Abstract
Confluent endothelial cells respond poorly to the proliferative signals of VEGF. Comparing isogenic endothelial cells differing for vascular endothelial cadherin (VE-cadherin) expression only, we found that the presence of this protein attenuates VEGF-induced VEGF receptor (VEGFR) 2 phosphorylation in tyrosine, p44/p42 MAP kinase phosphorylation, and cell proliferation. VE-cadherin truncated in β-catenin but not p120 binding domain is unable to associate with VEGFR-2 and to induce its inactivation. β-Catenin–null endothelial cells are not contact inhibited by VE-cadherin and are still responsive to VEGF, indicating that this protein is required to restrain growth factor signaling. A dominant-negative mutant of high cell density–enhanced PTP 1 (DEP-1)//CD148 as well as reduction of its expression by RNA interference partially restore VEGFR-2 phosphorylation and MAP kinase activation. Overall the data indicate that VE-cadherin–β-catenin complex participates in contact inhibition of VEGF signaling. Upon stimulation with VEGF, VEGFR-2 associates with the complex and concentrates at cell–cell contacts, where it may be inactivated by junctional phosphatases such as DEP-1. In sparse cells or in VE-cadherin–null cells, this phenomenon cannot occur and the receptor is fully activated by the growth factor.
Keywords: endothelium; cadherins; catenins; vascular endothelial growth factor; proliferation
Introduction
VEGF plays an important role in the formation of new vessels during embryogenesis and in proliferative diseases in the adult (Ferrara and Alitalo, 1999; Carmeliet and Jain, 2000; Dor et al., 2002). This effect is mediated by the capacity of the growth factor to induce endothelial cell proliferation and differentiation in vascular structures.
VEGF acts by binding to two high affinity receptor tyrosine kinases: VEGF receptor (VEGFR)* 1 also called Flt-1, and VEGFR-2, also called Flk-1/KDR. Although the role of VEGFR-1 in VEGF signaling is still debated, VEGFR-2 is effective in transducing signals regulating cell division and inhibition of cell death.
Contact-inhibited cells, including the endothelium, have a reduced response to specific growth factors when they reach confluence (Fagotto and Gumbiner, 1996; Vinals and Pouyssegur, 1999). It is likely that the establishment of intercellular contacts transfers negative intracellular signals, which restrains the capacity of the cells to respond to proliferative signals. Cadherins have been implicated in this phenomenon. These adhesive transmembrane proteins are specifically located at intercellular adherens junctions and, once the cells get in contact, form zipper-like structures along intercellular contacts.
Endothelial cells express an endothelial-specific cadherin called vascular endothelial cadherin (VE-cadherin) (Lampugnani et al., 1992). This protein, like the other members of the family, is linked through its cytoplasmic tail to p120, β-catenin, and γ-catenin (Gottardi and Gumbiner, 2001). β- and γ-catenins, by binding to α-catenin, mediate the anchorage of VE-cadherin to the actin cytoskeleton. In addition, β-catenin, and in some conditions γ-catenin and p120, when released in the cytosol, can translocate to the nucleus and modulate cell transcription (Gottardi and Gumbiner, 2001).
β-Catenin is considered an oncogene and, in tumor cells, can induce transcription of genes important in cell cycle regulation (Polakis, 2000; Conacci-Sorrell et al., 2002; van de Wetering et al., 2002). Cadherins would therefore signal in an indirect way by linking β-catenin at the membrane and limiting in this way its translocation to the nucleus.
Epithelial cadherin (E-cadherin) was found to induce contact inhibition of cell growth (St. Croix et al., 1998; Mueller et al., 2000). This effect requires binding of β-catenin to the cadherin cytoplasmic tail (Gottardi et al., 2001; Stockinger et al., 2001) and inhibition of its transcriptional activity.
Several studies indicate that adhesive proteins, such as integrins, can physically interact with growth factor receptors and synergistically modulate cell proliferation, migration, and survival (Schwartz and Baron, 1999). Although less studied, cadherins or the cadherin–catenin complex can interact with growth factor receptors and some effectors of their signaling pathways (Hoschuetzky et al., 1994; Pece and Gutkind, 2000; Vasioukhin et al., 2001). In endothelial cells, VE-cadherin can associate with VEGFR-2 upon activation of the cells with VEGF. This interaction increases phosphatidylinositol 3 (PI3) kinase activation by the growth factor and improves cell survival (Carmeliet et al., 1999).
In this paper, we show that VE-cadherin exerts a more complex role on VEGF signaling through VEGFR-2. We found that VE-cadherin expression and clustering at intercellular junctions blocks the proliferative response of the cells to this growth factor, and inhibition of VEGFR-2 phosphorylation in tyrosine likely contributes to this effect. We propose that VE-cadherin acts, in part, by forming a complex with VEGFR-2 and bringing the receptor close to junctional phosphatases. VE-cadherin may, therefore, modulate VEGFR-2 signaling by increasing cell survival while inducing contact inhibition of cell growth.
Results
VE-cadherin expression and clustering inhibit VEGF-induced endothelial proliferation
Confluent endothelial cells respond poorly to growth factors as compared with sparse cells (D'Amore, 1992; Vinals and Pouyssegur, 1999), suggesting that intercellular junctions may transfer growth inhibitory signals. To investigate whether VE-cadherin could play a role in this phenomenon, we compared syngenic endothelial cell lines differing for expression of VE-cadherin only. VE-cadherin–null and –positive cells (VEC null and VEC positive, respectively) retained the expression of all the endothelial-specific markers tested (Lampugnani et al., 2002). In addition, transfected VE-cadherin reached levels comparable to wild-type endothelium and was correctly organized at intercellular contacts (Lampugnani et al., 2002).
As shown in Fig. 1 A, VEC-positive cells arrested their growth at confluence, whereas VEC-negative cells maintained a sustained growth rate reaching a two to threefold higher density than positive cells. Confluent VEC-positive cells behaved like wild-type endothelium (Caveda et al., 1996; Vinals and Pouyssegur, 1999), showing a markedly lower DNA synthesis upon VEGF stimulation than sparse cells (Fig. 1 B). In contrast, VEC-null cells were highly responsive to VEGF both in sparse and confluent conditions. Thus, VE-cadherin substantially reduces the response of confluent, but not sparse, endothelium to VEGF.
Figure 1.
Confluence and VE-cadherin expression inhibit endothelial proliferation induced by VEGF. (A) Growth curve of endothelial cells expressing (VEC positive) or not expressing (VEC null) VE-cadherin. Cells (seeding 30,000/cm2) were cultured in complete culture medium. At the indicated time point, cells were detached and counted. The standard deviation in three independent experiments was between 5 and 10% of the mean values. (B) Confluent (100,000/cm2) and sparse (20,000/cm2) VEC-positive and VEC-null cells were stimulated with VEGF (80 ng/ml) for 24 h. BrdU (30 μM) was added during the last 4 h. A total of 300 nuclei in random fields for each treatment were scored. The mean values of three independent experiments ± SD are shown.
VE-cadherin expression inhibits VEGFR-2 phosphorylation
VEGFR-2 plays a major role in the growth factor–induced endothelial cell proliferation (Ferrara, 1999) and p44/42 MAP kinase activation (Takahashi et al., 1999b, 2001). In Fig. 2 A, we show that the presence of VE-cadherin markedly reduced tyrosine phosphorylation of VEGFR-2 after VEGF activation of the cells. We then tested whether the correct clustering of VE-cadherin at junctions, and not only its expression, could affect receptor activation. As shown in Fig. 2 B, sparse VEC-positive cells were able to respond to VEGF like null cells. Similarly, when freshly isolated human umbilical vein endothelial cells (HUVEC) were tested, VEGFR-2 phosphorylation was higher in sparse than in confluent cells (Fig. 2 C).
Figure 2.
