Abstract
During T-cell activation, a number of cytokine-activated signaling cascades, including the Jak-STAT, phosphoinositol 3-kinase (PI 3-kinase), and mitogen-activated protein kinase (MAPK) pathways, play important roles in modulating the expression of target genes and mediating a cellular response. We now report that interleukin 2 (IL-2) and IL-15, but not IL-7, rapidly activate the p90 ribosomal S6 kinases, Rsk1 and Rsk2, in human T lymphocytes. Surprisingly, mouse spleen T cells transduced with either the wild-type or a dominant-negative (DN) Rsk2-expressing retrovirus could not be recovered, in contrast to the normal survival of T cells transduced with retroviruses expressing wild-type or DN mutants of Rsk1 or Rsk3. Examination of Rsk2 knockout (KO) mice revealed normal T-cell development, but these T cells had delayed cell-cycle progression and lower production of IL-2 in response to anti-CD3 and anti-CD28 stimulation in vitro. Moreover, Rsk2 KO mice had defective homeostatic T-cell expansion following sublethal irradiation in vivo, which is known to involve T-cell receptor (TCR), IL-2, and/or IL-15 signals, each of which we demonstrate can rapidly and potently activate Rsk2 in mouse T cells. These results indicate an essential nonredundant role of Rsk2 in T-cell activation.
Introduction
Interleukin-2 has pleiotropic actions on T cells, B cells, and natural killer (NK) cells.1–5 Three IL-2 receptor subunits have been identified, including the IL-2 receptor α chain (IL-2Rα), IL-2Rβ, and the common cytokine receptor γ chain, γc.1–3 High-affinity (IL-2Rα/IL-2Rβ/γc) and intermediate-affinity (IL-2Rβ/γc) IL-2 receptors are functional receptors, whereas low-affinity (IL-2Rα) receptors are not.1–3 Interestingly, IL-2Rβ is also shared by the IL-15 receptor, whereas γc is also shared by the receptors for IL-4, IL-7, IL-9, IL-15, and IL-21,6,7 and is the protein whose gene is mutated in humans with X-linked severe combined immunodeficiency (XSCID).8
Like other type 1 cytokine receptor proteins, IL-2Rβ and γc lack intrinsic catalytic activities. IL-2–induced phosphorylation events are instead mediated by kinases that are either associated with receptor subunits or part of downstream signaling cascade(s). IL-2 can rapidly activate the Jak-STAT, phosphoinositol 3-kinase (PI 3-kinase), and Ras-MAP kinase pathways, and together these modulate biologic actions of IL-2.4,5 Much attention has focused on Jak-STAT signaling, and IL-2 mediates the activation of Jak1 and Jak3,9,10 with resulting tyrosine phosphorylation of Stat5a and Stat5b.4,11
One of the serine kinase pathways activated by IL-2 is the Ras-MAP kinase pathway, which is widely used by growth factors, hormones, neurotransmitters, and stress stimuli,12 and within the immune system, this pathway is activated via antigen receptors as well as cytokines, and is known to be essential for the development, differentiation, and function of T cells.4,13–16
Interestingly, mitogen-activated protein kinases (MAPKs) are found in both the cytoplasm and nucleus,17 and this dual localization is shared by 85 kDa to 92 kDa serine/threonine kinases downstream of MAPKs that are known as p90 ribosomal S6 kinases (p90Rsks).17 These latter kinases have 2 catalytic domains,18,19 and simultaneous mutation of both adenosine triphosphate (ATP) binding sites abrogates kinase activity20 and results in a dominant-negative mutant. Full catalytic activity of Rsks requires activation by both extracellular signal-regulated kinase (ERK)21,22 and PDK1 (3-phosphoinositide–dependent protein kinase 1).23,24
Rsk family kinases have multiple cellular functions. They are involved in the phosphorylation of histone H3 and remodeling of chromatin in response to epidermal growth factor (EGF)25 and can regulate gene expression by phosphorylating transcription factors, including c-Fos,26,27 cAMP-response element-binding protein (CREB),28–30 CREB-binding protein,31,32 estrogen receptor,33 ATF4,34 NFATc4,35 and NF-κB/IκBα.36,37 p90Rsk2 was also reported to phosphorylate the p34cdc2 inhibitory kinase Myt1 in frog oocytes, leading to activation of the cyclin-dependent kinase p34cdc2 that then promotes cell-cycle progression of oocytes through the G2/M phase of meiosis.38 Phosphorylation of the proapoptotic protein, Bcl-XL/Bcl-2–associated death promoter (Bad) by p90Rsk2 suppresses Bad-mediated apoptosis in neurons.39 In addition, phosphorylation of the Ras GTP/GDP-exchange factor, SOS, by p90Rsk2 in response to EGF may be involved in negative feedback regulation of the MAP kinase signaling pathway.40 Finally, loss-of-function mutations of p90Rsk2 in humans cause the Coffin-Lowry syndrome,41 an X-linked form of mental retardation that is associated with delayed bone age, delayed closure of fontanelles, and short stature.42,43
We now show that IL-2 and IL-15 rapidly activate Rsk1 and Rsk2 in human T cells. Moreover, our data indicate a nonredundant role for Rsk2 in TCR/cytokine signaling based on expression of various Rsk constructs as well as analysis of Rsk2-deficient mice.
Methods
Cell culture
Human peripheral blood lymphocytes (PBLs) were isolated from healthy donors using Ficoll-Hypaque, and T cells were enriched using nylon wool. Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS; HyClone, Logan, UT), 2 mM glutamine, 100 U/mL penicillin and streptomycin (complete medium), and 2 μg/mL phytohemmaglutinin (PHA; Boehringer Mannheim, Mannheim, Germany) for 2 days and maintained in complete medium with 20 U/mL recombinant (r) IL-2 (Roche, Indianapolis, IN). NK-like YT cells44 were grown in complete RPMI-1640 medium and 293T+ cells in complete Dulbecco modified Eagle medium (DMEM).
Plasmids, oligonucleotides, and mutagenesis
pRV is a bicistronic murine stem cell leukemia retroviral expression vector.45 pCL-Eco is a retroviral packaging vector plasmid.46 Mouse Rsk1, Rsk2, and Rsk3 retroviral vectors were generated by polymerase chain reaction (PCR) amplification from cDNAs using PfuUltra II Fusion HS DNA Polymerase (Stratagene, La Jolla, CA) or AccuPrime Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA) and primers including the Kozak consensus sequence 5′ to each ATG, with XhoI sites (for Rsk1 and Rsk2) or SalI sites (for Rsk3) at 5′ and 3′ ends, and subcloned into the XhoI site of pRV. Correct orientations and sequences were confirmed by DNA sequencing. Dominant-negative (DN) Rsk mutants were generated using oliogonucleotide-mediated (Table 1) site-directed mutagenesis (Stratagene) to replace K94 and K447 in Rsk1, K100 and K451 in Rsk2, and K91 and K444 in Rsk3 with alanines.20
Table 1.
