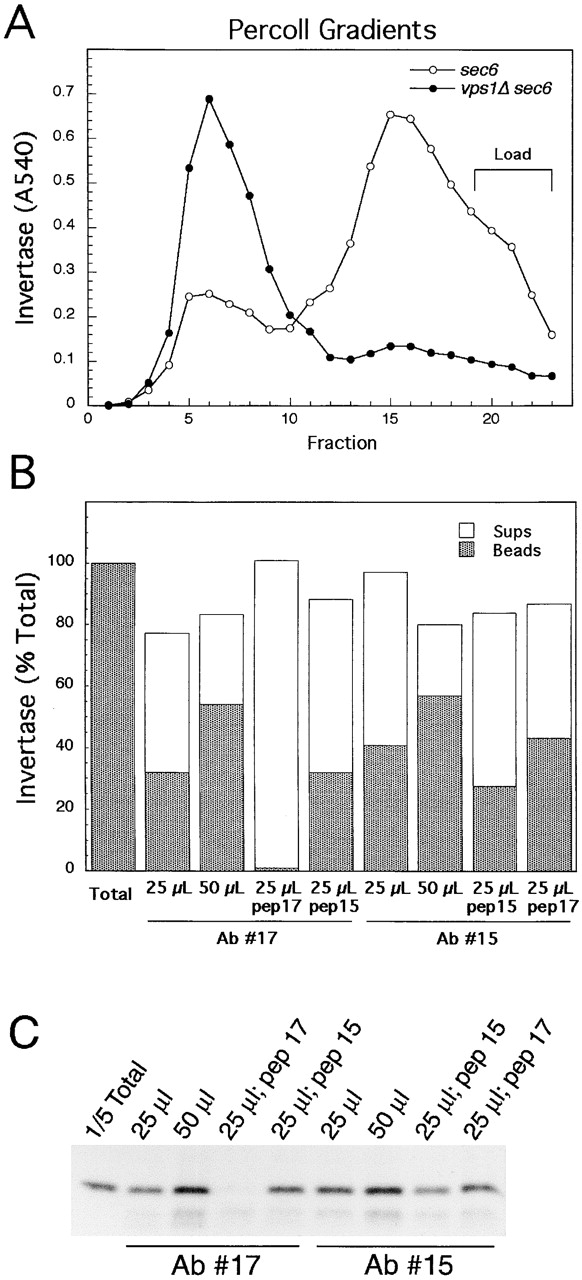
Vesicles immunoisolated from vps1Δ sec6-4 cells using anti-Pma1p monoclonal antibodies contain invertase and Bgl2p. (A) EHY225 (vps1Δ sec6-4) and EHY227 (sec6-4) cells were shifted to 37°C for 40 min and fractionated on 20–55% Percoll step gradients as described in Materials and methods; fractions were collected from the top and assayed for enzyme activities. (B) Two different anti-Pma1p monoclonal antibodies (Ab #15 and Ab #17) bound to Dynabeads protein G were used to immunoisolate Pma1p-containing membranes from the invertase peak fraction (#6) from a vps1 sec6 Percoll gradient fractionation. Either 25 or 50 μl beads were used as indicated. All incubations contained the same amount of membrane with invertase activity shown as “Total.” In antibody competition experiments, peptides corresponding to the mapped monoclonal epitopes (pep15, pep17) were preincubated with the beads and included in the immunoisolation reactions. (C) Western blot to detect Bgl2p in the immunoisolation reactions described in B.
Figure 5.
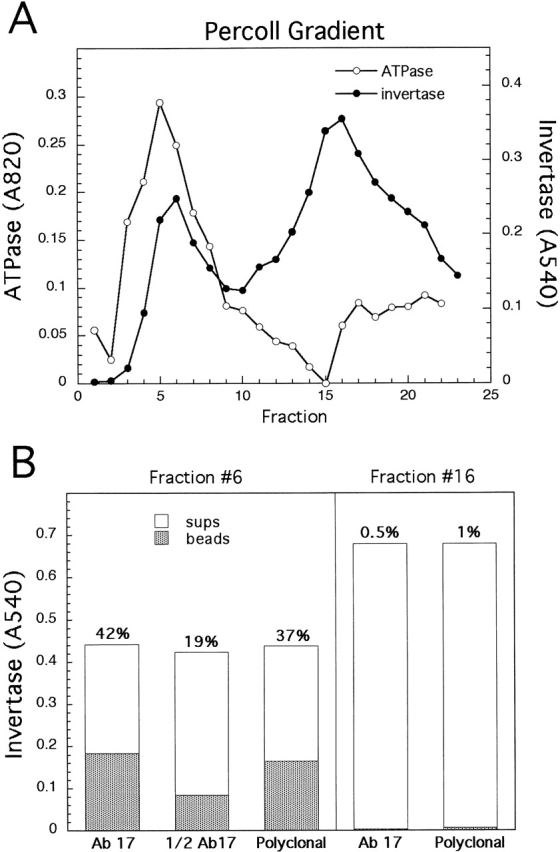
Light but not dense invertase-containing vesicles can be immunoisolated from vps4-ts sec6-4 cells. (A) 20–55% Percoll gradients from the fractionation of a vps4-ts sec6-4 strain (EHY348) contain both light and dense invertase-containing vesicles (as in Nycodenz gradiens; Fig. 2) and light invertase-containing vesicles cofractionate with ATPase (Pma1p) activity. Cells in the gradient shown were shifted to 37°C for 30 min, including ∼5 min warm-up time. (B) Membranes (equal volumes) from invertase peak fractions (#6 or #16 as indicated) from the gradient in A were incubated with undersaturating Dynabeads M500 coated with either monoclonal antibody #17 or with affinity-purified anti-Pma1p polyconal antibodies. The percent invertase bound to the beads is shown above the bars.
Pma1p is an abundant cargo protein and may appear at some level in all classes of secretory vesicles. Therefore, we compared the isolation efficiency of invertase in dense vesicles to that of invertase missorted into light vesicles using an undersaturating amount of anti-Pma1p beads (Fig. 5). To evaluate vesicles from a single gradient and the same strain, we fractionated a vps4-ts sec6 strain, which after short shift times contains both light and dense invertase vesicles (Fig. 2 and Fig. 5 A). We found that almost none of the dense vesicles were bound to the beads.
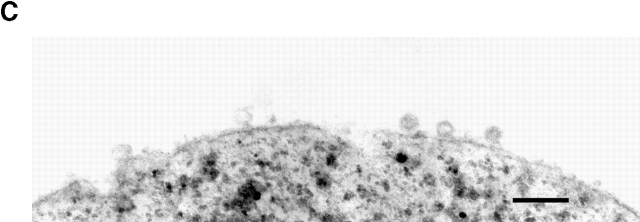
Thin section electron microscopic examination of immunoisolated membranes from vps4Δ sec6-4 cells indicated 100-nm vesicles and some tubular membranes bound to the beads (Fig. 6) .
Figure 6.
Thin section EM of vesicles immunoisolated with anti-Pma1p monoclonal antibody #17 bound to Dynabeads protein G. A vps4Δ sec6-4 strain (EHY327) was fractionated as in the legend to Fig. 5, and membranes from the light invertase peak fraction were immunoisolated using undersaturating beads. Bar, 200 nm.