VE-cadherin expression and clustering inhibit VEGFR-2 tyrosine phosphorylation. (A) Confluent VEC-null and -positive endothelial cells were stimulated with VEGF (80 ng/ml) for the indicated time intervals. Cell extracts were immunoprecipitated with antibodies to VEGFR-2 (IP αVEGFR-2) and immunoblotted (IB) with antibodies to phosphotyrosine (αphosphoTyr) and VEGFR-2 (αVEGFR-2). A similar experimental procedure was used for B–D. In the representative experiment shown, tyrosine-phosphorylated VEGFR-2 normalized over total VEGFR-2 was 0.5-, five-, and twofold more in VEC-null than in VEC-positive cells at time 0, 5, and 30 min, respectively. In 15 independent experiments, the range of increase at 5 min was from two- to sevenfold. (B) Tyrosine phosphorylation of VEGFR-2 in response to VEGF (80 ng/ml for 5 min) in sparse VEC-null and -positive endothelial cells was comparable. (C) In HUVEC, phosphorylation of VEGFR-2 in response to VEGF (80 ng/ml for 5 min) was lower in confluent than in sparse cultures (range three- to fivefold lower in four experiments). (D) Addition of antibodies to VE-cadherin (anti-VEC, 100 μg/ml) for 1 h increased receptor phosphorylation by VEGF (80 ng/ml, for 5 min). In response to VEGF, the phosphotyrosine content in VEGFR-2 was higher (from three- to fourfold in three experiments) in cells pretreated with VE-cadherin antibody. The antibody to VEGFR-2 recognized two bands at a molecular mass of ∼200 kD. Only the higher molecular mass band, representing the mature form of the receptor, was phosphorylated in tyrosine, as also described by Takahashi and Shibuya (1997). In the following figures, we therefore show only the heavier band of the doublet, which represents the phosphorylable pool of VEGFR-2.
We have previously reported that the addition of VE-cadherin blocking antibodies to confluent endothelial cells abolished localization and clustering of the protein at intercellular contacts (Corada et al., 2002). This effect was not accompanied by cell retraction or redistribution of other junctional proteins (Corada et al., 2001, 2002). As shown in Fig. 2 D, the addition of a blocking VE-cadherin monoclonal antibody to confluent HUVEC induces a three to fourfold increase in VEGFR-2 phosphorylation. Together, these data indicate that not only VE-cadherin expression, but also its correct organization and clustering at junctions, is required for an optimal inhibitory effect on VGFR-2 phosphorylation.
VEGFR-2 was similarly accessible to its ligand in confluent VEC-null and -positive cells. When a monoclonal antibody to the VEGF binding domain of the receptor (DC101) (Witte et al., 1998) was added in vivo to both cell types, it could recognize a comparable amount of VEGFR-2 (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1).
VE-cadherin counteracts p44/42 MAP kinase phosphorylation induced by VEGF
Activation of MAP kinases is an important event in endothelial cell proliferation induced by growth factors (Vinals and Pouyssegur, 1999). As reported in Fig. 3 A and Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1), in confluent but not in sparse cells, VE-cadherin expression reduced the peak and duration of MAP kinase phosphorylation induced by VEGF. Consistently, upon VEGF stimulation, sparse HUVEC presented higher MAP kinase activation than confluent cells (Fig. 3 B).
Figure 3.
VE-cadherin expression and clustering reduce the extent of p44/42 MAP kinase phosphorylation in response to VEGF. (A) Confluent and sparse VEC-null and VEC-positive endothelial cells were stimulated with VEGF (80 ng/ml for 10 min), and phospho p44/42 MAP kinase and total MAP kinase were assayed by Western blot with specific antibodies. The columns represent the ratio between phosphorylated and total values as fold increase over the ratio calculated in sparse unstimulated VEC null. VEGF-stimulated phosphorylation of MAP kinases was reduced at confluence only in VEC-positive cells. The mean ± SD of three independent experiments is reported. In a total of 12 experiments, the increase of p44/42 MAP kinase phosphorylation in VEC-null cells ranged from three- to sixfold over VEC-positive cells. (B) In confluent HUVEC, phosphorylation of p44/42 MAP kinase in response to VEGF (80 ng/ml for 10 min) was reduced about threefold in comparison with sparse cells. Column values are as in A.
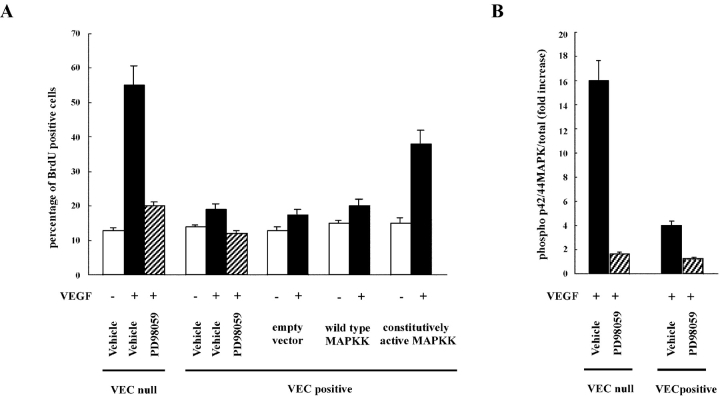
Treatment of VEC-null and -positive cells with PD98059 significantly reduced p44/42 MAP kinase activation and DNA synthesis after VEGF (Fig. 4, A and B). In addition, transfection of VEC-positive cells with a constitutive active MAP kinase kinase, but not with the wild-type construct or the empty vector, partially but significantly rescued cell proliferation.
Figure 4.
Inhibition of p44/42 MAP kinase phosphorylation correlates with reduction of BrdU incorporation in VEC-null endothelial cells. (A) Confluent VEC-null or -positive cells were treated with PD98059 (100 μM) for 20 min before challenge with VEGF (80 ng/ml for 10 min). VEC-positive cells were transfected with a constitutive active or wild-type MAP kinase kinase (MAPKK) construct or the empty vector. Nuclear incorporation of BrdU was evaluated after 24 h as in the legend to Fig. 1. PD98059 strongly inhibited proliferation of VEC-null cells in response to VEGF. Proliferation of VEC-positive cells was very low and was inhibited by the drug. Constitutively active MAPK kinase partially restored BrdU incorporation in response to VEGF in VEC-positive cells. (B) The concentration of PD98059 used in A was able to strongly inhibit phosphorylation of p44/42 MAP kinase after a 10-min stimulation with VEGF (80 ng/ml). Each column represents the fold increase of the ratio between phosphorylated and total values in VEGF-stimulated over the respective unstimulated condition. Data are means ± SD of three independent experiments. The vehicle is DMSO added at the same final concentration (0.1%) as in PD98059-treated cells.
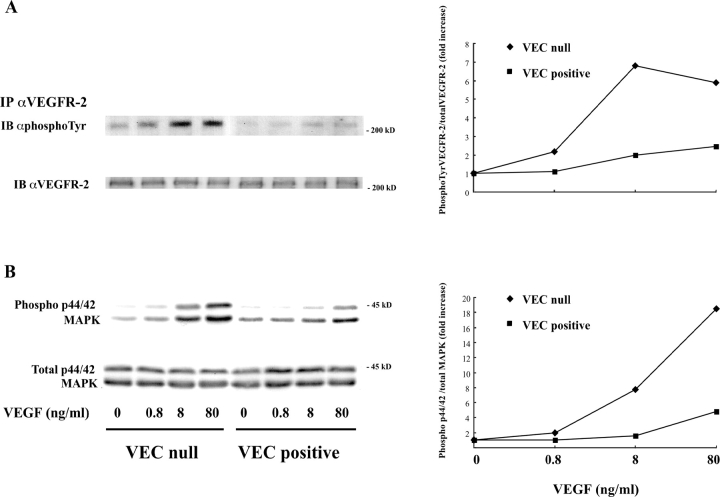
Changing VEGF concentrations, both VEGFR-2 and p44/42 MAP kinase phosphorylation presented a dose-dependent response (Fig. 5). VEC-null cells always presented higher receptor phosphorylation and MAP kinase activation at all concentrations of VEGF used.
Figure 5.