Oligonucleotides used for cloning and site-directed mutagenesis to generate retroviral expression vectors
| Name | Sequence |
|---|---|
| mRsk1Kozak-T | ATA TAT CTC GAG GCC GCC ACC ATG CCG CTC GCC CAG CTC AAG |
| mRsk1-B | ATA TAT CTC GAG TCA CAG GGT GGT GGA TGG CAG CTT CC |
| mRsk1 K94A-T | GGG CAC TTG TAT GCC ATG GCA GTA TTA AAG AAG GCC ACG |
| mRsk1 K94A-B | CGT GGC CTT CTT TAA TAC TGC CAT GGC ATA CAA GTG CCC |
| mRsk1 K447A-T | ATG GAG TAT GCT GTC GCG GTC ATC GAC AAG AGC |
| mRsk2 Kozak: | GGA ATT AGA TCT CTC GAG GCC GCC ACC ATG CCG CTG GCG CAG CTG |
| mRsk2 K100A-T: | AGA CAG CTT TAT GCC ATG GCA GTA TTA AAG AAG GCC ACG CTG |
| mRsk2 K100A-B: | CAG CGT GGC CTT CTT TAA TAC TGC CAT GGC ATA AAG CTG TCT |
| mRsk2 K451A-T: | AAC ATG GAG TTT GCC GTG GCG ATT ATT GAT AAA AGC AAG AG |
| mRsk2 K451A-B: | CTC TTG CTT TTA TCA ATA ATC GCC ACG GCA AAC TCC ATG TT |
| mRsk1 K447A-B | GCT CTT GTC GAT GAC CGC GAC AGC ATA CTC CAT |
| mRsk3 Kozak-T | ATA TAT GTC GAC GCC GCC ACC ATG GAG CTG AGC ATG AAG AAG TTC |
| mRsk3-B | ATA TAT GTC GAC CTA CAA CCT GGT AGA CGT GAG TCT C |
| mRsk3 | K91A-T GGT CAG CTC TAC GCC ATG GCG GTC CTG AAG AAA GCC ACC |
| mRsk3 | K91A-B GGT GGC TTT CTT CAG GAC CGC CAT GGC GTA GAG CTG ACC |
| mRsk3 | K444A-T GAT GCT GAG TAT GCT GTG GCG ATC ATC GAT AAG AGC AAA |
| mRsk3 | K444A-B TTT GCT CTT ATC GAT GAT CGC CAC AGC ATA CTC AGC GTC |
| mRsk1 121 | CAC CCG TTC GTG GTG AAG |
| mRsk1 271 | CCC CAG TTT CTG AGC ACG |
| mRsk1 421 | TGT AAG CGT TGT GTC CAC |
| mRsk1 571 | GCA CCT GAG GTC CTG AAG |
| mRsk2 121 | ATC TTG GTA GAA GTC AAT C |
| mRsk2 271 | AAA GAT CGT AAA GAA ACA |
| mRsk2 421 | GGA TAT GAA GTA AAA GAG |
| mRsk2 571 | GAA AAT GGT CTT CTC ATG |
| mRsk3 121 | ATT GTC AAG CTG CAT TAT G |
| mRsk3 271 | ATC CTC AAA GCC AAG CTG |
| mRsk3 421 | ATC GGG GTG GGC TCC TAC |
| mRsk3 571 | CCC TGC TAT ACT GCA AAC |
| pRV 5′ Seq: | TTA TCC AGC CCT CAC TCC TTC |
Underlined nucleotides are mutations. Sequencing primers are also listed.
Antibodies, immunoprecipitation, Western blotting, and in vitro kinase assay
Antibodies to Rsk1 (C-21), Rsk2 (C-19 and E-1), Rsk3 (C20), pRsk (T359/S363), pRsk (T577), Erk (K23), Actin (I19), and antiphosphotyrosine antibody (pY99) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Stat5 monoclonal antibody was from Transduction Laboratories (Lexington, KY). Antibodies for human Stat5a and Stat5b were described elsewhere.47 For immunoprecipitation and Western blotting,48 cells were washed in cold phosphate-buffered saline (PBS) and lysed in 50 mM Tris, pH 7.0, 150 mM NaCl, 0.5% NP40, 2.5 mM metabisulphite, 20 mM β-glycerophosphate, 0.5 mM Na3VO4, 5 mM benzamidine HCl, 20 mM NaF, and 1 mM AEBSF at 4°C for 30 minutes. Lysates were clarified by centrifugation at 25 000g at 4°C for 20 minutes. Immunoprecipitations were performed using 1 μg antibody and 40 μL rProtein-G agarose slurry (Invitrogen) or Goat ExactaCruz D IP Matrix (Santa Cruz Biotechnology), and then washed, resolved by 4% to 20% Nu-PAGE (Invitrogen), transferred to Immobilon-P membranes, blotted with indicated antibodies, and developed by enhanced chemiluminescence (ECL) reagent (Millipore, Billerica, MA).
For on-membrane in vitro kinase assays,49 total cell lysates were denatured, fractionated by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF membranes, denatured with 7 M guanidine HCl, and renatured on the membranes. After blocking with 5% bovine serum albumin (BSA), 30 mM Tris, pH 7.5, membranes were incubated with kinase buffer (30 mM Tris, pH 7.5, 10 mM MgCl2, 2 mM MnCl2) containing 50 μCi/mL [γ32-P]ATP (3000-6000 Ci/mmol; TBg/mmole-222 TBg/mmole) at room temperature for 1 hour. Kinase reactions were stopped by immersing blots in 30 mM Tris, pH 7.5, followed by washing for 10 minutes each at room temperature with 30 mM Tris pH 7.5, 30 mM Tris pH 7.5 containing 0.05% NP40, and 30 mM Tris pH 7.5 buffers. To further reduce background, membranes were washed sequentially with 1 M KOH, H2O, and 10% acetic acid, and kinase activity was detected by autoradiography.