Clathrin-coated vesicles transport invertase
Clathrin is involved in TGN-to-endosome transport of lysosomal precursors in mammalian cells (Kornfeld and Mellman, 1989), and it plays a similar role in the transport of vacuolar hydrolases in yeast (Seeger and Payne, 1992). A mutant with a temperature-sensitive chc1 (clathrin heavy chain) allele rapidly and severely missorts vacuolar hydrolases after a shift to a restrictive temperature but over time appears to regain the ability to properly sort vacuolar proteins (Seeger and Payne, 1992). A sec6-4 mutant with this temperature-sensitive chc1 allele missorted invertase into light-density vesicles (Fig. 7) , consistent with other vps mutants that block transport to endosomes. Gradients obtained after both the 30- and 60-min shifts also displayed a small peak of invertase in the same fractions where invertase peaked in vps27Δ sec6-4 (Fig. 1) and vps27-ts sec6-4 (Fig. 2) gradients, indicating that invertase may accumulate in both light and intermediate-density vesicles in this mutant.
Figure 7.
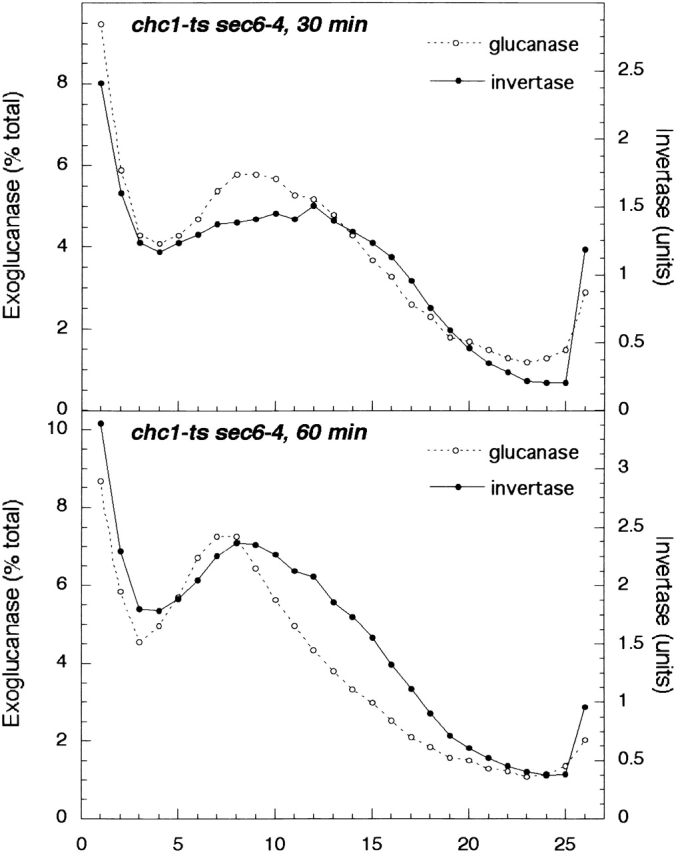
Nycodenz gradient fractionation (performed as in the legend to Figs. 1 and 2) of a chc1-ts sec6-4 strain (EHY242). The gradient profiles of invertase and exoglucanase show a missorting of these exocytic cargo molecules, indicating that clathrin plays a role in invertase transport, as was observed for vps sec6-4 strains (Figs. 1 and 2).
Invertase may be transported by clathrin-coated vesicles, or clathrin may play a less direct role in invertase transport by, for example, recycling Golgi proteins required for invertase transport. Previous immunoisolations of clathrin-coated vesicles suggested that clathrin is not involved directly in invertase transport (Deloche et al., 2001). However, invertase in transport vesicles in wild-type cells may represent a very small fraction of the total invertase in the high speed spin membrane fractions used. Furthermore, it is possible that insufficient osmotic support was provided to prevent leakage of invertase, which we found to be released easily from membranes. Therefore, we immunoisolated clathrin-coated vesicles from gradient fractions of membranes isolated from wild-type cells (Fig. 8) . A high buoyant density fraction of invertase cofractionated with clathrin light chain but not with the cis/medial Golgi marker, Gda1p (Fig. 8 A). Immunoisolation of clathrin-coated vesicles from fraction #14 isolated up to 30% of the invertase (Fig. 8 B); nearly identical results were obtained in immunoisolations from neighboring gradient fractions (fractions #13–17; unpublished data). Anti-GST antibodies isolated from the same serum from which anti-Clc1p antibodies were purified was used as a negative control. The clathrin-coated vesicle cargo protein Vps10p was somewhat more efficiently isolated in the same experiment (Fig. 8 B), as expected, because at steady state invertase should be distributed more evenly among secretory organelles than a recycling receptor. Pma1p contained in the same gradient fraction was not likewise immunoisolated; thus, only a subset of exocytic cargo molecules are trafficked via clathrin-coated vesicles. Thin section EM of the isolated membranes showed that essentially all membranes bound to the beads were ∼40-nm vesicles (Fig. 8 C). We had difficulty detecting coats on the surfaces of the vesicles, most likely because they did not preserve or stain well during processing for microscopy.
Figure 8.
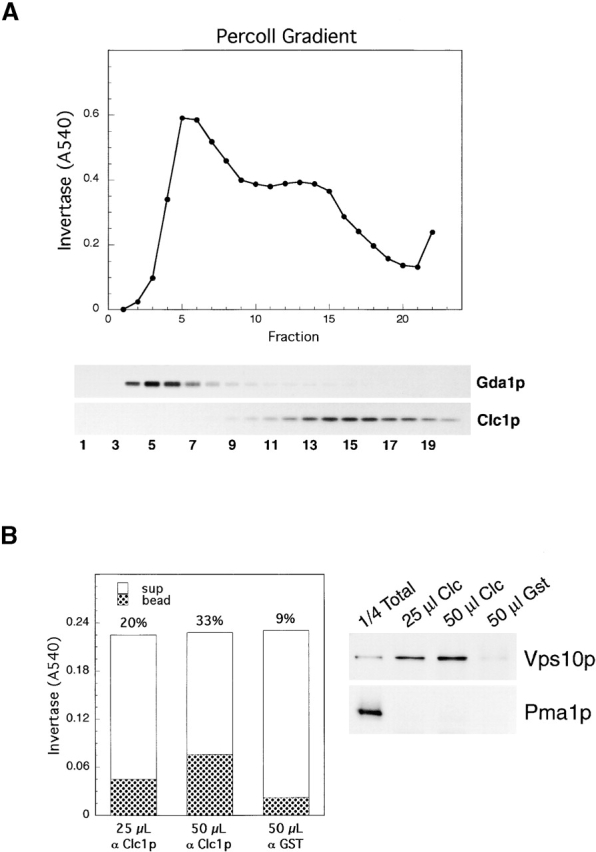
Clathrin-coated vesicles transport invertase. (A) Percoll gradient fractionation of a wt strain (EHY191) for identifying fractions enriched for clathrin-containing membranes. A 25–55% Percoll step gradient was formed in a buffer optimized for clathrin coat stabilization. Fractions were collected from the top and assayed for invertase activity and by Western blotting to detect clathrin light chain (Clc1p) and GDPase (Gda1p). (B) Immunoisolated clathrin-coated vesicles (from fraction #14 in the gradient in A) contain invertase and Vps10p but not Pma1p (immunoblots and enzyme assays are from the same immunoisolation experiment). (C) Thin section EM of immunoisolated clathrin-coated vesicles. Bar, 100 nm.