VE-cadherin expression down-regulates tyrosine phosphorylation of VEGFR-2 and phosphorylation of p44/42 MAP kinase over a range of VEGF concentrations. Confluent VEC-null and -positive endothelial cells were challenged for 5 min with different concentrations of VEGF (0.8, 8, and 80 ng/ml). Phosphorylated and total VEGFR-2 (A) and p44/42 MAP kinase (B) were assayed as described in the legend to Figs. 2 and 3. The fold increases of receptor and p44/42 MAP kinase phosphorylation in respect to unstimulated cells are reported on the right side of each panel. The SD was 5–15% of the means (three independent experiments).
VE-cadherin forms a complex with VEGFR-2
In previous work (Carmeliet et al., 1999), we found that VE-cadherin can be coimmunoprecipitated with VEGFR-2. We investigated whether this interaction is required for inhibition of VEGFR-2 phosphorylation. To this end, we analyzed VEC-null endothelial cells transfected with different mutants of VE-cadherin cytoplasmic tail. As described previously in detail (Lampugnani et al., 2002), mutant proteins are expressed at levels comparable to the wild type and are correctly clustered to cell–cell contacts. As reported in Fig. 6 (A and C), when the region responsible for binding to β-catenin was truncated (from aa 703 to 784, Δ-βcat), VEGFR-2 could not associate with VE-cadherin. In contrast, when the region responsible for p120 association was deleted (from aa 621 to 702, Δ-p120), the receptor was still coimmunoprecipitated with VE-cadherin. Compared with null cells, expression of Δ-βcat did not change VEGFR-2 phosphorylation, whereas transfection of Δ-p120 reduced this parameter, although not as effectively as VE-cadherin wild type (Fig. 6 A).
Figure 6.
VEGFR-2 association and dephosphorylation requires the β-catenin binding domain of VE-cadherin. VEC-null cells were transfected with VE-cadherin wild type or truncated mutants lacking β-catenin (Δ-βcat) or p120 (Δ-p120) binding domains. Intra, intracellular region; extra, extracellular region (C). (A) After stimulation with VEGF (80 ng/ml) for 5 and 30 min, cell extracts were immunoprecipitated (IP) with antibodies to VEGFR-2 (αVEGFR-2) and immunoblotted (IB) with antibodies to phosphotyrosine (αphosphoTyr), VEGFR-2 (αVEGFR-2), and VE-cadherin (αVEC). Wild-type (molecular mass, ∼120 kD) and Δ-p120 VE-cadherin (molecular mass, ∼100 kD) were coimmunoprecipitated with VEGFR-2 (A, lower panel). Receptor phosphorylation was significantly reduced in VEC-positive and Δ-p120, but not in Δ-βcat, in comparison with VEC-null cells. The quantification of receptor phosphorylation data from three experiments ± SD is shown in A on the right. The values represent the ratio between the phosphorylated and total amount of VEGFR-2 and are normalized to the ratio calculated in untreated VEC-positive cells. The peak of VEGFR-2 phosphorylation at 5 min is similar in VEC-null and Δ-βcat, but lower in Δ-p120. At longer stimulation (30 min), the level of phosphorylation of VEGFR-2 in Δ-p120 was comparable to VEC-positive cells. Incubation of VEC-positive cell extract with nonimmune (NI) rabbit immunoglobulin (matched with VEGFR-2 antibody used for IP) did not precipitate bands corresponding to either VEGFR-2 or VE-cadherin, last lane from the left (IP NI). (B) VE-cadherin mutants modulate endothelial growth induced by VEGF. VEC-null and Δ-βcat had comparable effects and were the most permissive mutations in terms of cell proliferation (>160% increase over VEC-positive cells). Mutations that affected binding of p120 (Δ-p120) allowed cell proliferation, but to a lower extent (60% increase over stimulation of VEC-positive cells). Proliferation was measured as BrdU incorporation as described in the legend to Fig. 1. Mean ± SD of three independent experiments, each in duplicate, is shown.
The differences in receptor phosphorylation paralleled those in cell proliferation. Fig. 6 B shows that Δ-βcat transfectants responded to VEGF as null cells, whereas Δ-p120 cells were partially inhibited. Coimmunoprecipitation experiments confirmed that Δ-βcat and Δ-p120 VE-cadherin mutants were unable to bind β-catenin or p120, respectively (Lampugnani et al., 2002).
β-Catenin is required for inhibition of VEGFR-2 phosphorylation
To investigate directly the role of β-catenin in VEGFR-2 phosphorylation, we tested β-catenin–null endothelial cells. These cells were isolated and cultured from animals carrying a null mutation of the β-catenin gene in endothelial cells specifically. Conditional gene inactivation was obtained by crossing mice carrying floxed β-catenin gene (Brault et al., 2001) with transgenics expressing Cre under an endothelial-specific promoter (Tie-2 Cre) (Kisanuki et al., 2001; unpublished data; see Materials and methods for details).
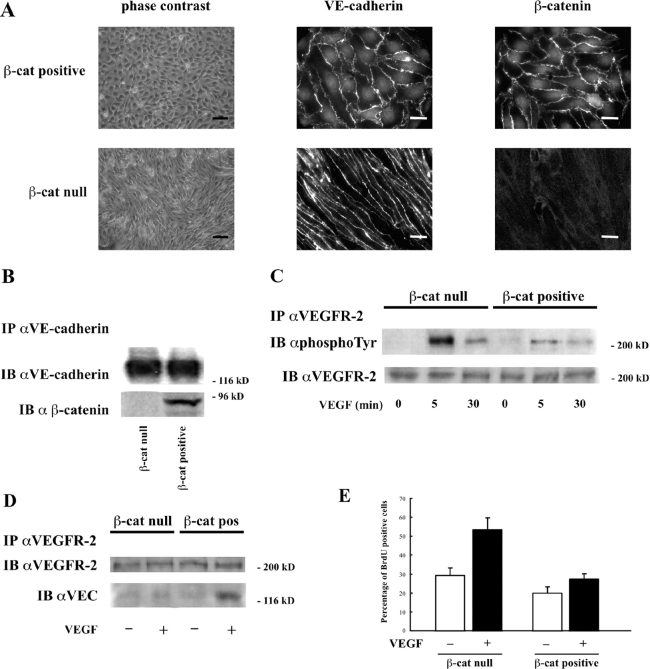
As reported in Fig. 7 (A and B), β-catenin was absent in these mutants whereas VE-cadherin expression was comparable. β-Catenin–null cells retained the endothelial cell markers tested (including VEGFR-2, Tie2, and CD34) and correctly organized junctions (as detected by the localization of VE-cadherin, ZO-1, occludin, PECAM, and JAM) (Fig. 7 A; unpublished data). β-Catenin–null cells presented higher density at confluence and elongated morphology in comparison with the positive cells (Fig. 7 A).
Figure 7.
The absence of β-catenin enhances VEGF-induced phosphorylation of VEGFR-2 and cell proliferation. Endothelial cells derived from β-catenin–null embryos (β-cat null) did not express β-catenin in comparison with cells obtained from β-catenin–positive (β-cat positive) littermate animals. (A) By immunofluorescence analysis, VE-cadherin was expressed at a comparable level and was concentrated at cell–cell contacts in both β-catenin–null and –positive cells. Cell junctions were negative for β-catenin in null cells. Bars: (phase contrast) 100 μm; (VE-cadherin and β-catenin) 20 μm. (B) Immunoprecipitation (IP) of cell extracts with VE-cadherin antibodies followed by Western blot (IB) with anti–VE-cadherin (αVE-cadherin) or β-catenin (αβ-catenin) antibodies showed absence of the last protein in the complex. (C) The absence of β-catenin enhanced the extent and duration of VEGFR-2 phosphorylation in response to VEGF (80 ng/ml). IP with anti–VEGFR-2 and Western blot with antiphosphotyrosine and anti–VEGFR-2 antibodies. (D) VE-cadherin could be coimmunoprecipitated with VEGFR-2 only in β-positive cells after VEGF (80 ng/ml for 5 min). IP with anti–VEGFR-2 and Western blot with anti–VEGFR-2 and anti–VE-cadherin antibodies. (E) Confluent β-cat–null endothelial cells incorporated BrdU 2–2.5-fold more than β-cat–positive cells in response to stimulation with VEGF (80 ng/ml for 24 h). Incorporation of BrdU was measured and calculated (mean of three independent experiments ± SD) as in Fig. 1. Two independent pairs of both β-positive and β-null endothelial cells obtained from littermate embryos of different mothers have been tested with comparable results.