Transfection of 293T+ cells and reporter assay
293T+ cells were cotransfected47 with a luciferase reporter containing 3 copies of the IL-2Rα GASn motif,50 the Renilla luciferase reporter control plasmid pSV-RL, and plasmids expressing IL-2Rβ, γc, Jak3, and wild-type (WT) or serine mutants of Stat5a using Effectene (Qiagen, Valencia, CA). At 40 hours after transfection, cells were either not treated or treated with 100 U/mL IL-2 for 6 to 24 hours, and lysed. Cleared lysates were used to determine reporter activity using the dual luciferase assay kit (Promega, Madison, WI).
Retroviral transduction and FACS analysis
Retroviral supernatants were produced and mouse T cells transduced as described.51 pCL-Eco was cotransfected with WT or mutant pRV-Rsk1, pRV-Rsk2, or pRV-Rsk3, into 293T+ cells using Effectene (Qiagen). One day later, medium was changed to RPMI-1640 medium containing 7.5% fetal bovine serum (FBS), glutamine, streptomycin, and penicillin, and the cells incubated at 32°C. Retrovirus-containing supernatant was harvested 48 and 72 hours after transfection. Splenocytes were isolated from 8- to 12-week-old WT C57BL/6 mice and T cells were either enriched by panning with goat anti–mouse IgG (Sigma, St Louis, MO) or purified using CD90 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), grown in complete RPMI medium containing 50 μM 2 ME, and activated with 5 μg/mL plate-bound anti-CD3 plus 1 μg/mL anti-CD28 for 24 hours. Activated T cells were transduced with viral supernatant in the presence of 4 μg/mL polybrene (Sigma), spinning at 713g for 45 minutes at 30°C, and grown in complete RPMI medium containing 2 μg/mL anti-CD3, 1 μg/mL anti-CD28, and 40 U/mL IL-2. A second retroviral transduction was performed 24 hours after the first, and the cells were grown in complete medium with 40 U/mL IL-2 for 2 to 3 days. One day after the second retroviral transduction, transduced T cells were identified by staining with anti-CD3 allophycocyanin (APC) and annexin V PE (BD Biosciences, San Jose, CA) and analyzed by FACSort using CellQuest software (BD Biosciences). To determine the retroviral titer, NIH3T3 cells were incubated with a range of volumes of the retroviral supernatant in the presence of 4 μg/mL polybrene overnight and grown in complete medium for an additional 24 hours before determining GFP+ cells using a FACSort (BD Biosciences, San Jose, CA).
Analysis of Rsk2-deficient mice
Wild-type C57BL/6 or Balb/c male mice used for mating were from the Jackson Laboratory (Bar Harbor, ME). All experiments were performed under protocols approved by the National Heart, Lung, and Blood Institute (NHLBI) Animal Use and Care Committee and followed the National Institutes of Health (NIH) guidelines for using animals in intramural research. Rsk2-deficient mice were generated at the NHLBI Mouse Core Facility using Rsk2−/− embryonic stem (ES) cells (provided by Dr Beverly H. Koller, University of North Carolina, and Dr Christian Bjorbaek, Harvard University), as described.52 Rsk2 KO mice were identified by PCR amplification using the following primers: Rsk2 5′ primer, CTTGATGAAGAAGGTCACATCAAG; Rsk2 3′ primer, AACTACTTCTGGAGCCATGTATTC; Neo 5′ primer, ACAAGATGGATTGC ACGCAGGTTC; Neo 3′ primer, TCTTCGTCCAGATCATCCTGATCG. Heterozygous female mice were backcrossed to C57BL/6 or Balb/c male mice for 5 to 9 generations. All Rsk2-deficient mice used were male, as Rsk2 is on the X chromosome.53
Thymic and splenic cellularities were determined. Total splenic T cells and CD4+ and CD8+ subpopulations were isolated from 6- to 12-week-old Rsk2-deficient mice and age-matched littermates. Cells were stimulated with plate-bound anti-CD3 (2 μg/mL) or plate-bound anti-CD3 (2 μg/mL) plus anti-CD28 (1 μg/mL) for 16 or 40 hours, followed by the measurement of mRNA levels for IL-2, IL-4, and IFNγ by real-time reverse transcription–polymerase chain reaction (RT-PCR; TaqMan; Applied Biosystems, Foster City, CA) using the following primers: MuIL-2 FW (461) TTGAGTGCCAATTCGATGATG melting temperature (Tm) = 58°C), MuIL-2 RV (541) AGATGATGCTTTGACAGAAGGCTAT (Tm = 59°C), MuIL-2 TapMan probe (485) CAGCAACTGTGGTGGACTTTCTGAGGAGAT (Tm = 69°C), MuIL-4 FW (231) CGGAGATGGATGTGCCAAAC (Tm = 60°C), MuIL-4 RV (311) GCACCTTGGAAGCCCTACAG (Tm = 58°C), MuIL-4 TaqMan probe (257) ACAGCAACGAAGAACACCACAGAGAGTGAG (Tm = 68°C), Mu IFNγ FW (91) CCTGCGGCCTAGCTCTGA (Tm = 59°C), Mu IFNγ RV (171) CAGCCAGAAACAGCCATGAG (Tm = 58°C), Mu IFNγ TaqMan probe (120) CTACACACTGCATCTTGGCTTTGCAGCTC (Tm = 69°C), MuIL-15 FW (80) CTGAGGCTGGCATTCAT TCT (Tm = 59°C), MuIL-15 RV (155) TCTATCCAGTTGGCCTCTGTTTTA (Tm = 58°C), and MuIL-15 TaqMan probe (102) CATTTTGGGCTGTGTCAGTGTAGGTCTCC (Tm = 68°C). The real-time RT-PCR primer for IL-7 was purchased from Applied Biosystems. The following real-time RT-PCR primers for mouse rpl7 were used to normalize input cDNA: MuRPL7 FW (312) ATGTGCCCGCAGAACCAA (Tm = 60°C), MuRPL7 RV (407) GACGAAGGAGCTGCAGAACCT (Tm = 60°C), and MuRPL7 TaqMan probe (351) TTCGAGGTATCAATGGAGTAAGCCCAAAGG (Tm = 69°C). IL-2 protein was measured using the OptEIA Mouse IL-2 ELISA Kit (BD Biosciences).