Mutants that block the ALP-transporting pathway to the vacuole do not missort invertase
All of the VPS proteins discussed so far are required for the transport of CPY to the vacuole via an endosomal intermediate. However, the vacuolar membrane protein ALP is transported by an alternate route that bypasses endosome(s) (Cowles et al., 1997b; Piper et al., 1997). Vps1p is required for the normal transport of both CPY and ALP to the vacuole, but Pep12p, Vps4p, and Vps27p are not required for ALP transport. Proteins required for the transport of ALP but not of CPY include Vps41p (Cowles et al., 1997b) and components of the AP-3 adaptin complex (Cowles et al., 1997a; Stepp et al., 1997). We fractionated an apl6Δ sec6-4 mutant, which lacks the β-subunit of the AP-3 complex, to determine whether blocking the ALP pathway to the vacuole affects invertase transport. As shown in Fig. 9 A, this mutant sorted invertase properly into dense vesicles. The slightly lowered level of exoglucanase activity in the light vesicles was reproducible in two experiments; however, these vesicles contained abundant ATPase activity, so the light vesicles were formed properly (unpublished data). Similar results for invertase sorting were obtained for vps41-ts sec6-4 (Fig. 9 B) and vps41Δ sec6-4 (unpublished data), indicating that blocking the ALP pathway does not have a significant effect on the exocytic pathway. Although the sorting of CPY is not affected by a vps41-ts mutation after short shifts to a restrictive temperature, CPY missorting is severe in vps41Δ (Cowles et al., 1997b; Radisky et al., 1997), whereas invertase sorting is only slightly affected. Such differences in missorting are also noted among vacuolar hydrolases that traffic by the CPY (endosome-mediated) pathway. In vps41Δ, the secretion of the soluble vacuolar hydrolase, PrA, is much less severe than that of CPY (Radisky et al., 1997).
Figure 9.
Gradient fractionation of mutants blocked in the ALP-transporting pathway to the vacuole. apl6Δ sec6 (EHY351) and vps41-ts sec6 (EHY350) cells (A and B) were shifted to 37°C for 1 h; vam3-ts sec6 (EHY436) cells were shifted to 38°C for 30 min (C) or 60 min (D). Cells were fractionated as described in the legend to Fig. 1, and gradient fractions were assayed for enzyme activities.
A syntaxin homologue associated with vacuolar membranes, Vam3p, is required for the transport of both CPY and ALP (Darsow et al., 1997; Piper et al., 1997) and believed to function in the final docking and/or fusion step at the vacuole (Darsow et al., 1997). Most invertase accumulated in dense vesicles in a vam3-ts sec6–4 mutant after 30 min at the restrictive temperature (Fig. 9 C), although after 60 min invertase was no longer sorted properly and fractionated as a broad peak similar to the density of light exocytic vesicles (Fig. 9 D). In contrast, close to half of the accumulated invertase was missorted in the vps4-ts sec6-4 and pep12-ts sec6-4 mutants after 30 min at the restrictive temperature (Fig. 2). Because the vam3-ts mutant is blocked in transport immediately upon shifting to a restrictive temperature (Darsow et al., 1997), a lack of significant missorting after a 30-min shift suggests that the effect of vam3-ts on invertase transport after a longer shift is indirect.
Discussion
Clathrin and VPS proteins required for the CPY pathway to the vacuole also function in invertase transport
This work demonstrates that a subset of yeast mutants defective in the biosynthetic pathway to the vacuole/lysosome is also defective in at least one branch of the exocytic pathway. Cargo that is normally present in dense exocytic vesicles accumulated by the sec6-4 mutant is missorted into light exocytic vesicles in mutants that are blocked in the transport of CPY and other hydrolases to an endosomal compartment en route to the vacuole. These mutants include vps1, chc1, and pep12, which are believed to be blocked in the formation, targeting, or fusion of vesicles trafficking to endosomes. Missorting of invertase and exoglucanase into light-density vesicles in these mutants is due either to defective biogenesis of the dense vesicles or to a defect in cargo packaging into dense vesicles.
A likely explanation for the requirement of these genes in one branch of the late exocytic pathway is that a subset of exocytic cargo transits through an endosomal compartment before reaching the cell surface. Invertase and vacuolar proteins in the CPY pathway may be transported together from the late Golgi to endosomes where they are sorted to the cell surface and to the vacuole, respectively. When the common pathway to endosomes is blocked, both invertase and CPY are rerouted to the cell surface via light secretory vesicles. Consistent with this model, we find that two mutants that are blocked in a vacuolar pathway that bypasses endosomes, apl6 and vps41, have little or no effect on the sorting of invertase. A class E vps mutant, vps4-ts, which blocks the exit of vacuolar, endocytosed, and recycling Golgi proteins from endosomes, rapidly missorts invertase into light vesicles, presumably because components required for normal invertase sorting or transport are not recycled from endosomes. Two other newly synthesized yeast proteins, a mutant form of the plasma membrane ATPase Pma1p (Luo and Chang, 2000) and the iron oxidase Fet3p (Radisky et al., 1997; Yuan et al., 1997), have been proposed to reach the cell surface from a post-Golgi compartment.
An alternative explanation for the missorting of exocytic cargo in vps mutants is that traffic between the Golgi and endosomes is somehow required for the maintenance of the normal sorting competence of the Golgi. Although we cannot rule out this possibility, the rapid onset of invertase missorting in conditional vps mutants suggests a more direct role of VPS proteins in invertase transport. The characterization of organelles that accumulate in vps mutants may provide further evidence for such a direct role, but our preliminary attempts to do so were hindered by the relatively small amounts of accumulated proteins (consistent with efficient missorting in many vps mutants). However, we have immunoisolated clathrin-coated vesicles from wild-type cells and showed that they contain invertase. Clathrin has been shown to play a role in Golgi-to-endosome transport in both yeast and mammalian cells (Kornfeld and Mellman, 1989; Seeger and Payne, 1992) but also mediates other transport routes by associating with different adaptin complexes (Robinson and Bonifacino, 2001).
Invertase and CPY transport in the vps10 sec6 mutant
A vps10 sec6 mutant, which lacks the sorting receptor for CPY (Marcusson et al., 1994), accumulates both invertase and CPY in dense vesicles, although this result was variable and influenced by the pH of the growth medium (unpublished data). An initial expectation for this mutant was the missorting of CPY into both exocytic pathways, or primarily into light vesicles, which are the most abundant membranes in our gradients (Harsay and Bretscher, 1995). However, CPY can be sorted into dense vesicles even in the absence of Vps10p, indicating a Vps10p-independent sorting step. Therefore, there may be two sorting steps for CPY, first at the Golgi and then at the early endosome, and only the second sorting step may be directly dependent on Vps10p. The effect of growth conditions on sorting may reflect secondary effects due to defective transport of Vps10p-dependent cargoes. Another possibility is that Vps10p functions at both the Golgi and endosome, but an alternative pH-sensitive sorting mechanism also functions at the Golgi. A pH-sensitive sorting step has been indicated for another soluble vacuolar hydrolase, proteinase A, which is sorted to the vacuole in the absence of Vps10p in low pH media, but its efficient sorting requires Vps10p when the pH of the growth medium is >5.0 (Seaman et al., 1997).
Although Vps10p has been proposed to function in sorting from the Golgi (Marcusson et al., 1994; Cooper and Stevens, 1996), it is not surprising that it may also sort cargo at early endosomes. A recycling pathway from endosomes to the plasma membrane has been demonstrated for yeast (Holthuis et al., 1998b; Chen and Davis, 2000; Wiederkehr et al., 2000); therefore, proteins en route to the vacuole need to be sorted from recycling proteins at the endosome. The mannose 6-phosphate receptor, which sorts soluble lysosomal enzymes from the Golgi to early endosomes (Press et al., 1998), is found predominantly in late rather than early endosomes (Bleekemolen et al., 1988; Griffiths et al., 1988) consistent with a role in early endosome to late endosome sorting before recycling back to the Golgi.
