Abstract
Upon viral stimulation, the natural interferon (IFN)-α/β–producing cells (IPCs; also known as pre-dendritic cells (DCs 2) in human blood and peripheral lymphoid tissues rapidly produce huge amounts of IFN-α/β. After performing this innate antiviral immune response, IPCs can differentiate into DCs and strongly stimulate T cell–mediated adaptive immune responses. Using four-color immunofluorescence flow cytometry, we have mapped the developmental pathway of pre-DC2/IPCs from CD34+ hematopoietic stem cells in human fetal liver, bone marrow, and cord blood. At least four developmental stages were identified, including CD34++CD45RA− early progenitor cells, CD34++CD45RA+ late progenitor cells, CD34+CD45RA++CD4+interleukin (IL)-3Rα++ pro-DC2, and CD34−CD45RA++ CD4+IL-3Rα++ pre-DC2/IPCs. Pro-DC2s have already acquired the capacity to produce large amounts of IFN-α/β upon viral stimulation and to differentiate into DCs in culture with IL-3 and CD40 ligand. CD34++CD45RA− early progenitor cells did not have the capacity to produce large amounts of IFN-α/β in response to viral stimulation; however, they can be induced to undergo proliferation and differentiation into IPCs/pre-DC2 in culture with FLT3 ligand.
Keywords: dendritic cells, interferon α/β–producing cells, stem cells, FLT3 ligand, interferon-producing cell
Introduction
Dendritic cells (DCs) represent heterogeneous populations of hematopoietic-derived cells that display potent ability to induce primary T cell activation, polarization, and in certain circumstances, tolerance 1 2 3 4. The distinct capacity of DCs to induce immunity versus tolerance or Th1 versus Th2 responses depends on their maturation stage 2 5, signals that induce or inhibit DC maturation 2 5 6, as well as the lineage origin of DCs 7 8 9 10 11 12. A lymphoid DC developmental pathway was suggested by the finding that mouse thymic lymphoid precursors can give rise to both T cells and CD8+CD11b− DCs 13 14. In addition, a well-established myeloid DC pathway giving rise to CD8−CD11b+ DCs has been defined 15 16 17. Recent studies suggest that CD8+CD11b− lymphoid DCs and CD8−CD11b+ myeloid DCs may have different functions in T cell activation/tolerance or Th1/Th2 differentiation 7 9 18 19 20 21 22 23 24.
In humans, two distinct populations of DC precursors exist in the blood. Monocytes (pre-DC1), which belong to the myeloid lineage, differentiate into immature DC1 after 5 d of culture in GM-CSF and IL-4 25 26. Upon CD40 ligand activation, immature myeloid DC1 undergo maturation and produce large amounts of IL-12 27 28. The mature DC1 induced by CD40 ligand are able to polarize naive CD4+ T cells into Th1 cells 29. The second type of DC precursor cells, pre-DC2 (previously known as plasmacytoid T/monocytes) are characterized by a unique surface phenotype (CD4+IL-3Rα++CD45RA+HLA-DR+ lineage marker-negative and CD11c−), and at the ultrastructural level resemble Ig-secreting plasma cells 30 31. Several lines of evidence suggest that pre-DC2s are of lymphoid origin: (a) pre-DC2 lack expression of the myeloid antigens CD11c, CD13, CD33, and mannose receptor 30 32, (b) pre-DC2 isolated from the thymus express the lymphoid markers CD2, CD5, and CD7 32, (c) pre-DC2 have little phagocytic activity 30, (d) pre-DC2 do not differentiate into macrophages after culture with GM-CSF and M-CSF 30, (e) pre-DC2 express pre–TCR-α transcripts 32 33, and (f) development of pre-DC2, T and B cells, but not myeloid DCs is blocked by ectopic expression of inhibitor of DNA binding (Id)2 or Id3, as shown by Spits et al., in this issue 34. Pre-DC2 differentiate into immature DC2 when cultured with monocyte conditional medium 35, IL-3 29 30 36, IFN-α/β, and TNF-α, or viruses like herpes simplex virus or influenza virus 37. Upon CD40 ligand activation, immature DC2 undergo maturation 30, but produce only low levels of IL-12 29. Mature DC2 are able to polarize naive CD4+ T cells into a Th2 phenotype 10 29. Recent studies showed that the pre-DC2 are the elusive natural IFN-producing cells (IPCs), capable of producing high amounts of IFN-α/β upon viral stimulation 38 39. Taken together, pre-DC2/IPCs represent a unique hematopoietic lineage, capable of performing crucial functions both in innate and in adapted immunity.