In contrast to positive cells, when β-catenin–null cells were challenged with VEGF, VEGFR-2 could not be coimmunoprecipitated with VE-cadherin (Fig. 7 D) and its phosphorylation was markedly increased (Fig. 7 C). Consistently, endothelial cell proliferation in response to VEGF was increased in β-catenin–null cells (Fig. 7 E). Comparable results were obtained using another β-catenin–null and another control cell line isolated from independent mice.
To test whether Cre expression may contribute to the observed functional behavior of β-catenin–null cells, we infected control cell lines with an adenoviral vector containing Cre cDNA as described previously (Anton and Graham, 1995). Using two adeno Cre infections at 2 pfu/cell concentration at a 9-h interval, we did not observe significant changes in cell growth or response to VEGF (unpublished data). Overall, these data indicate that β-catenin expression and binding to VE-cadherin is required for inhibition of VEGFR-2 phosphorylation and down-regulation of cell proliferation.
Inhibition of VEGFR-2 phosphorylation is due to phosphatase activity: the role of DEP-1/CD148
Treatment of VE-cadherin–positive cells with pervanadate (PV; from 100 μM vanadate and 200 μM H2O2), a general inhibitor of phosphatases (Volberg et al., 1992), restored the capacity of VEGF to induce VEGFR-2 phosphorylation at an even higher level than VE-cadherin–null cells (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1). These data link the inhibitory role of VE-cadherin to the activation or localization of phosphotyrosine phosphatase (PTP) activity.
Among different PTPs possibly implicated in this process, we studied the role of high cell density–enhanced PTP 1/CD148. This receptor-like PTP is of particular interest because it is up-regulated by cell density (Ostman et al., 1994), is expressed in endothelial cells of different origin, and, most importantly, codistributes with VE-cadherin at interendothelial junctions (Takahashi et al., 1999a).
To investigate the role of DEP-1 in VEGFR-2 phosphorylation, we transfected VE-cadherin–positive cells with either wild-type DEP-1 or a dominant-negative form of this PTP (DEP C/S). These constructs have been previously characterized in detail for expression and biological activity (Kume et al., 1996; Trapasso et al., 2000; Baker et al., 2001). In addition, VE-cadherin–positive cells have been targeted with DEP-1–directed short interfering RNA (siRNA) or corresponding scramble oligonucleotides. None of the transfected cell lines presented changes in the morphology, retraction, or junctional distribution of VE-cadherin and β-catenin (Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1).
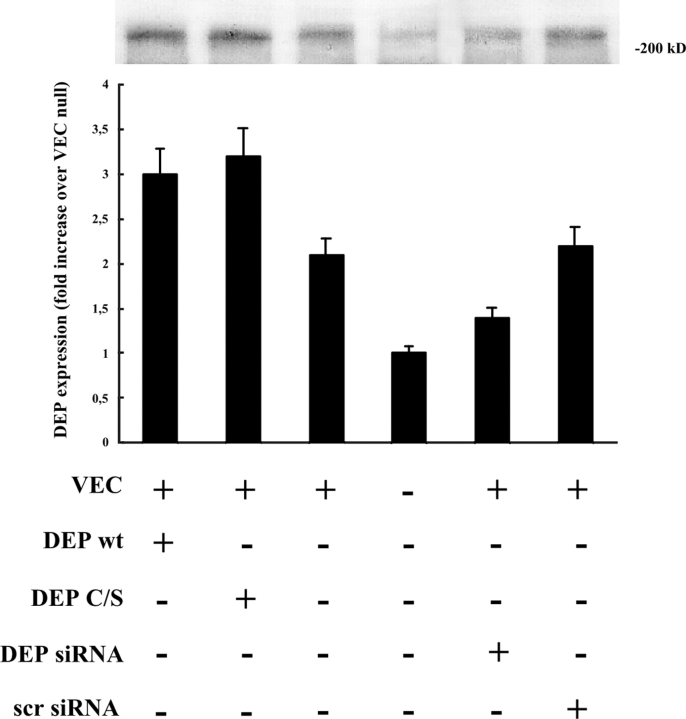
As reported in Fig. 8, expression of VE-cadherin increased DEP-1 protein levels by 80–90% by Western blot analysis. Transfection of VE-cadherin–positive cells with wild-type and mutant DEP-1 constructs resulted in increased levels of the corresponding proteins by Western blot (∼50% in comparison with VEC positive). siRNA directed against DEP-1 reduced DEP-1 production by ∼40% in VE-cadherin–positive cells, whereas the scramble oligonucleotides did not have a significant effect.
Figure 8.
Expression of tyrosine phosphatase DEP-1 in VEC-positive and -negative cells and effect of transfection of wild-type and DEP-1 C/S constructs and DEP-1 RNAi. In VEC-positive endothelial cells (third lane), DEP-1 was 80–90% higher than in VEC null (fourth lane). In VEC-positive cells, transfection of wild-type (wt, first lane) or point-mutated (C/S, second lane) DEP-1 constructs resulted in increased expression of the protein (50–60% more than in control VEC positive). siRNA directed against DEP-1 (DEP siRNA) resulted in 35–40% inhibition of DEP expression (fifth lane). Scramble oligonucleotides (scr siRNA), used as control (100 nM as DEP siRNA oligonucleotide), were ineffective. Plus and minus indicate the presence or the absence of the protein indicated on the left. The columns report the mean of four experiments ± SD.
We then tested the effect of DEP-1 on VEGFR-2 phosphorylation. As shown in Fig. 9, DEP-1 C/S and siRNA partially restored VEGF-induced receptor phosphorylation in VE-cadherin–positive cells. Wild-type DEP-1 (DEPwt) and the scrambled oligonucleotides slightly, but not significantly, reduced VEGFR-2 activation. We obtained similar results when we measured the phosphorylation of Tyr 996 or 951 of the receptor (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1).
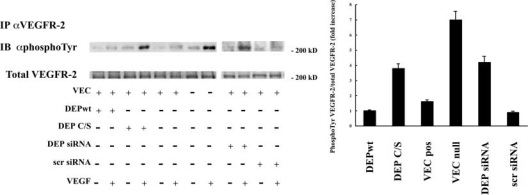
Figure 9.
Down-regulation of DEP-1 increases VEGFR-2 phosphorylation. Transfection of the dominant-negative DEP C/S mutant or DEP siRNA induced higher phosphorylation of VEGFR-2 upon VEGF (80 ng/ml for 5 min) than the respective controls, VEC positive, DEP wild type (DEP wt), and scramble siRNA (scr siRNA), respectively. Data were obtained by immunoprecipitation (IP) with anti–VEGFR-2 antibodies (αVEGFR-2) followed by Western blot (IB) with an antiphosphotyrosine antibody (αphosphoTyr). The graph was obtained by quantifying the gel bands and calculating the ratio of bands labeled with antiphosphotyrosine antibodies over total VEGFR-2. Each column represents the fold increase over unstimulated cells. Data are means ± SD of four experiments. Plus and minus indicate the presence or the absence of the protein indicated on the left.
Both DEP-1 C/S and siRNA had a partial, but significant, effect in restoring MAP kinase activation and BrdU uptake induced by VEGF in VEC-positive cells (Fig. 10, A and B). Wild-type DEP-1 (DEPwt) and the scrambled RNA did not have any effect.
Figure 10.