The role of class E VPS proteins in exocytic and vacuolar cargo transport
A better understanding of the functions of class E VPS proteins may help to clarify the roles of early and late endosomes in yeast. The different effects of two class E mutants, vps4 and vps27, on invertase transport suggests that Vps4p and Vps27p may regulate different transport steps either from the same compartment or from different compartments. Although vps4 and vps27 are the most well-characterized class E mutants and have very similar phenotypes (Piper et al., 1995; Babst et al., 1997), the same prominent phenotype (accumulation of the “class E compartment”) may be a more direct effect of one mutation than of the other, or the mutations may have different effects on additional organelles. Perhaps one mutation blocks exit primarily from early endosomes, whereas the other blocks exit from late endosomes or from a different type of early endosome. Several types of early endosomes have been recognized in mammalian cells based on their distinct morphologies and functions (Brown et al., 2000; Lemmon and Traub, 2000). Yeast endosomes are much less clearly characterized; however, the existence of three different Rab proteins that function in the early endocytic pathway (Singer-Krüger et al., 1994) suggests a similar complexity. The vps27 mutant appears to have a more severe effect on invertase transport than other vps mutants, and its mammalian homologue, Hrs, has been localized to early rather than late endosomes (Hayakawa and Kitamura, 2000; Raiborg et al., 2001). Cargo in the invertase-transporting pathway may, therefore, transit early endosomes rather than (or in addition to) late endosomes. Direct traffic from the TGN to early endosomes is also consistent with other examples in which newly synthesized proteins transit an endosome before exocytosis (Futter et al., 1995; Leitinger et al., 1995; Sariola et al., 1995). However, we found a strong effect on invertase sorting by pep12 mutations (thought to block vesicle fusion with late endosomes), so it is possible that invertase is transported from early to late endosomes from which it then reaches the cell surface. CPY and other vacuolar proteins may likewise traffic through both early and late endosomes as has been shown for lysosomal hydrolases in mammalian cells (Ludwig et al., 1991; Press et al., 1998). If such is the case, then some VPS proteins are likely to function primarily in early endosome-to-late endosome transport, and missorting takes place in early endosomes rather than at the TGN.
Why transport exocytic cargo through endosomes?
All previous examples of newly synthesized proteins transiting through endosomes before reaching the cell surface are of membrane proteins that have special functions in, or recycle through, endosomal compartments, so it is not entirely unexpected that they are targeted to endosomes directly from the Golgi. However, no prior evidence exists for newly synthesized soluble exocytic proteins transiting endosomes en route to the cell surface, and this is thought to be a specialized rather than a general route. Why might soluble cargo transit through an early endosomal compartment? The answer to this question may also provide an explanation for why yeast exocytic cargo is sorted into separate pathways.
One reason for divergent exocytic pathways in yeast may be that different types of cargo may have different processing requirements and are therefore routed through different compartments. Alternatively, sorting into divergent routes may reflect the need to regulate differentially a pathway mediating surface expansion and a pathway exporting soluble proteins destined for release from the cell. This possibility is consistent with the nature of the cargo thus far identified in the two pathways. Another feature of markers in dense exocytic vesicles is that they are required only under certain physiological conditions (invertase in low glucose and acid phosphatase in low phosphate). Although both secreted invertase and acid phosphatase are regulated at the transcriptional level (Johnston and Carlson, 1992), a second level of regulation in the form of differential trafficking may exist also, and this form of regulation may be important for other cargo that share this pathway. One such cargo may be the general amino acid permease Gap1p, which is regulated both transcriptionally and posttranslationally by differential sorting in the late secretory pathway (Roberg et al., 1997). When cells are grown on urea, Gap1p is transported to the plasma membrane, whereas in cells grown on glutamate Gap1p is sorted to the vacuole. Perhaps proteins that are required only under certain growth conditions are first transported to early endosomes, which receive traffic from and may have sorting properties affected by the external environment, and from this point the various cargoes are sorted either to the plasma membrane or to late endosomes for vacuolar degradation. This type of regulation may be useful especially for proteins whose external levels need to be rapidly adjusted to environmental conditions or for soluble proteins, which unlike membrane proteins cannot be efficiently retrieved from the cell surface if they are no longer needed.
The involvement of VPS proteins in one branch of the yeast exocytic pathway shows promise for the isolation of additional mutants that block transport of only a subset of exocytic cargo from the Golgi or from endosomes. We have yet to identify mutants that specifically block the major, Pma1p-transporting, pathway, and screening for a complete secretory block in a vps mutant background may facilitate the isolation of such mutants. The characterization of more mutants blocked uniquely in one exocytic pathway should contribute to our knowledge of exocytic cargo sorting and vesicle formation at the late Golgi.
Materials and methods
Materials
Yeast growth media were prepared as described by Guthrie and Fink (1991). Reagents for media were obtained from Difco Laboratories, Inc. Rich medium (YPD) contained 1% Bacto-yeast extract, 2% Bacto-peptone, and 2% glucose. Where noted, the pH was adjusted to 4.5 with HCl (∼1 ml 6 N HCl per 500 ml of medium). Minimal media (synthetic dextrose; SD) contained 0.67% yeast nitrogen base with ammonium sulfate, 2% glucose, and required amino acids. Restriction and modifying enzymes were from Boehringer or New England Biolabs, Inc. Nycodenz was from Life Technologies, Inc., Percoll was from Amersham Pharmacia Biotech, Zymolyase-100T was from US Biological, and BSA (protease- and IgG-free) was from Jackson ImmunoResearch Laboratories. All other reagents were obtained from Sigma Aldrich unless otherwise noted. Optical density and absorbance values were read on a Genesys 5 spectrophotometer (Spectronic Instruments).
Yeast strains and plasmids
Yeast strains used in this study are listed in Table I. Standard yeast genetic techniques were used to perform crosses and tetrad analysis (Guthrie and Fink, 1991). Yeast transformations were by the lithium acetate method (Schiestl and Gietz, 1989). For plasmid construction, DNA fragments were isolated using a QIAEX II kit from QIAGEN, and plasmid DNA was isolated using a QIAPrep Spin kit (QIAGEN). Escherichia coli transformations were performed using INVαF' competent cells from Invitrogen. All other recombinant DNA techniques were performed as described by Ausubel et al. (1987).