The pathway underlying the development of pre-DC2/IPCs from CD34+ hematopoietic stem cells has not been elucidated. Caux et al. 40 showed that cord blood CD34+ hematopoietic progenitor cells cultured in GM-CSF, stem cell factor (SCF), and TNF-α differentiate along two DC pathways: (a) the Langerhans cell (LC) pathway, in which intermediate CD14−CD1a+ DC precursors differentiated into LCs characterized by the expression of CD1a, Birbeck granules, the Lag antigen, and E cadherin; and (b) the dermal DC pathway, in which intermediate CD14+CD1a− DC precursors differentiate into dermal DCs characterized by the expression of CD1a, CD9, CD68, CD2, and factor XIIIa 41. Recently, a common human lymphoid progenitor in the bone marrow was described that expresses both CD45RA and CD10 42. These cells develop into T, B, NK cells, and DCs, but not into erythroid, megakaryocytic, and myeloid cells. In these experiments, exclusively CD1a+ LCs were generated with a cocktail of nine cytokines (IL-1, IL-3, IL-6, IL-7, SCF, GM-CSF, TNF, erythropoietin, and FLT3 ligand). Of these cytokines, the hematopoietic growth factor FLT3 ligand has been shown to play an important role in the proliferation, survival, and differentiation of early murine and human hematopoietic precursor cells 43 44. Interestingly, volunteer donors injected with FLT3 ligand had a 13-fold increase in pre-DC2 number and a 48-fold increase in CD11c+ myeloid DC number in the blood stream 45. Consistent with this finding, injection of mice with human FLT3 ligand led to dramatically increased numbers of both myeloid and lymphoid DCs not only in the peripheral blood, but also in the bone marrow, thymus, and secondary lymphoid tissues 7 9 46 47. Two recent reports revealed a five- to sixfold increase in pre-DC2/IPC numbers in the blood of G-CSF–treated donors 10 45. However, it is not clear from these studies whether FLT3 ligand and G-CSF enhance the differentiation of pre-DC2 from hematopoietic progenitor cells or promote the migration of pre-DC2 from bone marrow into blood.
The aims of this study are: (a) to trace the developmental pathway of pre-DC2/IPCs from CD34++ hematopoietic progenitor cells, and (b) to identify the stimuli that can induce CD34++ hematopoietic progenitor cells to differentiate into pre-DC2/IPCs.
In this paper, we describe the identification of CD34-expressing precursors of pre-DC2/IPCs from human fetal tissues and cord blood. In addition, we report on the generation of pre-DC2/IPCs from early hematopoietic stem cells in in vitro cultures.
Materials and Methods
Flow Cytometric Analysis and Cell Sorting of Human Cord Blood and Fetal Tissues.