Inhibition of DEP-1 enhances phosphorylation of p44/42 MAP kinase and BrdU nuclear incorporation in endothelial cells. (A) Phosphorylation of p44/42 MAP kinase in response to VEGF (80 ng/ml for 10 min) is increased by 80–90% in VEC-positive cells expressing DEP C/S in comparison with control VEC positive. A similar increase is observed after DEP siRNA transfection of the cells. The graph represents the ratio between phosphorylated and total p44/42 MAP kinase as fold increase over untreated VEC-null cells. The values are means ± SD of four independent experiments. Plus and minus indicate the presence or the absence of the protein indicated on the left. (B) Nuclear incorporation of BrdU was stimulated by 80–100% in VEC-positive cells expressing C/S DEP or after DEP siRNA. BrdU incorporation was assayed as in Fig. 1. Data are means ± SD of four experiments.
Finally, we tested whether the effect of DEP-1 inhibition could increase phosphorylation of the receptor also in sparse cells, indicating a nonspecific, general effect of the phosphatase. As reported in Fig. S6 (available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1), the effect of DEP-1 C/S was apparent only in confluent cells, and no further increase in the phosphorylation of VEGFR-2 was detected in sparse cultures.
Discussion
In this paper, we report that VE-cadherin expression is responsible, to a substantial extent, for the reduced response of confluent endothelial cells to VEGF-induced proliferation. VE-cadherin–positive or –negative cells respond equally well to the growth factor when they are sparse, but only VE-cadherin–positive cells become insensitive to VEGF at confluence. This indicates that the difference is not mediated by VE-cadherin expression, per se, but requires the establishment of intercellular contacts and cadherin clustering at junctions.
Other papers showed that expression of cadherins could strongly limit cell growth. E-cadherin and N-cadherin in different tumor cell lines induced contact inhibition of cell growth (St. Croix et al., 1998; Levenberg et al., 1999; Gottardi et al., 2001; Stockinger et al., 2001). This effect is mediated, at least in part, by the induction of cell cycle arrest at G1 phase likely due to dephosphorylation of retinoblastoma protein, elevation of the cyclin D1–dependent kinase inhibitor p27kip1, and late reduction of cyclin D1.
The growth inhibitory effects are believed to be mediated by the capacity of cadherins to link β-catenin at the membrane, limiting in this way its nuclear translocation. β-Catenin up-regulates transcription of cyclin D1 and c-myc (Polakis, 2000; Gottardi and Gumbiner, 2001; van de Wetering et al., 2002; Conacci-Sorrell et al., 2002), and inhibition of its activity would limit growth.
However, few observations do not perfectly fit in this picture. The addition of cadherin blocking antibodies restores growth in contact-inhibited cells (Caveda et al., 1996; St. Croix et al., 1998) but does not change the cadherin–β-catenin complex (St. Croix et al., 1998; Corada et al., 1999). Similarly, in sparse or confluent cells, the VE-cadherin–β-catenin complex remains unchanged despite profound differences in growth (Lampugnani et al., 1997).
In this paper, we propose an additional mechanism through which cadherin expression may modulate growth. We found that VE-cadherin expression in confluent endothelial cells is accompanied by a strong reduction of VEGFR-2 phosphorylation. This effect was not due to down-regulation of VEGFR-2 because equal amounts of the protein were found in VE-cadherin–positive and –negative cells or in sparse and confluent cells. The accessibility of the receptor to its ligand, measured as binding of a mAb directed to the active site, was also unchanged.
VE-cadherin clustering at junctions was, however, necessary. The addition of anti–VE-cadherin antibodies, able to disrupt VE-cadherin clusters and induce a diffuse redistribution of the protein on the cell membrane, restores VEGFR-2 phosphorylation. In addition, VE-cadherin–positive, sparse cells behave like VE-cadherin–null cells.
These data are consistent with previous work by Rahimi and Kazlauskas (1999), who showed that both removing divalent cations or adding VE-cadherin antibodies increased VEGFR-2 phosphorylation in endothelial cells. In addition, Takahashi and Suzuki (1996) reported that in human breast epithelial cells, inhibition of E-cadherin clustering restored EGF receptor phosphorylation and cell response to this growth factor.
We published previously that VE-cadherin can form a complex with VEGFR-2 upon activation of the cells with VEGF, and this paralleled VEGFR-2 localization at intercellular junctions (Carmeliet et al., 1999; Shay-Salit et al., 2002; Zanetti et al., 2002). We therefore investigated whether the association of VE-cadherin with VEGFR-2 influences its phosphorylation. When the cytoplasmic tail of VE-cadherin is truncated in the β-catenin–binding domain, the complex cannot form and receptor phosphorylation is not inhibited. In contrast, when VE-cadherin is truncated in the p120 binding domain, it can still associate with VEGFR-2 like the wild type. Receptor phosphorylation in these cells was decreased at 5 min and lasted for a shorter time in comparison with VE-cadherin–null cells. This effect paralleled a reduction in VEGF-induced cell proliferation in Δ-p120 compared with VE-cadherin–null cells. The data discussed above suggested a role of cadherin-associated β-catenin in the inhibition of VEGFR-2 activation. This was further confirmed using β-catenin–null cells. These cells presented normal levels of VE-cadherin and all the other endothelial cell markers tested. In addition, VE-cadherin clustering at junctions as well as its association with γ-catenin/plakoglobin were retained. However, in these cells, VEGFR-2 association with VE-cadherin and its phosphorylation were not inhibited, and cells could still respond to the proliferative signal of VEGF.
Together, these data indicate that β-catenin is necessary for VEGFR-2 dephosphorylation and for down-regulation of cell growth. This observation is somehow in contrast with the general concept that β-catenin expression would favor, and not contrast, cell proliferation. Indeed, there may be cell-specific differences in the response to β-catenin among different cell types and among transformed and nontransformed cells as previously reported by Posthaus et al. (2002). VE-cadherin–null, Δ-βcat, or β-catenin–null mutant cells behave similarly, suggesting that the effect on VEGFR-2 inactivation is related more to localization of β-catenin at the membrane than its nuclear translocation, which cannot occur in β-catenin–null cells. An attractive hypothesis is that the mechanism of action of the VE-cadherin–β-catenin–p120 complex comprises tethering VEGFR-2 to specific phosphatases, inhibiting in this way the degree and duration of its phosphorylation. This is supported by the observation that the general phosphatase inhibitor pervanadate blocks receptor inactivation by VE-cadherin.
Several phosphatases (PTPμ, PTP-K, SHP-1, SHP-2, PTP-LAR, and PTP-1B) (Brady-Kalnay et al., 1995; Balsamo et al., 1996; Fuchs et al., 1996; Kypta et al., 1996; Ukropec et al., 2000; Tonks and Neel, 2001) have been found to be directly or indirectly associated with the cadherin–catenin complex. Among these phosphatases, DEP-1/CD148 (Ostman et al., 1994) is of particular interest in endothelial cells. This type I membrane-associated phosphatase is expressed in many endothelial cell lines from different sources, is up-regulated by confluence, and is located at intercellular junctions (Takahashi et al., 1999a). Inactivation of its encoding gene induces a vascular phenotype characterized by enlarged, oversized vessels with abnormally high endothelial cell proliferation (Takahashi et al., 2003). This suggests that the lack of DEP-1 activity leads to uncontrolled endothelial cell growth, and the data reported here may explain these observations. We show that DEP-1 contributes to VE-cadherin–induced inhibition of VEGFR-2 activation. When its activity or expression is inhibited, VEGFR-2 phosphorylation, activation of p44/42 MAP kinase, and cell proliferation are increased.
In previous papers, DEP-1 was found to codistribute with VE-cadherin at endothelial cell junctions; however, chelation of ions and disruption of VE-cadherin clusters did not change DEP-1 distribution (Takahashi et al., 1999a). This suggests that this phosphatase is contiguous but not directly associated with VE-cadherin. We propose that VE-cadherin binding to VEGFR-2 concentrates the receptor at junctions and makes it available to DEP-1 and possibly other junctional-associated phosphatases that would limit its activation. The partial rescue of a functional response to VEGF by dominant-negative DEP-1 and DEP-1–directed RNA interference (RNAi) (as well as constitutively activated MAPK kinase) could indicate that other mechanisms besides the effect on VEGFR-2 phosphorylation could be involved in contact inhibition of growth in response to this mitogen. These mechanisms could be operated by junctional β-catenin and act in parallel to the direct effect on the receptor.