Table I. S. cerevisiae strains used in this study.
| Strain | Relevant genotypea | Source |
|---|---|---|
| DBY1829 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | T. Huffakerb |
| NY10 | MATα ura3-52 | P. Novickc |
| NY17 | MATa sec6-4 ura3-52 | Salminen and Novick, 1987 |
| CBY31 | MATα pep12Δ::HIS3 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 | Burd et al., 1997 |
| CCY255 | MATα apl6Δ::HIS3 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 | Cowles et al., 1997a |
| WSY41 | MATα vps41Δ::LEU2 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 | Cowles et al., 1997b |
| MBY3 | MATα vps4Δ::TRP1 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 | Babst et al., 1997 |
| RPY2 | MATa vps27-123(ts) leu2-3,112 ura3-52 his4-519 ade6 | Piper et al., 1995 |
| TDY2 | MATα vam3Δ::LEU2 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 [vam3-ts URA3] | Darsow et al., 1997 |
| EHY47 | MATa his3-Δ200 leu2-3,112 trp1-1 ura3-52 | NY17 × DBY1829 |
| EHY50 | MATa sec6-4 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | NY17 × DBY1829 |
| EHY52 | MATα sec6-4 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | NY17 × DBY1829 |
| EHY62 | MATα sec6-4 vps10Δ::TRP1 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | EHY52, integ. pEMY10-3 |
| EHY188 | MATα sec6-4 TPI::SUC2::HIS3 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | EHY52, integ. pEH117 |
| EHY191 | MATa ade2-1 his3-11,15 leu2-3,112 trp1-Δ ura3-1 pep4Δ::TRP1 TPI::SUC2::HIS3 | RSY 620, integ. pEH117 |
| EHY225 | MATα sec6-4 vps1::LEU2 TPI::SUC2::HIS3 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-5 | EHY188, integ. pCKR2 |
| EHY226 | MATa sec6-4 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | EHY50, integ. pEH119 |
| EHY227 | MATa sec6-4 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | EHY50, integ. pEH118 |
| EHY232 | MATa sec6-4 pep12Δ::HIS3 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1 ura3-52 | EHY227 × CBY31 |
| EHY239 | MATa TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | EHY47, integ. pEH118 |
| EHY242 | MATα sec6-4 chc1-521(ts) TPI::SUC2::URA3 his3-Δ200 ura3-5 | Materials and methods |
| EHY282 | MATα sec6-4 vps10Δ::TRP1 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | EHY62, integ. pEH119 |
| EHY309 | MATa sec6-4 vps27Δ::LEU2 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | EHY227, integ. pKJH2 |
| EHY326 | MATα sec6-4 vps41Δ::LEU2 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 | EHY226 × WSY41 |
| EHY327 | MATα sec6-4 vps4Δ::TRP1 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 | EHY226 × MBY3 |
| EHY348 | MATα sec6-4 vps4Δ::TRP1 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 [vps4-ts HIS3] | EHY327, pMB59 |
| EHY351 | MATα sec6-4 apl6Δ::HIS3 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 | EHY227 × CCY255 |
| EHY374 | MATa sec6-4 vps27-123(ts) TPI::SUC2::TRP1 leu2-3,112 ura3-52d | EHY227 × RPY2 |
| EHY376 | MATa TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 [HA::KEX2 LEU2] | EHY227 × RPY2 |
| EHY413 | MATa sec6-4 pep12Δ::HIS3 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 [pep12-ts TRP1] | EHY226 × CBY31, pCB49 |
| EHY432 | MATa sec6-4 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 [HA::KEX2 URA3] | EHY227, pSN218 |
| EHY436 | MATa sec6-4 vam3Δ::LEU2 TPI::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 [vam3-ts URA3] | EHY227 × TDY2 |
| EHY350 | MATα sec6-4 vps41Δ::LEU2 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 [vps41-ts TRP1] | EHY326, integ. pVPS41-85 |
| EHY478 | MATα sec6-4 vps4Δ::TRP1 TPI::SUC2::URA3 his3-Δ200 leu2-3,112 trp1 ura3-52 PMA1-HA::LEU2 | EHY327, integ. HA-PMA1 |
Genes born by plasmids are indicated by brackets.
Cornell University, Ithaca, NY.
Yale University, New Haven, CT.
May also be his4-519 and/or his3-Δ200.
The integrating plasmids pEH117, pEH118, and pEH119 contained the SUC2 gene fused to the strong constitutive promoter of triose-phosphate isomerase (TPI1) and were constructed as follows: pDB31 (Brada and Schekman, 1988) was cut with SmaI and EcoRI to release a 6-kb fragment containing TPI::SUC2; this fragment was ligated into the SmaI-EcoRI sites of pRS303, pRS304, and pRS306 (Sikorski and Hieter, 1989) to generate pEH117, pEH118, and pEH119, respectively. To integrate the plasmids into the SUC2 locus, we converted plasmid DNA to linear form with AgeI (which cuts within SUC2) before transformation. Epitope tagging of PMA1 was performed as described (Ziman et al., 1996). Plasmid pVPS41-85 was described by Cowles et al. (1997b), pMB59 by Babst et al. (1997), pVPS45–28 by Cowles et al. (1994), pCB49 by Burd et al. (1997), and pSN218, pSN222 by Nothwehr et al. (1995); all six plasmids have YCp (low copy) backbones. The integrating plasmid pKJH2 was used for disrupting VPS27 as described by Raymond et al. (1992), pCKR2 was used to disrupt VPS1 as described by Rothman et al. (1990), and pEMY10-3 was used to disrupt VPS10 as described by Marcusson et al. (1994). In all cases where a vpsΔ mutant was generated with an integrating plasmid rather than a by a cross, integrants were analyzed for the secretion of CPY by colony blot overlay as described by Roberts et al. (1991) to confirm the proper targeting of the disruption fragment.
To construct a temperature-sensitive clathrin mutant in our strain background, we used the integrating plasmid YIpchc521-ΔClaI (Tan et al., 1993) to replace the wild-type copy of CHC1 in the strain NY10, congenic with NY17 (Salminen and Novick, 1987); the resulting strain was then crossed with EHY226 (sec6-4) to generate the chc1-521 sec6-4 mutant (confirmed by complementation analysis).
Subcellular fractionation
Overnight primary yeast cultures (OD600 ∼0.5–2) were inoculated into YPD or SD with required amino acids (500 ml culture per gradient) and grown for 12–16 h at 24°C to OD600 0.5–0.7. For SD cultures, growth was continued for an additional 2 h at 24°C in 250 ml YPD to allow cells to adjust to rich medium. Cells were then shifted to 37°C for 60 min (unless otherwise indicated) in 250 ml prewarmed YPD to induce the sec6-4 secretory block. All cells constitutively expressed invertase (Suc2p), so it was not necessary to derepress SUC2 by lowering glucose concentrations. Conversion of cells to spheroplasts and lysis was performed as described by Harsay and Bretscher (1995) except spheroplasts were washed only once and lysis was performed with 15 strokes in a Dounce homogenizer, sufficient to result in ∼60–90% cell lysis. Fractionation of secretory vesicles was performed as described (Harsay and Bretscher, 1995) with the following changes: for differential centrifugation, a 700-g spin was not performed routinely so that the first spin was at 13,000 g for 20 min. The supernatant from this spin was divided into two 17-ml tubes per gradient (SW28.1; Beckman Coulter), and a 70 μl cushion of 40% Nycodenz in lysis buffer was placed through the sample onto the bottom of each tube with a Pasteur pipette. After centrifugation at 100,000 g av for 90 min, the supernatants were removed by pipetting, leaving ∼400 μl at the bottom of each tube. The small cushion, which was mixed with the membranes before loading gradients, allowed easy resuspension so that incubation on ice to loosen pellets was not necessary. Linear 15–30% Nycodenz/0.8 M sorbitol gradients were formed in 12.5-ml tubes (SW41; Beckman Coulter) using a two-chambered gradient mixer attached to an Auto Densi-Flow II C fractionator (Labconco). Membranes were mixed with 80% Nycodenz in lysis buffer so that the load volume for each gradient was 1.5 ml in ∼32% Nycodenz. The membranes were loaded through the gradients and centrifuged at 100,000 g av for 15–16 h (to equilibrium); 0.4-ml fractions were collected from the top using an Isco Model 640 density gradient fractionator. Fraction densities were determined by reading refractive indices on a Bausch and Lomb refractometer and converting these values to g/ml based on a standard curve generated by five weighed standards. The equation for the standard curve is ρ = 3.384η−3.536, where ρ is density in g/ml and η is the refractive index.