Fetal tissue (16–22 wk of gestation) and cord blood were obtained from Advanced Bioscience Resources Inc. Mononuclear cells (MNCs) were isolated from these samples by Ficoll density gradient centrifugation (1.077 g/ml Lymphoprep; Amersham Pharmacia Biotech). MNCs were washed three times in PBS (BioWhittaker), and resuspended in PBS containing 2% (vol/vol) human serum (HS; Gemini Bioproducts) and 2 mM EDTA (PBS/HS/EDTA). Magnetic bead depletion was performed to remove lineage-positive cells. In brief, MNCs were incubated with a mixture of antibodies against CD3 (OKT-3 ascites), CD8 (OKT-8 ascites), CD14 (RPA-M1 ascites), CD16 (3G8; Immunotech), CD19 (4G7 ascites), CD56 (My31 ascites), CD66B (80H3; all from Immunotech), and glycophorin A (10F7MN ascites). After two washes, the cells were incubated with goat anti–mouse IgG coupled to magnetic beads (Dynabeads® M-450, goat anti–mouse IgG; Dynal) and isolated according to the manufacturer's instructions. The enriched cells were stained either to perform a four-color flow cytometric analysis or to purify different subsets by cell sorting (FACS®; Becton Dickinson). Cells were incubated with a cocktail of the following FITC-conjugated antibodies: CD3 (Leu-4), CD14 (Leu-M3), CD15 (Leu-M1), CD16 (Leu-11a), CD20 (Leu-16), CD57 (Leu-7; all from Becton Dickinson), and CD11c (3.9; Caltag Laboratories). To analyze expression of different antigens on the lineage FITC-negative cells, the cells were stained with CD34-allophycocyanin (APC; HPCA-2; Becton Dickinson) and CD45RA-Tricolor (MEM 56; Caltag), and in addition, different PE-conjugated antibodies: anti–HLA-DR (Becton Dickinson), CD4 (Leu-3a; Becton Dickinson), and IL-3Rα (9F5l; BD PharMingen). For cell sorting, the enriched cells were stained with CD34-APC (HPCA-2), CD45RA-PE (Leu-18), and CD4-biotin (Leu-3a), all from Becton Dickinson. Expression of CD4 was revealed after a second step staining using the streptavidin-alexa594 (Molecular Probes) or avidin–Texas red (BD PharMingen) conjugate.
DC2 Culture Conditions.
For generation of DC2, sorted cells were cultured for 5 d in the presence of IL-3 (10 ng/ml; R&D Systems) and CD40 ligand–transfected L cells (10,000/well, irradiated at 7,000 rads) in 25 μl Yssel's medium 48 containing 2% HS in microwell plates (Robbins Scientific Corporation).
Proliferation Assay.
Sorted cells (15,000/well) were cultured in duplicate for the indicated durations in the presence of IL-3 (10 ng/ml; R&D Systems), GM-CSF (800 U/ml; a gift from Schering-Plough, Kenilworth, NJ), FLT3 ligand (100 ng/ml; a gift from S. Menon, DNAX Research Insitute of Molecular and Cellular Biology), and SCF (10 ng/ml; R&D Systems) in 200 μl Yssel's medium 48 containing 2% HS in 96-well round-bottomed culture plates (Falcon®; Becton Dickinson). [3H]Thymidine (1 μCi/well; Amersham Pharmacia Biotech) was added during the last 8 h of the culture. Proliferation data were calculated in counts per minute.
IFN-α Production.
Cells (106/ml) were cultured in 25 μl Yssel's medium 48 containing 2% HS in microwell plates (Robbins Scientific). HSV-1, KOS strain, attenuated by γ irradiation (a gift from R. Chase, Schering-Plough, Kenilworth, NJ) was added at 10 PFU/cell. After 24 h, supernatants were collected and frozen at −20°C before analysis by an IFN-α–specific sandwich ELISA (Biosource International). Appropriate dilutions of the supernatants were made if necessary.
Pre-DC2/IPC Generation from Stem Cells.
Sorted cells (25,000–50,000/well) were cultured in 200 μl Yssel's medium containing 2% HS in 96-well round-bottomed culture plates. Cytokines were added at the following concentrations: FLT3 ligand (100 ng/ml; provided by S. Menon, DNAX Research Institute of Molecular and Cellular Biology), GM-CSF (800 U/ml; a gift from Schering-Plough), SCF (10 ng/ml; R&D Systems), IL-3 (10 ng/ml; R&D Systems), IL-7 (10 ng/ml; R&D Systems), and G-CSF (5 ng/ml; R&D Systems). Cell cultures were refreshed every 5 d by demi-depletion and splitted if necessary.
Results
Identification of Pro-DC2, the CD34-expressing Immediate Precursors of Pre-DC2/IPCs.