It has been recently reported that DEP-1 can dephosphorylate the hepatocyte growth factor receptor Met at specific sites. This suggests that this phosphatase has a general role in regulating signaling from growth factor receptors (Palka et al., 2003).
VEGFR-2 triggers different signaling pathways that mediate cell growth and survival. Activation of PLCγ (Takahashi et al., 2001), and in some cases Ras (Meadows et al., 2001), would induce activation of a MAP kinase cascade and cell division. In contrast, PI3 kinase activation and Akt phosphorylation are mostly responsible, with some exception (Dayanir et al., 2001), for promoting cell survival over apoptosis (Gerber et al., 1998a,b).
Inhibition of VEGFR-2 activation by VE-cadherin would repress all its downstream signaling pathways. However, in previous work (Carmeliet et al., 1999), we observed that, instead, expression of VE-cadherin reinforced VEGFR-2 activation of PI3 kinase, accompanied by a marked decrease in endothelial cell sensitivity to apoptotic stimuli. This suggests that the effect of VE-cadherin is more complex and that this protein can direct VEGFR-2 signaling to specific pathways, inhibiting others. A possible explanation is that the phosphatase activity associated with the VE-cadherin–catenin complex may be specific for some VEGFR-2 tyrosines and not others. This would inhibit receptor interaction with some effectors without affecting other pathways. For instance, some authors reported that phosphorylation of tyrosine 1173 is crucial for PLCγ but not for PI3 kinase binding to VEGFR-2 (Takahashi et al., 2001). In contrast, tyrosine 799 constitutes a binding site for the p85 element of PI3 kinase but not for PLCγ (Dayanir et al., 2001). In this paper, we analyzed the behavior of tyrosine 951 and 996 because specific antibodies were available. These two tyrosines contribute to PLCγ and MAP kinase activation. We found that phosphorylation of both tyrosines was restrained by VE-cadherin expression and that this activity was restored by the DEP-1 dominant-negative mutant. A systematic analysis of the phosphorylation of all different tyrosines in VEGFR-2 in the presence or absence of VE-cadherin is underway.
In conclusion, in this paper we report that VE-cadherin expression and clustering may strongly modulate VEGFR-2 signaling. In confluent endothelial cells, VEGFR-2 would preferentially signal through PI3 kinase for survival, whereas in sparse cells, or in cells lacking VE-cadherin, it would mostly promote cell growth through MAP kinase activation. Thus, the same receptor, in the same cells and upon addition of the same agonist, would behave differently if associated or not with VE-cadherin. This introduces the idea that cadherins may contribute to receptor tyrosine kinase signaling by providing a docking platform for binding effectors and inhibitors, which may mediate the diversification of signaling pathways. The absence of VE-cadherin at junctions, as in VE-cadherin–null animals (Carmeliet et al., 1999) or in animals treated with blocking antibodies (Corada et al., 1999), causes profound changes in vascular organization. The lack of modulation of VEGF signaling pathways may play an important role in these effects that lead to early lethality in the embryo and extensive vascular damage in the adult.
Materials and methods
Cell preparation and characterization
Endothelial cells were derived from murine embryonic stem cells with homozygous null mutation of the VE-cadherin gene (VEC null) as described in detail by Balconi et al. (2000). Wild-type and mutant forms of VE-cadherin were introduced in these cells using the retroviral vector PINCO, obtained through the courtesy of P.G. Pelicci (European Institute of Oncology, Milan, Italy) after the authorization of G.P. Nolan (Stanford University, Stanford, CA). Cell types that expressed the following proteins were generated: wild-type VE-cadherin (VEC positive), Δ-p120 (amino acid deletion 621–702, corresponding to the binding region of p120), and Δ-βcat (amino acid deletion 703–784, corresponding to the binding region of β-catenin). The details of the production and characterization of these cells were described by Lampugnani et al. (2002).
The genes for human DEP/CD-148 wild type (DEP wt) and with cysteine 1239 mutated to serine (DEP C/S) (Ostman et al., 1994) were introduced in VEC-positive endothelial cells. The original constructs (Takahashi et al., 1999a) were cloned into PINCO retroviral vector, which also expressed GFP. Control cells were obtained by transfection with PINCO vector expressing GFP only. After infection, the GFP-positive cells were sorted. Expression of DEP, endogenous and neo-expressed, was assayed by Western blot with a mouse monoclonal antibody to ectodomain (clone D3F) and rabbit polyclonal antibody to cytoplasmic domain, which gave comparable results. Both antibodies recognized the human and mouse form of DEP.
The genes for hamster wild-type MAP kinase kinase (p45 MAPKK) and constitutively active MAP kinase kinase (with serine 218 and serine 222 both mutated to aspartic acid, D; Brunet et al., 1994) were also introduced in VEC-positive cells. The constructs, obtained through the courtesy of G. Pages (CNRS-UMR 6543, Nice, France), were cloned into PINCO vector, which also expressed the resistance to zeocin. Resistant cells were selected by culture in the presence of zeocin (300 μg/ml) for 20 d.
β-Catenin–positive and –null cells, wild type and homozygously null mutated in the β-catenin gene (Brault et al., 2001), respectively, were isolated from 9.5-d postcoitum littermate embryos, as described in detail by Balconi et al. (2000). Expression of β-catenin protein was tested with the anti–β-catenin antibody by Transduction Laboratories (BD Biosciences), expression of VE-cadherin was tested with BV 13 (Corada et al., 1999), and expression of VEGFR-2 was tested with sc504 by Santa Cruz Biotechnology, Inc. These cells have also been analyzed for the expression of β-catenin mRNA by PCR, expression of other endothelial markers (PECAM, Tie-2, and endoglin), as well as expression and distribution of junctional molecules (α-catenin, plakoglobin, ZO-1, and JAM).
For all the endothelial cells of murine origin, culture medium was DME (GIBCO BRL) with 10% fetal calf serum (HyClone), heparin (100 μg/ml, from porcine intestinal mucosa; Sigma-Aldrich), and endothelial cell growth supplement (5 μg/ml, made from calf brain) (complete culture medium). Culture medium for HUVEC was M199 (GIBCO BRL) with the same supplements.
RNAi
RNAi of DEP-1 expression was induced with siRNA directed against DEP. Positive 21-nucleotide siRNA (GGG.CCA.GGU.CCU.GUG.CGC.A.dT.dT. and U.GCG.CAC.GGA.CCA.GGU.CCC.dT.dT) targeted murine DEP-1 sequence 86 nucleotides downstream of the start codon. As a negative control, the Scramble II Duplex, siACE-RNAi, was used. Both positive and control oligonucleotides were from Dharmacon Research.
VEC-positive cells were seeded at a density of 20,000/cm2 the day before transfection, and they were ∼40% confluent at the time of transfection with 100 nM positive or scramble oligonucleotides in Oligofectamine (Invitrogen) and Optimem (GIBCO BRL), without serum or BSA. Before transfection, cells were washed once with Optimem. Transfection medium was maintained on cells for 4 h. Then it was removed and cells were cultured in complete medium. Transfection was repeated every 24 h for 2 d, and cells were used for the experiment after another 24 h. DEP expression was tested by Western blot as described above.
Proliferation assay
Cells (50,000 cell/cm2) were cultured for 48 h in culture medium (1 ml) on fibronectin-coated (7 μg/ml; Sigma-Aldrich) glass coverslips set in a 24-well plate. Cell layers were washed once in MCDB 131 (GIBCO BRL) and cultured for another 24 h in MCDB 131 with 1% BSA, with a wash and change of medium after 24 h. Fresh MCDB 131 with 1% BSA was then added containing VEGF (80 ng/ml; PeproTech) when indicated. Cells were confluent during the stimulation (∼100,000 cell/cm2).