For Percoll density gradient fractionation, we shifted vps1Δ sec6-4 and sec6-4 cells for 40 min and vps4-ts sec6-4 for 30 min in YPD at 37°C. A high speed spin (100,000 g av) membrane fraction was prepared as above except rather than a Nycodenz cushion a 200 μl 67% Percoll solution in lysis buffer was placed through the samples into the bottoms of the SW28.1 tubes. When preparing the 67% Percoll solution, we adjusted the sorbitol buffer concentration to account for the significant volume taken up by Percoll particles. A 25 ml 67% Percoll solution contained 16.75 ml Percoll, 7.5 ml 3× sorbitol-triethanolamine lysis buffer (3× is 2.4 M sorbitol, 30 mM triethanolamine, 3 mM EDTA, pH 7.2), and 0.75 ml H2O; final pH was adjusted to pH 7.2 with acetic acid. The membranes were adjusted to 58% Percoll in 3.5 ml (475 μl membranes, 3.025 ml 67% Percoll solution), placed into the bottom of an SW41 tube, and the following gradient steps were added: 2 ml 55%, 1.5 ml 50%, 1.5 ml 40%, 2 ml 30%, and 1.5 ml 20% Percoll. The steps were prepared by mixing lysis buffer containing 2 mg/ml BSA with 67% Percoll solution that contained 2 mg/ml BSA. The gradients were spun for 1 h at 100,000 g av, and 0.5-ml fractions were collected from the top. Percoll gradient fractionation of wild-type cells for the isolation of clathrin-coated vesicles was performed in a similar manner with the following changes. Cells were grown at 30°C in YPD. Lysis was performed in a buffer optimized for clathrin coat stability (as in Payne and Schekman, 1985; except with higher sorbitol concentration): 100 mM MES-NaOH, pH 6.5, 0.8 M sorbitol, 0.5 mM MgCl2, 1 mM EGTA, 2 mM DTT, and protease inhibitor cocktail (Compete Mini, EDTA free; Roche). The 67% Percoll solution was made up with this lysis buffer in the manner described above for sorbitol-triethanolamine lysis buffer. The high speed spin membranes were adjusted to 58% Percoll in 2.6 ml, loaded into an SW41 tube, and 1.5 ml of each of the following steps were added: 55, 50, 40, 35, 30, and 25% Percoll. The gradients were spun for 1 h at 100,000 g av, and 0.5 ml fractions were collected from the top.
Immunoisolations
Anti-Pma1p monoclonal antibodies (#15 and #17) (Serrano et al., 1993) were purified from hybridoma tissue culture supernatants by ammonium sulfate precipitation (60% saturation) followed by protein A chromatography using binding and elution buffers optimized for mouse IgG1 (Pierce Chemical Co.). Peak fractions from the protein A column were exchanged into 50 mM sodium phosphate, pH 6.9, using Excellulose desalting columns (Pierce Chemical Co.) and stored in this buffer with 5 mg/ml BSA, 10 mM NaN3. Affinity purified polyclonal anti-Clc1p antibodies were prepared as follows: antiserum against GST-Clc1p (Deloche et al., 2001) was preadsorbed against fresh cells with a deletion of CLC1, and immunoglobulins were purified on T-Gel columns (Pierce Chemical Co.). GST and GST-Clc1p were purified according to a protocol from Amersham Pharmacia Biotech and crosslinked to Amino-Link Plus coupling gel (Pierce Chemical Co.) for preparation of affinity columns. Anti-GST antibodies were purified from the immunoglobulin preparation, and the remaining IgG was used for affinity-purifying anti-Clc1p according to Harlow and Lane (1988)(. Antibodies were concentrated on protein A columns, desalted, and stored as described above for anti-Pma1p.
Immunoisolations were performed with magnetic Dynabeads (Dynal). Dynabeads protein G were incubated overnight with purified antibodies, and bound antibodies were quantified by SDS-PAGE. Anti-Pma1p beads contained 0.1 μg antibody/μl beads; anti-Clc1p and anti-GST beads contained 0.2 μg antibody/μl beads. In one experiment (Fig. 6), Dynabeads M-500 subcellular were used, which were prepared according to the manufacturer's instructions and contained 40 ng primary antibody/μl beads. For immunoisolation of Pma1p-containing vesicles, we prepared a 1-ml reaction containing Dynabeads (amounts as specified), lysis buffer, 5 mg/ml BSA, and 30 μl membranes from Percoll gradient peak fractions obtained by fractionating 1 g of cells. The reactions were rotated gently at 4°C for 2 h and washed twice over 2 h. The beads were resuspended in 200 μl lysis buffer (5× original concentration). For immunoisolation of clathrin-coated vesicles, we mixed Dynabeads with 200 μl membranes from clathrin-enriched Percoll gradient fractions obtained by fractionating 1 g of cells in a final reaction volume of 1 ml. Incubations and washes were the same as described for anti-Pma1p, and washed beads were resuspended in 250 μl lysis buffer (4× original concentration).
Peptide competition experiments to confirm the specificity of the anti-Pma1p immunoisolations were as follows: peptides corresponding to the mapped epitopes of each antibody (VSAHQPTQEKPAKTYDDAAS for antibody #15 and IEELQSNHGVDDEDSDNDG for antibody #17 [New England Peptide]) were dissolved in water at 2 mg/ml. Beads bound with antibodies were preincubated with peptide (40 μg peptide per 1 μg bound antibody, 400 μl volume), and 100 μg peptide (50 μl stock) was included in a 1-ml immunoisolation reaction containing 2.5 μg bound antibody.
EM of immunoisolated membranes was performed as described (Harsay and Bretscher, 1995) except the beads were mixed 1:1 with 4% intermediate melting temperature agarose after the primary fixation and were processed as ∼1 mm3 cut agarose chunks.
Protein and enzyme assays
For invertase reactions, Nycodenz gradient fractions were assayed as described (Goldstein and Lampen, 1975; Harsay and Bretscher, 1995). Units are expressed as nmol glucose produced per min, per μl fraction, normalized to 1 g wet cells. Samples from Percoll gradient fractions and immunoisolations were assayed in a similar manner except longer reaction times were used (20–50 min), and the reactions were spun after the boiling stop bath to remove precipitated Percoll or beads before the glucose assay. Exoglucanase activity was determined as described (Harsay and Bretscher, 1995) except that rather than 6-h incubations, 10–20 μl of undiluted fraction in 250 μl reaction buffer was incubated for 9–12 h at 30°C; activities are expressed as the percent total of the activity measured in the fractions. ATPase and GDPase activities were assayed as described (Harsay and Bretscher, 1995); units are expressed as pmol inorganic phosphate produced per min per μl fraction, normalized to 1 g wet cells. The bottom three fractions of the gradients (load volume) contained high levels of soluble enzymes and were not routinely assayed.
Immunoblotting was performed as described (Harsay and Bretscher, 1995) except a tank transfer apparatus (Amersham-Pharmacia Biotech) was used to transfer proteins. Rabbit polyclonal antisera were used to detect Bgl2p, Sec61p, and CPY (this laboratory) and Tlg1p (Holthuis et al., 1998a). Monoclonal antibodies from Molecular Probes, Inc. were used to detect ALP, Vps10p, and Pep12p. A monoclonal antibody (Brennwald and Novick, 1993) was used to detect Sec4p. Anti-HA monoclonal antibody HA.11 from Covance was used to detect HA-Kex2p and HA-Pma1p. HRP-conjugated secondary antibodies were from Amersham Pharmacia Biotech. Blots were developed using the standard ECL or ECL-Plus kits (Amersham Pharmacia Biotech).