For in vitro proliferation experiments, 5,6-carboxyfluorescein diacetate-succinimyl ester (CFSE)–labeled T cells (purified by negative selection using a pan–T-cell isolation kit; Miltenyi Biotec) were stimulated by plate-bound anti-CD3 (2 μg/mL) plus anti-CD28 (1 μg/mL). Cell division of CD4+ and CD8+ T-cell subpopulations was measured by assessing CSFE dilution on days 1 to 4 by flow cytometric analyses of cells stained with CD4-CyChrom and CD8-APC. T-cell survival was determined using an Annexin V–PE Apoptosis Detection Kit (BD Pharmingen, San Diego, CA) on day 1 and day 2 after stimulation with plate-bound anti-CD3 (2 μg/mL) plus anti-CD28 (1 μg/mL). For homeostatic proliferation in vivo, 8- to 12-week-old WT or Rsk2-deficient mice were sublethally irradiated (600 Rad), and splenocyte recovery was evaluated 14 to 16 days later. Total splenocytes, T cells, and CD4+ and CD8+ T-cell subpopulations, as well as B220+, DX5+, Mac1+, Gr1+, and Ter119+ cells were evaluated by flow cytometry. For cytokine injection experiments, sublethally irradiated WT or Rsk2-deficient mice were intraperitoneally injected54 daily for 15 days with 10 μg IL-2, IL-15 in 0.2 mL PBS or 0.2 mL PBS and total splenocytes, T cells, or CD4+ and CD8+ T-cell subpopulations were evaluated by flow cytometry.
Results
IL-2 and IL-15, but not IL-7, rapidly activate Rsk1 and Rsk2 in human T cells and YT cells
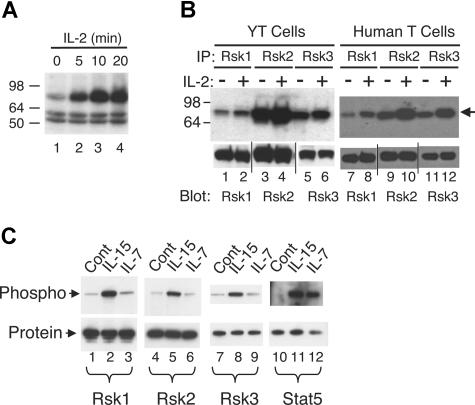
To identify serine/threonine kinase(s) that are activated by IL-2, an on-membrane in vitro kinase assay that preferentially detects serine/threonine protein kinases49 was performed. As shown in Figure 1A, a 78 kDa to 80 kDa major band was revealed in total cell lysate of YT cells and its activity was rapidly and markedly increased by IL-2 stimulation (Figure 1A lanes 2-4 vs lane 1). Bands of 16 kDa (not shown) and 50 kDa to 60 kDa were also detected, but IL-2 did not increase their intensities (Figure 1A). To try to identify the 78 kDa to 80 kDa putative kinase, we tested antibodies to Raf1, RafB, p70S6 kinase (p70S6K), p90Rsk1, p90Rsk2, and p90Rsk3, which are serine/threonine kinases with relatively similar molecular weights. Of these, complexes immunoprecipitated with antibodies to Rsk1, Rsk2, and Rsk3 exhibited basal kinase activity that was induced by IL-2 in YT cells (Figure 1B lanes 1-6) and primary human T cells (lanes 7-12), whereas IL-2 did not induce activation of Raf1, RafB, and p70S6K in this assay (not shown). However, evaluation of these Rsk antibodies revealed that antibodies to Rsk1 and Rsk2 were specific for endogenous and overexpressed Rsk1 and Rsk2 proteins (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article), respectively, whereas the antibody to Rsk3 could also recognize endogenous Rsk2 (Figure S1A) as well as overexpressed Rsk1 and Rsk2 (Figure S1A,B). We also examined 2 other cytokines whose receptors share γc and found that Rsk1 and Rsk2 were also activated in human T cells by IL-15 (Figure 1C lanes 2 and 5 vs lanes 1 and 4) but not by IL-7 (lanes 3 and 6). Because of possible cross-reactivity, no definitive conclusions could be made regarding the activation of Rsk3 (lanes 7-9). As expected, both IL-15 and IL-7 activated Stat5 in these cells (lanes 11 and 12 vs lane 10).
Figure 1.
Rsk1, Rsk2, and Rsk3 are activated in human T cells by IL-2 and IL-15 but not by IL-7. (A) Total lysates from YT cells not treated or treated with IL-2 for various time points were subjected to an on-membrane in vitro kinase assay. The arrow indicates the IL-2–induced protein kinase activity. (B) Total lysates from YT cells or primary human T cells not treated or treated with IL-2 for 15 minutes were immunoprecipitated with anti-Rsk1, anti-Rsk2, or anti-Rsk3 antibodies and the corresponding immune complexes then subjected to an on-membrane in vitro kinase assay. The arrow indicates the protein kinase activity. Levels of immunoprecipitated Rsk1, Rsk2, and Rsk3 were determined by blotting an aliquot with antibodies as indicated. Vertical lines have been inserted to indicate repositioned gel lanes. (C) Total lysates from primary human T cells not treated or treated with IL-15 or IL-7 for 20 minutes were immunoprecipitated with anti-Rsk1, anti-Rsk2, anti-Rsk3, or anti-Stat5a/b antibodies. Phospho-Rsk1 and -Rsk2 were detected by blotting with anti-pRsk (Thr577), phospho-Rsk3 with anti-pRsk (Thr359/Ser363), and phospho-Stat5 with pY99 as indicated. Equal amounts of protein immunoprecipitated by each antibody were confirmed by blotting with each corresponding antibody as shown.
Rsks do not alter IL-2–dependent GAS-driven reporter activity but the WT and a DN Rsk2 mutant failed to transduce mouse T cells
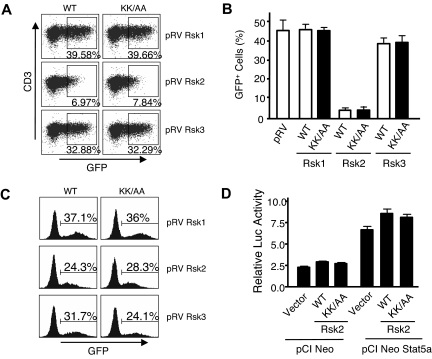
To further evaluate the biologic significance of IL-2–induced phosphorylation of the Rsks, we generated retroviruses expressing wild-type (WT) or DN mutants of Rsk1, Rsk2, and Rsk3, with similar viral titers and expression levels in 293T+ cells (not shown). Infected GFP+ T cells were readily recovered from Rsk1, Rsk3, DN-Rsk1, and DN-Rsk3, but unexpectedly, essentially no GFP+ cells were recovered from T cells transduced with either the WT Rsk2 or a DN Rsk2 mutant (Figure 2A,B). The same retroviral supernatants could infect NIH3T3 cells with similar efficiency (Figure 2C), indicating that all these retroviral constructs could produce comparable levels of infectious viral particles in 293T+ cells. Although the basis for the effect of the Rsk2 is unknown, it is presumably independent of Stat5a phosphorylation, as neither the WT Rsk2 nor the DN Rsk2 affected Stat5-dependent reporter activity (Figure 2D).