When subconfluent cultures were tested, cells at seeding were 5,000 cell/cm2, which reached a density of ∼20,000 cell/cm2 at the time of VEGF stimulation. At 20 h from the beginning of the stimulation, BrdU (30 μM) was added, and the incubation continued for another 4 h. Cells were then fixed with 3% paraformaldehyde and treated for 10 min with 2 N HCl. BrdU incorporation into nuclear structures was put in evidence with anti-BrdU antibodies (mouse monoclonal, Amersham Biosciences), followed by TRITC-conjugated antibody to mouse immunoglobulin (DakoCytomation) in the presence of Hoechst 33258 (0.1 μg/ml). Coverslips were mounted in Mowiol 4-88 (Calbiochem), and total and BrdU-positive cells were counted in random fields up to a number of 300 total nuclei/sample.
p44/42 MAP kinase phosphorylation assay
Cells (50,000 cell/cm2 and 5,000 cell/cm2, to obtain confluent and subconfluent cultures at the time of stimulation, see previous section) were cultured on gelatin-coated (0.1%; Difco) plastic tissue culture vessels for 48 h in culture medium and for another 48 h in 1% BSA-MCDB 131, as described in detail in the Proliferation assay section. 2 h before stimulation, cells were washed once with MCDB 131 and incubated in MCDB 131 with 1% BSA. Medium was replaced with fresh 1% BSA-MCDB 131 just before stimulation with VEGF (80 ng/ml). Control cells had exactly the same treatment without VEGF. At different times after VEGF, as indicated in the specific sections, cells were washed twice with PBS and extracted in boiling Laemli sample buffer containing DTT (50 mM). Cells were scraped and further boiled for 10 min. A parallel sample for each cell type was extracted without DTT for assay of protein content with BCA reagent by Pierce Chemical Co. A total of 15 μg protein was loaded for each lane of a 12% polyacrylamide gel, separated by SDS electrophoresis, and transferred to nitrocellulose (Schleicher & Schuell). To evaluate the phosphorylation of p44/42 MAP kinase, the antibodies (rabbit polyclonal) from Cell Signaling, which specifically recognize the Thr202 and the Tyr204 of p44/42 MAP kinase when phosphorylated, were used. The total content of MAP kinase was assayed in a parallel run using anti–total MAP kinase (rabbit polyclonal; Cell Signaling). Western blots were developed with HRP-conjugated anti–rabbit immunoglobulin (Cell Signaling) followed by ECL (Pierce Chemical Co.). The signals on film (Hyperfilm ECL; Amersham Biosciences) were scanned and quantified using the NIH Image 1.62 program for Macintosh (developed at the National Institutes of Health and freely available at http://rsb.info.nih.gov/nih-image/). The gel images presented have been prepared using Adobe Photoshop 7.0® and combined with Excel graphics in Canvas 8. All the programs were versions for Macintosh.
VEGFR-2 phosphorylation assay
Cells were cultured exactly as described in the previous section for assaying p44/42 MAP kinase phosphorylation. To measure the level of total phosphotyrosine in VEGFR-2, cells were extracted in lysis buffer, as described in detail by Zanetti et al. (2002), and immunoprecipitated with antibodies to VEGFR-2 (sc504, mapping at the carboxy terminus 1158–1345; Santa Cruz Biotechnology, Inc.). Protein content was measured (BCA reagent; Pierce Chemical Co.), and ∼1 mg cell extract for each sample was immunoprecipitated with 20 μg anti–VEGFR-2 conjugated to 20 μl protein A agarose (Amersham Biosciences). Two thirds of the Laemmli sample buffer in which the immunoprecipitates were finally recovered (Zanetti et al., 2002) was run in SDS-PAGE (7% polyacrylamide) for blot with antiphosphotyrosine (PY20; Transduction Laboratories). The remaining one third was run in parallel and blotted with antibodies to VEGFR-2, to normalize the level of phosphorylation to the level of total VEGFR-2 immunoprecipitated. Quantification of the specific bands was as described in the previous section.
Online supplemental material
The supplemental material (Figs. S1–S6) is available at http://www.jcb.org/cgi/content/full/jcb.200209019/DC1. A concise description of the data presented in each supplemental figure is introduced upon citation in the text. Details on the experimental procedure and further comments on the data reported can be found in the online legends.
Supplemental Material
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro; European Community (QLRT-2001-02059, QLG1-CT-1999-01036, and QT-CT-1999-00020); Agenzia Spaziale Italiana; Associazione Duchenne Parent Project; Italian Ministry of Health; Ministry of University, Scientific and Technological Research; Telethon Italy; Consiglio Nazionale delle Ricerche/Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) (CNR.02.731.DEJA); MIUR/Fondo per gli Investimenti della Ricerca di Base (RBNE01MAWA_009 and RBNE01F8LT_007); and Cofin 2002 (2001053777-002).
M.G. Lampugnani and A. Zanetti contributed equally to this work.
The online version of this manuscript includes supplemental material.
Footnotes
Abbreviations used in this paper: DEP-1, high cell density–enhanced PTP 1; E-cadherin, epithelial cadherin; HUVEC, human umbilical vein endothelial cells; PI3, phosphatidylinositol 3; PTP, phosphotyrosine phosphatase; RNAi, RNA interference; siRNA, short interfering RNA; VE-cadherin, vascular endothelial cadherin; VEGFR, VEGF receptor.
References
- Anton, M., and F.L. Graham. 1995. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J. Virol. 69:4600–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J.E., R. Majeti, S.G. Tangye, and A. Weiss. 2001. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cγ1 phosphorylation. Mol. Cell. Biol. 21:2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi, G., R. Spagnuolo, and E. Dejana. 2000. Development of endothelial cell lines from embryonic stem cells: a tool for studying genetically manipulated endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 20:1443–1451. [DOI] [PubMed] [Google Scholar]
- Balsamo, J., T. Leung, H. Ernst, M.K. Zanin, S. Hoffman, and J. Lilien. 1996. Regulated binding of PTP1B-like phosphatase to N-cadherin: control of cadherin-mediated adhesion by dephosphorylation of β-catenin. J. Cell Biol. 134:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay, S.M., D.L. Rimm, and N.K. Tonks. 1995. Receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J. Cell Biol. 130:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault, V., R. Moore, S. Kutsch, M. Ishibashi, D.H. Rowitch, A.P. McMahon, L. Sommer, O. Boussadia, and R. Kemler. 2001. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 128:1253–1264. [DOI] [PubMed] [Google Scholar]
- Brunet, A., G. Pages, and J. Pouyssegur. 1994. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 9:3379–3387. [PubMed] [Google Scholar]
- Carmeliet, P., and R.K. Jain. 2000. Angiogenesis in cancer and other diseases. Nature. 407:249–257. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., M.G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 98:147–157. [DOI] [PubMed] [Google Scholar]
- Caveda, L., I. Martin-Padura, P. Navarro, F. Breviario, M. Corada, D. Gulino, M.G. Lampugnani, and E. Dejana. 1996. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J. Clin. Invest. 98:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell, M., J. Zhurinsky, and A. Ben-Ze'ev. 2002. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Invest. 109:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada, M., M. Mariotti, G. Thurston, K. Smith, R. Kunkel, M. Brockhaus, M.G. Lampugnani, I. Martin-Padura, A. Stoppacciaro, L. Ruco, et al. 1999. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA. 96:9815–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada, M., F. Liao, M. Lindgren, M.G. Lampugnani, F. Breviario, R. Frank, W.A. Muller, D.J. Hicklin, P. Bohlen, and E. Dejana. 2001. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 97:1679–1684. [DOI] [PubMed] [Google Scholar]
- Corada, M., L. Zanetta, F. Orsenigo, F. Breviario, M.G. Lampugnani, S. Bernasconi, F. Liao, D.J. Hicklin, P. Bohlen, and E. Dejana. 2002. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood. 100:905–911. [DOI] [PubMed] [Google Scholar]
- D'Amore, P.A. 1992. Mechanisms of endothelial growth control. Am. J. Respir. Cell Mol. Biol. 6:1–8. [DOI] [PubMed] [Google Scholar]
- Dayanir, V., R.D. Meyer, K. Lashkari, and N. Rahimi. 2001. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J. Biol. Chem. 276:17686–17692. [DOI] [PubMed] [Google Scholar]