Acknowledgments
We are very grateful to Tony Bretscher for supporting initial studies that led to this work. We also thank Scott Emr, Tom Stevens, and members of their laboratories for providing vps mutants, constructs, and discussion, Eric Bensen and Greg Payne for providing the plasmid for constructing the chc1-ts mutant, Steve Nothwehr for providing (via Gregory Jedd) the HA-KEX2 constructs, Hugh Pelham for anti-Tlg1p antibodies, Ramon Serrano for anti-Pma1p antibodies, Jon Bertsch for preparing anti-CPY antibodies, Patrick Brennwald for anti-Sec4p antibodies, Elmar Weiler for hybridoma cell lines, Ann Fischer for culturing hybridoma cells, and Frantisek Supek and other members of our laboratory for discussion.
This work was supported by grants from the National Institutes of Health (GM26755) and the Human Frontier Science Program (G-501-95) to R. Schekman. E. Harsay was supported in part by a National Research Service award. R. Scheckman is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: ALP, alkaline phosphatase; CPY, carboxypeptidase Y; SD, synthetic dextrose; TGN, trans-Golgi network; VPS, vacuolar protein sorting; YPD, yeast extract/peptone/dextrose.
References
- Ausubel, F.M., R. Brent, R. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. John Wiley & Sons Inc., New York.
- Babst, M., T.K. Sato, L.M. Banta, and S.D. Emr. 1997. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16:1820–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer, K.A., S.E. Rieder, S.D. Emr, and E.W. Jones. 1996. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol. Biol. Cell. 7:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleekemolen, J.E., M. Stein, K. von Figura, J.W. Slot, and H.J. Geuze. 1988. The two mannose 6-phosphate receptors have almost identical subcellular distributions in U937 monocytes. Eur. J. Cell Biol. 47:366–372. [PubMed] [Google Scholar]
- Brada, D., and R. Schekman. 1988. Coincident localization of secretory and plasma membrane proteins in organelles of the yeast secretory pathway. J. Bacteriol. 170:2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald, P., and P. Novick. 1993. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 362:560–563. [DOI] [PubMed] [Google Scholar]
- Brown, P.S., E. Wang, B. Aroeti, S.J. Chapin, K.E. Mostov, and K.W. Dunn. 2000. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 1:124–140. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., and T.H. Stevens. 1997. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J. Cell Biol. 136:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C.G., M. Peterson, C.R. Cowles, and S.D. Emr. 1997. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Biol. Cell. 8:1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, T.L., and R.B. Kelly. 1987. Constitutive and regulated secretion of proteins. Annu. Rev. Cell Biol. 3:243–293. [DOI] [PubMed] [Google Scholar]
- Cereghino, J.L., E.G. Marcusson, and S.D. Emr. 1995. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell. 6:1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., and N.G. Davis. 2000. Recycling of the yeast a-factor receptor. J. Cell Biol. 151:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear, E., and T.H. Stevens. 1998. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta. 1404:211–230. [DOI] [PubMed] [Google Scholar]
- Cooper, A.A., and T.H. Stevens. 1996. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, C.R., S.D. Emr, and B.F. Horazdovsky. 1994. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J. Cell Sci. 107:3449–3459. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., G. Odorizzi, G.S. Payne, and S.D. Emr. 1997. a. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 91:109–118. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., W.B. Snyder, C.G. Burd, and S.D. Emr. 1997. b. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 16:2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow, T., S.E. Rieder, and S.D. Emr. 1997. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, D., S. Sundarababu, and J.E. Gerst. 1998. Involvement of long chain fatty acid elongation in the trafficking of secretory vesicles in yeast. J. Cell Biol. 143:1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche, O., B.G. Yeung, G.S. Payne, and R. Schekman. 2001. Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell. 12:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter, C., C.N. Connolly, D.F. Cutler, and C.R. Hopkins. 1995. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J. Biol. Chem. 270:10999–11003. [DOI] [PubMed] [Google Scholar]
- Goldstein, A., and J.O. Lampen. 1975. β-D-Fructofuranoside fructohydrolase from yeast. Methods Enzymol. 42:504–511. [DOI] [PubMed] [Google Scholar]
- Graham, T.R., M. Seeger, G.S. Payne, V.L. MacKay, and S.D. Emr. 1994. Clathrin-dependent localization of alpha 1,3 mannosyltransferase to the Golgi complex of Saccharomyces cerevisiae. J. Cell Biol. 127:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, G., B. Hoflack, K. Simons, I. Mellman, and S. Kornfeld. 1988. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 52:329–341. [DOI] [PubMed] [Google Scholar]
- Guo, W., M. Sacher, J. Barrowman, S. Ferro-Novick, and P. Novick. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10:251–255. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and G.R. Fink. 1991. Guide to yeast genetics and molecular biology. Methods Enzymol. 194:1–863 [PubMed] [Google Scholar]
- Hannah, M.J., A.A. Schmidt, and W.B. Huttner. 1999. Synaptic vesicle biogenesis. Annu. Rev. Cell Dev. Biol. 15:733–798. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies. In A Laboratory Manual. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY. 313–315.
- Harsay, E., and A. Bretscher. 1995. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa, A., and N. Kitamura. 2000. Early endosomal localization of Hrs requires a sequence within the proline- and glutamine-rich region but not the FYVE finger. J. Biol. Chem. 275:29636–29642. [DOI] [PubMed] [Google Scholar]
- Holthuis, J.C., B.J. Nichols, S. Dhruvakumar, and H.R. Pelham. 1998. a. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis, J.C.M., B.J. Nichols, and H.R.B. Pelham. 1998. b. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol. Biol. Cell. 9:3383–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, M., and M. Carlson. 1992. Regulation of carbon and phosphate utilization. The Molecular and Cellular Biology of the Yeast Saccharomyces. Vol. 2. E.W. Jones, J.R. Pringle, and J.R. Broach, editors. Cold Spring Harbor Laboratory Press, Plainview, NY. 193–281.
- Jones, S.M., K.E. Howell, J.R. Henley, H. Cao, and M.A. McNiven. 1998. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 279:573–577. [DOI] [PubMed] [Google Scholar]
- Kaiser, C.A., R.A. Gimeno, and D.A. Shaywitz. 1997. Protein secretion, membrane biogenesis, and endocytosis. The Molecular and Cellular Biology of the Yeast Saccharomyces. J.R. Pringle, J.R. Broach, and E.W. Jones, editors. Cold Spring Harbor Laboratory Press, Plainview, NY. 91–228.