Figure 2.
Mouse spleen T cells transduced by a DN Rsk2 mutant could not be recovered. (A) Representative flow cytometric profiles of mouse spleen T cells transduced by WT or DN mutants (KK/AA) of mouse Rsk1, Rsk2, and Rsk3. The numbers indicate percentage of the GFP+ cells gated on CD3+ cells. (B) A summary of 3 independent retroviral transduction experiments. The error bars indicate mean plus or minus standard error of the mean (SEM). The WT and DN mutant for each retroviral construct was labeled as WT and KK/AA, respectively. (C) NIH3T3 cells were infected with 0.2 mL of the same retroviral supernatant produced in 293T+ cells as used in panel A and GFP+ cells were determined on day 2 after infection. (D) 293T+ cells were transfected with pME18S-IL-2Rβ, pME18S-γc, pME18S-Jak3, pEIB IL-2Rα PRRIII reporter, pCi NeoStat5a or vector control pCINeo, and pRL-SV together with either WT or the DN mutant (shown as KK/AA) of pCI neo Rsk2, as indicated. IL-2–induced relative luciferase activity from a representative transfection is shown as mean plus or minus SEM from triplicate samples.
Rsk2 is required for T-cell proliferation in vitro and homeostatic proliferation in vivo
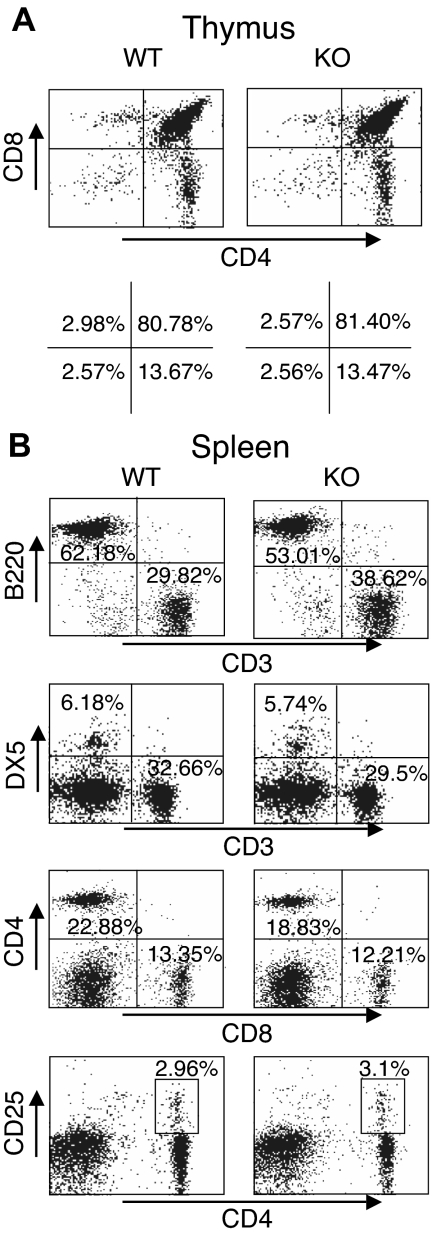
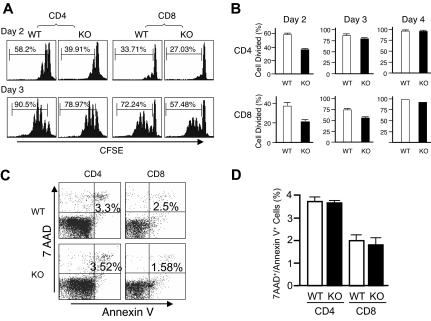
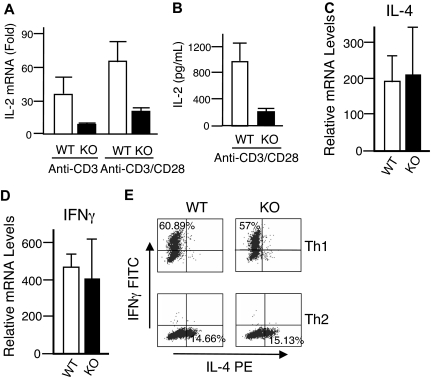
Given the unexpected lack of recovery of T cells transduced with either WT or DN Rsk2, to further analyze the role of Rsk2 on cell proliferation/survival, we next regenerated Rsk2 KO mice52 from Rsk2 KO ES cells. As expected, T cells from Rsk2 KO mice did not express Rsk2 but expressed similar levels of Rsk1, Rsk3, and Erk as their WT littermates (Figure S1A). These mice and WT littermates had similar cellularity and CD4/CD8 flow cytometric profiles in the thymus (Figure 3A) as well as similar numbers of T, B, and NK cells and CD4+, CD8+, and CD4+/CD25+ T-cell subpopulations in the spleen (Figure 3B). This indicates that Rsk2 is dispensable for normal lymphocyte development. Interestingly, however, when CFSE-labeled T cells were stimulated in vitro by anti-CD3 plus anti-CD28, both CD4+ and CD8+ T cells from Rsk2 KO mice had delayed cell division as compared with cells from WT littermates. This effect was evident at days 2 and 3 (Figure 4A-B), but not at day 4 (Figure 4B), indicating that Rsk2 contributes to the early phase of T-cell proliferation in response to stimulation with anti-CD3 plus anti-CD28. In addition, there was no increased apoptosis in either CD4 or CD8 T cells from Rsk2 KO mice after stimulation with anti-CD3 plus anti-CD28 (Figure 4C,D). Because of this delayed TCR-induced proliferation, we hypothesized that Rsk2 might be required for optimal production of IL-2. Indeed, stimulation of Rsk2 KO CD4+ T cells with anti-CD3 or anti-CD3 plus anti-CD28 resulted in diminished induction of IL-2 mRNA (Figure 5A) and with anti-CD3 plus anti-CD28 resulted in lower production of IL-2 protein (Figure 5B). In contrast, IL-4 mRNA (Figure 5C) and IFNγ mRNA (Figure 5D) induced by anti-CD3 plus anti-CD28 stimulation were expressed at comparable levels in both WT and Rsk2 KO T cells. Furthermore, differentiation of Th1 or Th2 CD4+ T cells was not defective, as indicated by normal anti-CD3 plus anti-CD28–mediated production of IFN-γ (Th1) and IL-4 (Th2) in differentiated Rsk2 KO CD4+ T cells (Figure 5E). These data indicate that Rsk2 plays an essential role in regulating expression of IL-2 but not IFN-γ or IL-4.