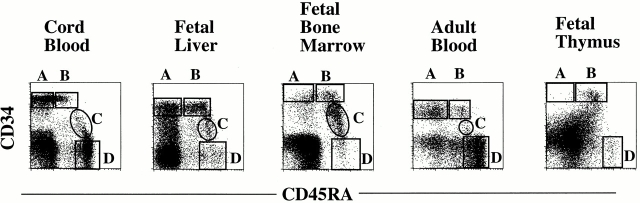
Pre-DC2/IPCs have been identified in tonsil, adult peripheral blood 29 30, and postnatal thymus 32. To define the developmental pathway of pre-DC2/IPCs from CD34++ hematopoietic progenitors, lineage-positive cells (including T, B, and NK cells, and monocytes, granulocytes, and erythrocytes) from fetal liver, bone marrow, cord blood, and adult blood were depleted using magnetic beads. The remaining lineage-negative cells were analyzed by four-color flow cytometry analysis after staining with: (a) FITC-conjugated antibodies against lineage markers (CD3, CD11c, CD14, CD15, CD16, CD20, and CD57), in order to exclude the remaining lineage-positive cells; (b) anti-CD34 antibody conjugated to APC, to follow the populations of hematopoietic progenitor cells; (c) anti-CD45RA antibody conjugated to Tricolor as a positive marker to identify pre-DC2/IPCs and to distinguish CD34++CD45RA− early progenitors from CD34++CD45RA+ late progenitors; and (d) various PE-conjugated antibodies known to detect antigens expressed on pre-DC2/IPCs, such as CD4 and IL-3Rα. After gating on FITC-negative cells, four populations of cells (A, B, C, and D) were identified (Fig. 1). CD34++ CD45RA− cells (population A) are enriched for early multipotent progenitor cells 49, whereas CD34++CD45RA+ cells (population B) are enriched for myeloid/lymphoid progenitors, which have lost the potential to develop into the erythroid lineage 42. These two populations of CD34++ progenitor cells expressed lower levels of CD4 and IL-3Rα than expressed by pre-DC2/IPCs derived from adult peripheral blood (Fig. 2) or tonsils (data not shown). A substantial proportion of CD34+CD45RA+ cells in population C and CD34−CD45RA+ cells in population D expressed a moderate level of CD4 and a high level of IL-3Rα, similar to the expression level detected on pre-DC2/IPCs from adult peripheral blood (Fig. 2) or tonsils (data not shown). The CD4− and IL-3Rα− fraction of cells in populations C and D expressed NKRP1A (data not shown), and most likely represent cells of the NK lineage 50. Thus, according to these phenotypical analyses, the CD34−CD45RA+CD4+IL-3Rα++ cells in population D may represent pre-DC2/IPCs. Furthermore, because of the low CD34 expression, the CD34+CD45RA+CD4+ IL-3Rα++ cells in population C may represent the immediate progenitors of pre-DC2/IPCs. Accordingly, the CD34+CD45RA+CD4+IL-3Rα++ cells in population C will be referred to as pro-DC2 (for progenitor of pre-DC2). Both pre-DC2 in fraction D and pro-DC2 in fraction C display a similar size profile (intermediate size between lymphocytes and monocytes) revealed by forward scatter (FSC)/side scatter (SSC) (Fig. 2) and a plasmacytoid morphology by Giemsa staining (not shown). The presence of pro-DC2 could be detected in cord blood, fetal bone marrow, fetal liver, and at very low numbers in peripheral blood, but not in fetal thymus (Fig. 1). All tissues analyzed contained pre-DC2 in population D (Fig. 1 and data not shown).
Figure 1.
Expression of CD34 and CD45RA on lineage-negative cells reveals four different subpopulations in human fetal liver, fetal bone marrow, fetal thymus, cord blood, and adult peripheral blood samples. Lineage-positive cells (including T, B, and NK cells, and monocytes, granulocytes, and erythrocytes) were depleted using magnetic beads. The enriched cells were analyzed by four-color flow cytometric analysis after staining with an FITC-conjugated cocktail of antibodies against lineage markers (including CD3, CD11c, CD14, CD15, CD16, CD20, and CD57), anti-CD34–APC, anti-CD45RA–Tricolor, and the PE-conjugated antibodies anti-CD4 and anti–IL-3Rα. After electronic gating on FITC-negative cells, four populations of cells (A, B, C, and D) were identified: population A, CD34++CD45RA−; population B, CD34++CD45RA+; population C, CD34+CD45RA+; and population D, CD34−CD45RA+.