- Dor, Y., V. Djonov, R. Abramovitch, A. Itin, G.I. Fishman, P. Carmeliet, G. Goelman, and E. Keshet. 2002. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 21:1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto, F., and B.M. Gumbiner. 1996. Cell contact-dependent signaling. Dev. Biol. 180:445–454. [DOI] [PubMed] [Google Scholar]
- Ferrara, N. 1999. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 77:527–543. [DOI] [PubMed] [Google Scholar]
- Ferrara, N., and K. Alitalo. 1999. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 5:1359–1364. [DOI] [PubMed] [Google Scholar]
- Fuchs, M., T. Muller, M.M. Lerch, and A. Ullrich. 1996. Association of human protein-tyrosine phosphatase κ with members of the armadillo family. J. Biol. Chem. 271:16712–16719. [DOI] [PubMed] [Google Scholar]
- Gerber, H.P., V. Dixit, and N. Ferrara. 1998. a. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 273:13313–13316. [DOI] [PubMed] [Google Scholar]
- Gerber, H.P., A. McMurtrey, J. Kowalski, M. Yan, B.A. Keyt, V. Dixit, and N. Ferrara. 1998. b. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 273:30336–30343. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., and B.M. Gumbiner. 2001. Adhesion signaling: how β-catenin interacts with its partners. Curr. Biol. 11:R792–R794. [DOI] [PubMed] [Google Scholar]
- Gottardi, C.J., E. Wong, and B.M. Gumbiner. 2001. E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J. Cell Biol. 153:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschuetzky, H., H. Aberle, and R. Kemler. 1994. β-Catenin mediates the interaction of the cadherin–catenin complex with epidermal growth factor receptor. J. Cell Biol. 127:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki, Y.Y., R.E. Hammer, J. Miyazaki, S.C. Williams, J.A. Richardson, and M. Yanagisawa. 2001. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230:230–242. [DOI] [PubMed] [Google Scholar]
- Kume, T., T. Watanabe, R. Sanokawo, D. Chida, T. Nakamura, and M. Oishi. 1996. Expression of the protein tyrosine phosphatase β2 gene in mouse erythroleukemia cells induces terminal erythroid differentiation. J. Biol. Chem. 271:30916–30921. [DOI] [PubMed] [Google Scholar]
- Kypta, R.M., H. Su, and L.F. Reichardt. 1996. Association between a transmembrane protein tyrosine phosphatase and the cadherin–catenin complex. J. Cell Biol. 134:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani, M.G., M. Resnati, M. Raiteri, R. Pigott, A. Pisacane, G. Houen, L.P. Ruco, and E. Dejana. 1992. A novel endothelial-specific membrane protein is a marker of cell–cell contacts. J. Cell Biol. 118:1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani, M.G., M. Corada, P. Andriopoulou, S. Esser, W. Risau, and E. Dejana. 1997. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J. Cell Sci. 110:2065–2077. [DOI] [PubMed] [Google Scholar]
- Lampugnani, M.G., A. Zanetti, F. Breviario, G. Balconi, F. Orsenigo, M. Corada, R. Spagnuolo, M. Betson, V. Braga, and E. Dejana. 2002. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol. Biol. Cell. 13:1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg, S., A. Yarden, Z. Kam, and B. Geiger. 1999. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 18:869–876. [DOI] [PubMed] [Google Scholar]
- Meadows, K.N., P. Bryant, and K. Pumiglia. 2001. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J. Biol. Chem. 276:49289–49298. [DOI] [PubMed] [Google Scholar]
- Mueller, S., E. Cadenas, and A.H. Schonthal. 2000. p21WAF1 regulates anchorage-independent growth of HCT116 colon carcinoma cells via E-cadherin expression. Cancer Res. 60:156–163. [PubMed] [Google Scholar]
- Ostman, A., Q. Yang, and N.K. Tonks. 1994. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc. Natl. Acad. Sci. USA. 91:9680–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palka, H.L., M. Park, and N.K. Tonks. 2003. Hepatocyte growth factor receptor tyrosine kinase Met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J. Biol. Chem. 278:5728–5735. [DOI] [PubMed] [Google Scholar]
- Pece, S., and J.S. Gutkind. 2000. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 275:41227–41233. [DOI] [PubMed] [Google Scholar]
- Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837–1851. [PubMed] [Google Scholar]
- Posthaus, H., L. Williamson, D. Baumann, R. Kemler, R. Caldelari, M.M. Suter, H. Schwarz, and E. Muller. 2002. β-Catenin is not required for proliferation and differentiation of epidermal mouse keratinocytes. J. Cell Sci. 115:4587–4595. [DOI] [PubMed] [Google Scholar]
- Rahimi, N., and A. Kazlauskas. 1999. A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells. Mol. Biol. Cell. 10:3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and V. Baron. 1999. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11:197–202. [DOI] [PubMed] [Google Scholar]
- Shay-Salit, A., M. Shushy, E. Wolfovitz, H. Yahav, F. Breviario, E. Dejana, and N. Resnick. 2002. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. USA. 99:9462–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Croix, B., C. Sheehan, J.W. Rak, V.A. Florenes, J.M. Slingerland, and R.S. Kerbel. 1998. E-cadherin–dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J. Cell Biol. 142:557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger, A., A. Eger, J. Wolf, H. Beug, and R. Foisner. 2001. E-cadherin regulates cell growth by modulating proliferation-dependent β-catenin transcriptional activity. J. Cell Biol. 154:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., and K. Suzuki. 1996. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp. Cell Res. 226:214–222. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., and M. Shibuya. 1997. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-γ pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 14:2079–2089. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., K. Takahashi, R. Mernaugh, V. Drozdoff, C. Sipe, H. Schoecklmann, B. Robert, D.R. Abrahamson, and T.O. Daniel. 1999. a. Endothelial localization of receptor tyrosine phosphatase, ECRTP/DEP-1, in developing and mature renal vasculature. J. Am. Soc. Nephrol. 10:2135–2145. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., H. Ueno, and M. Shibuya. 1999. b. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK- MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 18:2221–2230. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., S. Yamaguchi, K. Chida, and M. Shibuya. 2001. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A- dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., K. Takahashi, P.L. St. John, P.A. Fleming, T. Tomemori, T. Watanabe, D.R. Abrahamson, C.J. Drake, T. Shirasawa, and T.O. Daniel. 2003. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol. Cell. Biol. 23:1817–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks, N.K., and B.G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182–195. [DOI] [PubMed] [Google Scholar]
- Trapasso, F., R. Iuliano, A. Boccia, A. Stella, R. Visconti, P. Bruni, G. Baldassarre, M. Santoro, G. Viglietto, and A. Fusco. 2000. Rat protein tyrosine phosphatase η suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1). Mol. Cell. Biol. 20:9236–9246. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ukropec, J.A., M.K. Hollinger, S.M. Salva, and M.J. Woolkalis. 2000. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 275:5983–5986. [DOI] [PubMed] [Google Scholar]
- van de Wetering, M., W. de Lau, and H. Clevers. 2002. WNT signaling and lymphocyte development. Cell. 109(Suppl):S13–S19. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., C. Bauer, L. Degenstein, B. Wise, and E. Fuchs. 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell. 104:605–617. [DOI] [PubMed] [Google Scholar]
- Vinals, F., and J. Pouyssegur. 1999. Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol. Cell. Biol. 19:2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg, T., Y. Zick, R. Dror, I. Sabanay, C. Gilon, A. Levitzki, and B. Geiger. 1992. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J. 11:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte, L., D.J. Hicklin, Z. Zhu, B. Pytowski, H. Kotanides, P. Rockwell, and P. Bohlen. 1998. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 17:155–161. [DOI] [PubMed] [Google Scholar]
- Zanetti, A., M.G. Lampugnani, G. Balconi, F. Breviario, M. Corada, L. Lanfrancone, and E. Dejana. 2002. Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler. Thromb. Vasc. Biol. 22:617–622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.