Figure 3.
Normal T- and B-cell development in thymus and spleen of Rsk2 KO mice. Representative flow cytometric profiles of thymus (A) and spleen (B) from WT littermate and Rsk2-deficient mice (KO). The numbers show the percentage of each cell population with positive staining by indicated antibodies.
Figure 4.
Rsk2 is required for the early phase of T-cell proliferation and expression of IL-2 but is not required for IFNγ and IL-4 production. (A) Purified T cells isolated from WT and Rsk2 KO (KO) mice were labeled with CFSE and stimulated with plate-bound anti-CD3 (2 μg/mL) plus anti-CD28 (1 μg/mL). The rates of cell division for CD4+ and CD8+ T cells were determined by dilution of CFSE on days 2 and 3, respectively. Representative flow cytometric profiles are shown and the percentage of cells undergoing cell division is indicated. (B) Summary of the CFSE experiment for data derived from days 2 to 4. Shown is the mean plus or minus SEM (WT: n = 3; KO: n = 4). (C) Purified T cells from WT and Rsk2 KO mice were stimulated using 2 μg/mL plate-bound anti-CD3 plus 1 μg/mL anti-CD28 for 24 hours, and the cells were stained first with anti-CD4 APC or anti-CD8 APC and then with 7AAD and Annexin V–PE. Apoptotic cells were indicated as 7AAD+/Annexin V+ cells. (D) Shown are data from 3 independent experiments. Error bars represent SEM.
Figure 5.
Rsk2 is required for optimal IL-2 expression but not for IL-4 and IFNγ expression and Th1 and Th2 differentiation. (A,B) Shown are IL-2 mRNA (A) and protein (B) induction in T cells from WT and Rsk2 KO (KO) mice stimulated by anti-CD3, anti-CD3 plus anti-CD28 (A), and anti-CD3 plus anti-CD28 (B). For IL-2 protein production, equal volumes of supernatants collected from same number of cells were used for enzyme-linked immunosorbent assay (ELISA). The error bars display the standard error of mean (n = 9 for panel C and n = 3 for panel D). (C,D) Shown are a representative experiment for IL-4 mRNA (C) and IFNγ mRNA (D) expression stimulated by anti-CD3 plus anti-CD28 in T cells from WT and Rsk2 KO (KO) mice. Shown are the means plus or minus SEM (n = 3). (E) Purified CD4+ T cells from either WT or Rsk2 KO mice were polarized to Th1 and Th2 cells and expanded for a total of 6 days before overnight restimulation with anti-CD3 plus anti-CD28. Expression of IFNγ and IL-4 in Th1 and Th2 cells stimulated by anti-CD3 plus anti-CD28 was determined by intracellular staining. Representative flow cytometric profiles are shown, and the percentages of Th1 cells expressing IFNγ and of Th2 cells expressing IL-4 are indicated.
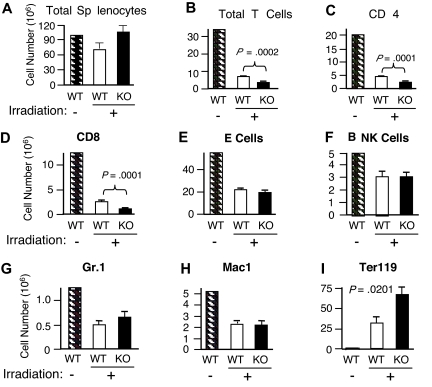
In view of the diminished cell-cycle progression observed in vitro (Figure 4A-B), we next investigated whether Rsk2 is also involved in the lymphopenia-mediated homeostatic proliferation of T cells in vivo. Mice were sublethally irradiated (600 rad) and then the recovery of cellular populations in the spleen was analyzed 16 days later. Total splenocytes were unexpectedly higher in irradiated Rsk2 KO mice than in WT mice (Figure 6A), but this apparently resulted, at least in part, from an increase in Ter119+ cells (Figure 6I). The basis for the increase in those erythroid lineage cells is unclear and an area for future investigation. The recovery of total T cells (Figure 6B) as well as both CD4 (Figure 6C) and CD8 (Figure 6D) subpopulations was significantly lower in irradiated Rsk2 KO mice than in WT mice, whereas there was no statistically significant difference in the recovery of B cells, NK cells, Gr1+ cells, and Mac1+ cells (Figure 6E-H). These data reveal a role for Rsk2 in the homeostatic proliferation of CD4+ and CD8+ T cells in vivo.
Figure 6.
Defective repopulation of total T cells as well as CD4 and CD8 subpopulations in irradiated Rsk2 KO mice and activation of Rsk2 by anti-CD3 plus anti-CD28 and IL-15 in mouse T cells but not by IL-7. WT and Rsk2 KO mice were sublethally irradiated and the cellularities of total splenocytes (A) and indicated subpopulation of cells (B-I) from these mice were determined 16 days after irradiation. A nonirradiated WT mouse was used as the control. Shown are means plus or minus SEM (n = 5).
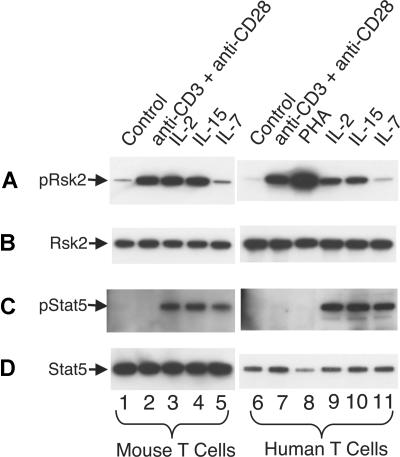
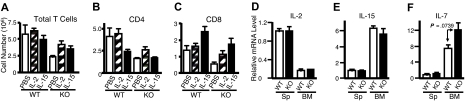
It is well known that signals for TCR activation and for IL-7 and IL-15 play vital roles in homeostatic proliferation of T cells in vivo.55,56 In addition to IL-2, anti-CD3 plus anti-CD28 and IL-15 can rapidly and potently activate Rsk2 in both mouse (Figure 7A lanes 2-4 vs lane 1) and human T cells (lanes 7-10 vs lane 6), whereas IL-7 did not (lanes 5 and 11). As expected, IL-2, IL-15, and IL-7 readily activated Stat5 in mouse (Figure 7C lanes 3-5 vs lane 1) and human T cells (lanes 9-11 vs lane 6) whereas antigen stimulations did not (lanes 2, 7, and 8). After sublethal irradiation, the number of total T cells in Rsk2 KO mice was lower as compared with WT mice, even with intraperitoneal injection with IL-2 or IL-15 (Figure 8A). However, these cytokines had some effect on total cellularity, with IL-2 having a grater effect on CD4+ T cells (Figure 8B) and IL-15 having a great effect on CD8+ T cells (Figure 8C). The ex vivo mRNA expression levels for IL-2, IL-15, and IL-7 in spleen and bone marrow were similar in WT and Rsk2 KO mice (Figure 8D-F), indicating that Rsk2 is not required for the basal expression of these cytokines in vivo. Thus, defective IL-2–, IL-15–, or TCR-mediated activation of Rsk2 may provide at least a partial explanation for the defective homeostatic proliferation of T cells in the Rsk2-deficient mice.