Figure 2.
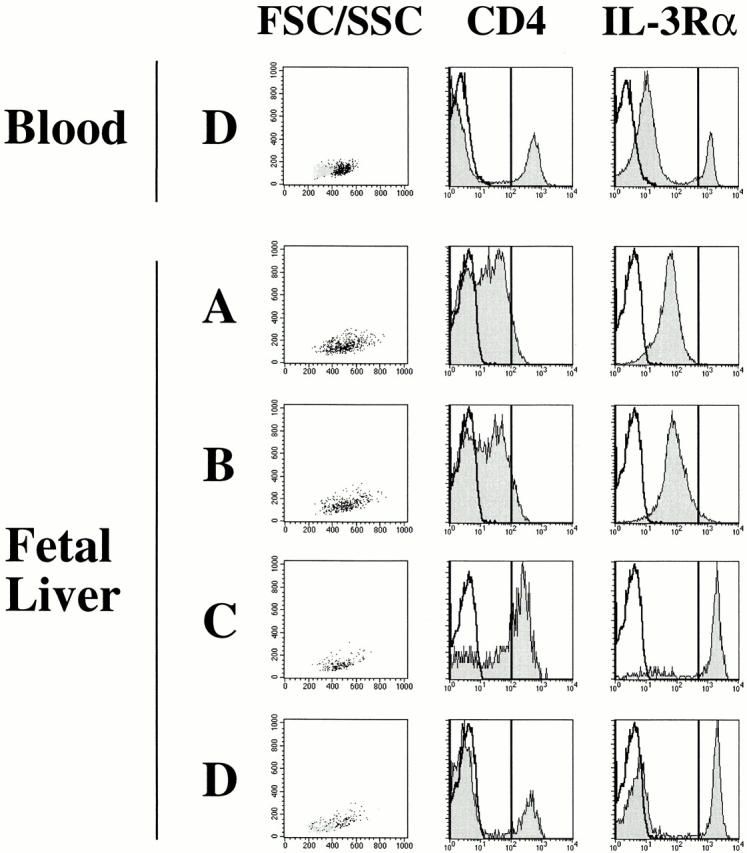
Phenotype of populations A–D. Cells in populations A–D of human fetal liver shown in Fig. 1 were electronically gated and analyzed for the FSC/SSC size profiles and the expression of CD4 and IL-3Rα. The black dots on the FSC/SSC profile represent the IL-3Rα++ fraction. The gray dots represent IL-3Rαlow lymphocyte fraction. Open histograms represent expression after staining with an isotype control antibody, and the shaded histograms represent expression of CD4 or IL-3Rα on populations A–D. The phenotype of populations A–D from human adult blood, bone marrow, and cord blood were similar to that of fetal liver.
Pro-DC2 and Pre-DC2 Produce Large Amounts of IFN-α after Viral Stimulation.
A key function feature of pre-DC2/IPCs is the rapid production of huge amounts of IFN-α/β in response to viral stimulation. 37 38. To determine if CD34+ pro-DC2s in population C have acquired this function, pro-DC2 were stimulated with irradiated HSV-1 (10 PFU/cell) for 24 h, in parallel with pre-DC2 and CD34++ progenitor cell populations A and B. CD34++CD45RA− (population A) isolated from either cord blood or fetal liver only produced low levels of IFN-α (population A, <17–111 pg/ml, n = 11; Table ). Similar low IFN-α levels were found for virally stimulated cord blood CD34++CD45RA+ (population B) cells (population B, 17–48 pg/ml, n = 4). However, the range of IFN-α production from fetal liver–derived CD34++CD45RA+ cells was more variable (population B, <17–4,257 pg/ml, n = 11). Pro-DC2 in population C and pre-DC2 in population D isolated from either cord blood or fetal liver produced huge amounts of IFN-α (pro-DC2, 172–90,464 pg/ml, n = 10; pre-DC2, 1,024–15,830 pg/ml, n = 8; Table ). No IFN-α was detectable from the CD4− cells in population D after purification and viral stimulation, suggesting that these cells are different from the pre-DC2 (data not shown).