- Keller, P., and K. Simons. 1997. Post-Golgi biosynthetic trafficking. J. Cell Sci. 110:3001–3009. [DOI] [PubMed] [Google Scholar]
- Keller, P., D. Toomre, E. Diaz, J. White, and K. Simons. 2001. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat. Cell Biol. 3:140–149. [DOI] [PubMed] [Google Scholar]
- Kleijmeer, M.J., S. Morkowski, J.M. Griffith, A.Y. Rudensky, and H.J. Geuze. 1997. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J. Cell Biol. 139:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, S., and I. Mellman. 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5:483–525. [DOI] [PubMed] [Google Scholar]
- Kreitzer, G., A. Marmorstein, P. Okamoto, R. Vallee, and E. Rodriguez-Boulan. 2000. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat. Cell Biol. 2:125–127. [DOI] [PubMed] [Google Scholar]
- Kuehn, M.J., and R. Schekman. 1997. COPII and secretory cargo capture into transport vesicles. Curr. Opin. Cell Biol. 9:477–483. [DOI] [PubMed] [Google Scholar]
- Laird, V., and M. Spiess. 2000. A novel assay to demonstrate an intersection of the exocytic and endocytic pathways at early endosomes. Exp. Cell Res. 260:340–345. [DOI] [PubMed] [Google Scholar]
- Leitinger, B., A. Hille-Rehfeld, and M. Spiess. 1995. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc. Natl. Acad. Sci. USA. 92:10109–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, S.K., and L.M. Traub. 2000. Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol. 12:457–466. [DOI] [PubMed] [Google Scholar]
- Ludwig, T., G. Griffiths, and B. Hoflack. 1991. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J. Cell Biol. 115:1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, W.-j., and A. Chang. 2000. An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol. Biol. Cell. 11:579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio, J.P., B.A. Rous, N.A. Bright, P.R. Pryor, B.M. Mullock, and R.C. Piper. 2000. Lysosome-endosome fusion and lysosome biogenesis. J. Cell Sci. 113:1515–1524. [DOI] [PubMed] [Google Scholar]
- Marcusson, E.G., B.F. Horazdovsky, J.L. Cereghino, E. Gharakhanian, and S.D. Emr. 1994. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 77:579–586. [DOI] [PubMed] [Google Scholar]
- McNiven, M.A., H. Cao, K.R. Pitts, and Y. Yoon. 2000. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 25:115–120. [DOI] [PubMed] [Google Scholar]
- Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625. [DOI] [PubMed] [Google Scholar]
- Mostov, K.E., M. Verges, and Y. Altschuler. 2000. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12:483–490. [DOI] [PubMed] [Google Scholar]
- Müsch, A., H. Xu, D. Shields, and E. Rodriguez-Boulan. 1996. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J. Cell Biol. 133:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoziani, P., F. Vilhardt, A. Llorente, L. Hilout, P.J. Courtoy, K. Sandvig, and D.B. van. 2000. Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell. 11:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S.F., E. Conibear, and T.H. Stevens. 1995. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol. 129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., C. Field, and R. Schekman. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 21:205–215. [DOI] [PubMed] [Google Scholar]
- Payne, G.S., and R. Schekman. 1985. A test of clathrin function in protein secretion and cell growth. Science. 230:1009–1014. [DOI] [PubMed] [Google Scholar]
- Piper, R.C., A.A. Cooper, H. Yang, and T.H. Stevens. 1995. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131:603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, R.C., N.J. Bryant, and T.H. Stevens. 1997. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J. Cell Biol. 138:531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press, B., Y. Feng, B. Hoflack, and A. Wandinger-Ness. 1998. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140:1075–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky, D.C., W.B. Snyder, S.D. Emr, and J. Kaplan. 1997. Characterization of VPS41, a gene required for vacuolar trafficking and high-affinity iron transport in yeast. Proc. Natl. Acad. Sci. USA. 94:5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., B. Bremnes, A. Mehlum, D.J. Gillooly, A. D'Arrigo, E. Stang, and H. Stenmark. 2001. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci. 114:2255–2263. [DOI] [PubMed] [Google Scholar]
- Raymond, C.K., I. Howald-Stevenson, C.A. Vater, and T.H. Stevens. 1992. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell. 3:1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg, K.J., N. Rowley, and C.A. Kaiser. 1997. Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J. Cell Biol. 137:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C.J., C.K. Raymond, C.T. Yamashiro, and T.H. Stevens. 1991. Methods for studying the yeast vacuole. Methods Enzymol. 194:644–661. [DOI] [PubMed] [Google Scholar]
- Robinson, M.S., and J.S. Bonifacino. 2001. Adaptor-related proteins. Curr. Opin. Cell Biol. 13:444–453. [DOI] [PubMed] [Google Scholar]
- Rothman, J.H., C.K. Raymond, T. Gilbert, P.J. O'Hara, and T.H. Stevens. 1990. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 61:1063–1074. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and P. Novick. 1987. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 49:527–538. [DOI] [PubMed] [Google Scholar]
- Sariola, M., J. Saraste, and E. Kuismanen. 1995. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J. Cell Sci. 108:2465–2475. [DOI] [PubMed] [Google Scholar]
- Saucan, L., and G.E. Palade. 1994. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J. Cell Biol. 125:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R.H., and R.D. Gietz. 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16:339–346. [DOI] [PubMed] [Google Scholar]
- Seaman, M.N., E.G. Marcusson, J.L. Cereghino, and S.D. Emr. 1997. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger, M., and G.S. Payne. 1992. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 11:2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, R., B.C. Monk, J.M. Villalba, C. Montesinos, and E.W. Weiler. 1993. Epitope mapping and accessibility of immunodominant regions of yeast plasma membrane H(+)-ATPase. Eur. J. Biochem. 212:737–744. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Krüger, B., R. Frank, F. Crausaz, and H. Riezman. 1993. Partial purification and characterization of early and late endosomes in yeast. J. Biol. Chem. 268:14376–14386. [PubMed] [Google Scholar]
- Singer-Krüger, B., H. Stenmark, A. Düsterhöft, P. Philippsen, J. Yoo, D. Gallwitz, and M. Zerial. 1994. Role of three Rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 125:283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., B. Horazdovsky, and S.D. Emr. 1995. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu. Rev. Cell Dev. Biol. 11:1–33. [DOI] [PubMed] [Google Scholar]
- Stepp, J.D., K. Huang, and S.K. Lemmon. 1997. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J. Cell Biol. 139:1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, P.K., N.G. Davis, G.F. Sprague, and G.S. Payne. 1993. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J. Cell Biol. 123:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D.R., T. Maurice, D. Roth, and P. Novick. 1996. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Traub, L.M., and S. Kornfeld. 1997. The trans-Golgi network: a late secretory sorting station. Curr. Opin. Cell Biol. 9:527–533. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., S. Avaro, C. Prescianotto-Baschong, R. Haguenauer-Tsapis, and H. Riezman. 2000. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, C.A., K. Redding, R. Wright, and R.S. Fuller. 1992. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol. Biol. Cell. 3:1353–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, P.R., and H.L. Ploegh. 1995. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol. 11:267–306. [DOI] [PubMed] [Google Scholar]
- Woodman, P.G. 2000. Biogenesis of the sorting endosome: the role of Rab5. Traffic. 1:695–701. [DOI] [PubMed] [Google Scholar]
- Yang, Z., H. Li, Z. Chai, M.J. Fullerton, Y. Cao, B.-H. Toh, J.W. Funder, and J.-P. Liu. 2001. Dynamin II regulates hormone secretion in neuroendocrine cells. J. Biol. Chem. 276:4251–4260. [DOI] [PubMed] [Google Scholar]
- Yoshimori, T., P. Keller, M.G. Roth, and K. Simons. 1996. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 133:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, D.S., A. Dancis, and R.D. Klausner. 1997. Restriction of copper export in Saccharomyces cerevisiae to a late Golgi or post-Golgi compartment in the secretory pathway. J. Biol. Chem. 272:25787–25793. [DOI] [PubMed] [Google Scholar]
- Ziman, M., J.S. Chuang, and R.W. Schekman. 1996. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell. 7:1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]