Figure 7.
TCR stimulation, IL-2, and IL-15, but not IL-7, potently activate Rsk2 in mouse and human T cells. Mouse T cells were first activated by 2 μg/mL plate-bound anti-CD3 and 1 μg/mL anti-CD28 for 2 days and human PBLs were first activated by 2 μg/mL PHA for 3 days. The activated cells were expanded with 40 U/mL of IL-2 for 4 days (mouse) or 7 days (human), washed, and rested for 24 hours (mouse) or 48 hours (human) before restimulation with each reagent for 20 minutes as indicated. Total cell lysates were prepared and equal amounts of lysates were immunoprecipitated with anti-Rsk2 (C19 antibody; A,B) or anti-Stat5a and anti-Stat5b (C,D) antibodies, and phospho-proteins were detected by blotting using anti-pRsk (Thr359/Ser363; panel A lanes 1 through 5) for mouse pRsk2, anti-pRsk (Thr577; panel A lanes 6 through 11) for human pRsk2, or pY99 antibodies (C) for pStat5, respectively. Equal loading was confirmed by using anti-Rsk2 (E1; B) or pan-Stat5 (D) as indicated.
Figure 8.
In vivo administration of IL-2 or IL-15 could not rescue defective homeostatic proliferation in Rsk2 KO mice, and Rsk2 mice had normal expression of IL-2, IL-7, and IL-15 in spleen and bone marrow cells. (A-C) Sublethally irradiated WT and Rsk2 KO (KO) mice were intraperitoneally injected daily for 15 days with either 10 μg of IL-2 or IL-15 in 0.2 mL PBS, or 0.2 mL PBS as control, and the cellularities of total T cells (A) and the CD4+ (B) and CD8+ (C) subpopulations from these mice were determined. (D-F) Ex vivo mRNA expression of IL-2 (D), IL-15 (E), and IL-7 (F) in splenocytes (Sp) and bone marrow (BM) from WT and Rsk2 KO (KO) mice was determined by RT-PCR, and the expression levels were shown as relative to the levels from splenocytes of WT mice.
Discussion
Although Rsk family proteins have been extensively studied, little is known of their role or roles in the immune system. We now report that Rsk1 and Rsk2 are catalytically activated by stimulation with TCR, IL-2, and IL-15 in T cells. Unexpectedly, primary splenic T cells transduced with this DN Rsk2 mutant could not be recovered, whereas cells expressing DN forms of Rsk1 and Rsk3 were readily recovered. Thus, Rsk2 appears to play an essential role in T-cell activation at least for cellular growth/survival. The inability to recover T cells after retroviral transduction with WT or DN Rsk2 prevented our using this approach to further evaluate the function of Rsk2 in normal T cells.
Both Erk1/2 and PDK1 are upstream of p90Rsks and are essential for the full activation of p90Rsks in response to extracellular stimuli. The vital roles of Erk1/2 and PDK1 in development and cellular functions have been demonstrated in knockout mice. Erk1 KO mice have defective thymocyte maturation and TCR-mediated thymocyte proliferation is severely impaired,57 whereas Erk2 and PDK1 KO mice are embryonic lethal.58–60 Interestingly, deleting PDK1 in T cells blocks thymocyte differentiation whereas hypomorphic expression allows thymocyte differentiation but blocks thymocyte expansion.61 Given the roles of Erk and PDK1 for activating all the Rsks, it is not surprising that the abnormalities in the Rsk2 KO mice are less severe than those in the Erk and PDK1-deficient mice. In this study, we showed that T, B, and NK cells from Rsk2 KO mice developed normally but that there is defective TCR-induced IL-2 production as well as a defect in homeostatic proliferation. Stimulation via the T-cell receptor, IL-7, and IL-15 can contribute to homeostatic proliferation55,56 and indeed we found that anti-CD3 plus anti-CD28 and IL-15 activated Rsk2 at least as potently as did IL-2 in both human and mouse T cells, which may partially explain the defect in homeostatic proliferation of T cells observed in Rsk2 mice upon sublethal irradiation. In contrast to IL-2 and IL-15, IL-7 did not activate Rsk2, which is consistent with previous reports that IL-7 does not activate the ERK/MAP kinase pathway in IL-7–dependent T-cell lines.62,63 Additional work is required to identify the specific critical substrates for Rsk2 and/or other functions for this protein to clarify the mechanistic basis. In summary, we have identified Rsk1 and Rsk2 as kinases that are activated by stimulation with anti-CD3 plus anti-CD28, IL-2, and IL-15, but not IL-7, and for the first time provided data indicating a critical role for Rsk2 in T-cell activation, with actions related to both cytokine and TCR signaling.
Supplementary Material
Acknowledgments
We thank Dr Hai-Hui Xue (National Heart, Lung, and Blood Institute [NHLBI]) for his valuable insights and suggestions; Drs Kuan-Teh Jeang (National Institute of Allergy and Infectious Diseases [NIAID]), Keji Zhao (NHLBI), and Larry E. Samelson (National Cancer Institute [NCI]) for their critical comments on the manuscript; Drs Robert L. Erickson for plasmid encoding for mouse Rsk2, Kenneth M. Murphy for pRV, and Inder M. Verma for pCL-Eco; Larry E. Samelson for his suggestion to use an on-membrane in vitro kinase assay; Chengyu Liu (Transgenic Core Facility, NHLBI) for regenerating Rsk2 KO mice; Hyok Joon Kwon for intraperitoneal injection; and Ms Constance Robinson for maintaining the mouse colony.
This work was supported by the Intramural Research Program of the NHLBI, NIH.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.-X.L. and R.S. designed and performed research, collected and analyzed data, and wrote the paper; W.J.L. designed research, analyzed data, and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren J. Leonard, NHLBI, NIH, Bldg 10, Rm 7B05, 10 Center Dr, Bethesda, MD 20892-1674; e-mail: wjl@helix.nih.gov.
References
- 1.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 2.Sugamura K, Asao H, Kondo M, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 3.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 5.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest. 2004;33:109–142. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 6.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 7.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 9.Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 10.Russell SM, Johnston JA, Noguchi M, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 11.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 12.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 14.Rincon M. MAP-kinase signaling pathways in T cells. Curr Opin Immunol. 2001;13:339–345. doi: 10.1016/s0952-7915(00)00224-7. [DOI] [PubMed] [Google Scholar]
- 15.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 16.Cantrell DA. Transgenic analysis of thymocyte signal transduction. Nat Rev Immunol. 2002;2:20–27. doi: 10.1038/nri703. [DOI] [PubMed] [Google Scholar]
- 17.Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SW, Erikson E, Blenis J, Maller JL, Erikson RL. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci U S A. 1988;85:3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjorbaek C, Zhao Y, Moller DE. Divergent functional roles for p90rsk kinase domains. J Biol Chem. 1995;270:18848–18852. doi: 10.1074/jbc.270.32.18848. [DOI] [PubMed] [Google Scholar]
- 21.Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 22.Gavin AC, Nebreda AR. A MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- 23.Jensen CJ, Buch MB, Krag TO, Hemmings BA, Gammeltoft S, Frodin M. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 1999;274:27168–27176. doi: 10.1074/jbc.274.38.27168. [DOI] [PubMed] [Google Scholar]
- 24.Richards SA, Fu J, Romanelli A, Shimamura A, Blenis J. Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr Biol. 1999;9:810–820. doi: 10.1016/s0960-9822(99)80364-9. [DOI] [PubMed] [Google Scholar]
- 25.Sassone-Corsi P, Mizzen CA, Cheung P, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 26.Chen RH, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci U S A. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen RH, Juo PC, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- 28.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 29.De Cesare D, Jacquot S, Hanauer A, Sassone-Corsi P. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Natl Acad Sci U S A. 1998;95:12202–12207. doi: 10.1073/pnas.95.21.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merienne K, Pannetier S, Harel-Bellan A, Sassone-Corsi P. Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities. Mol Cell Biol. 2001;21:7089–7096. doi: 10.1128/MCB.21.20.7089-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima T, Fukamizu A, Takahashi J, et al. The signal-dependent coactivator CBP is a nuclear target for pp90RSK. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 32.Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 33.Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Matsuda K, Bialek P, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 35.Yang TT, Xiong Q, Graef IA, Crabtree GR, Chow CW. Recruitment of the extracellular signal-regulated kinase/ribosomal S6 kinase signaling pathway to the NFATc4 transcription activation complex. Mol Cell Biol. 2005;25:907–920. doi: 10.1128/MCB.25.3.907-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoda L, Lin X, Greene WC. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IkappaBalpha and stimulates its degradation in vitro. J Biol Chem. 1997;272:21281–21288. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- 37.Schouten GJ, Vertegaal AC, Whiteside ST, et al. IkappaB alpha is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 40.Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene. 1997;15:373–383. doi: 10.1038/sj.onc.1201214. [DOI] [PubMed] [Google Scholar]
- 41.Trivier E, De Cesare D, Jacquot S, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 42.Coffin R, Phillips JL, Staples WI, Spector S. Treatment of lead encephalopathy in children. J Pediatr. 1966;69:198–206. doi: 10.1016/s0022-3476(66)80320-7. [DOI] [PubMed] [Google Scholar]
- 43.Lowry B, Miller JR, Fraser FC. A new dominant gene mental retardation syndrome: association with small stature, tapering fingers, characteristic facies, and possible hydrocephalus. Am J Dis Child. 1971;121:496–500. [PubMed] [Google Scholar]
- 44.Yodoi J, Teshigawara K, Nikaido T, et al. TCGF (IL 2)-receptor inducing factor(s), I: regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985;134:1623–1630. [PubMed] [Google Scholar]
- 45.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 46.Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B: reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–10744. [PubMed] [Google Scholar]
- 48.Lin JX, Leonard WJ. The immediate-early gene product Egr-1 regulates the human interleukin-2 receptor beta-chain promoter through noncanonical Egr and Sp1 binding sites. Mol Cell Biol. 1997;17:3714–3722. doi: 10.1128/mcb.17.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrell JEJ, Martin GS. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989;264:20723–20729. [PubMed] [Google Scholar]
- 50.John S, Robbins CM, Leonard WJ. An IL-2 response element in the human IL-2 receptor alpha chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO J. 1996;15:5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 51.Xue HH, Fink DWJ, Zhang X, Qin J, Turck CW, Leonard WJ. Serine phosphorylation of Stat5 proteins in lymphocytes stimulated with IL-2. Int Immunol. 2002;14:1263–1271. doi: 10.1093/intimm/dxf101. [DOI] [PubMed] [Google Scholar]
- 52.Dufresne SD, Bjorbaek C, El-Haschimi K, et al. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjorbaek C, Vik TA, Echwald SM, et al. Cloning of a human insulin-stimulated protein kinase (ISPK-1) gene and analysis of coding regions and mRNA levels of the ISPK-1 and the protein phosphatase-1 genes in muscle from NIDDM patients. Diabetes. 1995;44:90–97. doi: 10.2337/diab.44.1.90. [DOI] [PubMed] [Google Scholar]
- 54.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman Z, Min B, Meier-Schellersheim M, Paul WE. Concomitant regulation of T-cell activation and homeostasis. Nat Rev Immunol. 2004;4:387–395. doi: 10.1038/nri1355. [DOI] [PubMed] [Google Scholar]
- 56.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Pages G, Guerin S, Grall D, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 58.Yao Y, Li W, Wu J, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100:12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatano N, Mori Y, Oh-hora M, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 60.Lawlor MA, Mora A, Ashby PR, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat Immunol. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- 62.Crawley JB, Willcocks J, Foxwell BM. Interleukin-7 induces T cell proliferation in the absence of Erk/MAP kinase activity. Eur J Immunol. 1996;26:2717–2723. doi: 10.1002/eji.1830261125. [DOI] [PubMed] [Google Scholar]
- 63.Rajnavolgyi E, Benbernou N, Rethi B, et al. IL-7 withdrawal induces a stress pathway activating p38 and Jun N-terminal kinases. Cell Signal. 2002;14:761–769. doi: 10.1016/s0898-6568(02)00026-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.