Table 1.
IFN-α–producing Capacity of Purified Pro-DC2, Pre-DC2, and CD34++ Progenitor Subsets from Cord Blood and Fetal Liver
| CD34++CD45RA− | CD34++CD45RA+ | Pro-DC2 | Pre-DC2 | |
|---|---|---|---|---|
| pg/ml | pg/ml | pg/ml | pg/ml | |
| Cord blood | ||||
| 1 | 32 (±10) | 36 (±5) | 4,295 (±69) | 3,254 (±55) |
| 2 | 41 (±3) | 48 (±3) | 172 (±6) | 1,024 (±7) |
| 3 | 43 (±20) | 43 (±5) | 6,938 (±67) | 13,020 (±322) |
| 4 | − | − | 1,284 (±43) | 3,484 (±333) |
| 5 | − | − | 10,835 (±780) | 9,241 (±712) |
| 6 | 20 (±1) | 17 (±5) | − | − |
| Fetal liver | ||||
| 1 | <17 | 1,270 (±150) | 90,464 (±1391) | − |
| 2 | 76 (±6) | 1,241 (±22) | 39,577 (±678) | 15,830 (±1,121) |
| 3 | 111 (±9) | 41 (±37) | 20,308 (±714) | 900 (±48) |
| 4 | 81 (±32) | 1,496 (±41) | 20,308 (±714) | 900 (±48) |
| 5 | 32 (±3) | 4,257 (±95) | 90,010 (±856) | − |
| 6 | 23 (±3) | 46 (±9) | − | − |
| 7 | <17 | <17 | − | − |
CD34++ progenitor subsets, pro-DC2, and pre-DC2 were purified by flow cytometric cell sorting and stimulated with HSV-1 for 24 h. The amount of IFN (pg/ml) produced in the supernatants was measured by ELISA. The detection limit of the ELISA was 17 pg/ml. Numbers in parentheses represent standard deviations. −, not tested.
These data indicate that pre-DC2s from fetal liver and cord blood are similar to pre-DC2/IPCs isolated from adult blood and tonsils regarding their surface phenotype and function in antiviral innate immunity. Furthermore, although pro-DC2 express the CD34 antigen, these cells already acquired the functional capacity to produce a large amount of IFN-α in response to viral stimulation, and therefore pro-DC2 may represent the earliest IPCs during hematopoiesis. In addition, the ability to rapidly produce vast amounts of type 1 IFN is acquired during hematopoietic development, as CD34++ early hematopoietic progenitors only produced a low amount of IFN-α in response to viral stimulation.
Pro-DC2s Differentiate into Mature DCs upon IL-3 and CD40 Ligand Stimulation.
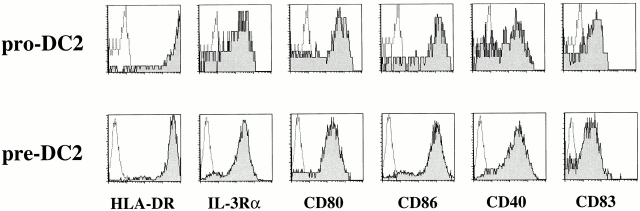
Adult blood and tonsil-derived pre-DC2 depend on IL-3 for their survival 30. Culture of pre-DC2 with IL-3– and CD40 ligand–transfected L cells results in their differentiation into mature DCs (DC2 [29, 30]). To determine whether pro-DC2 have the potential to differentiate into mature DCs, the cells were cultured for 5 d with IL-3 and CD40 ligand in parallel with pre-DC2s. After 5 d of culture, both pro-DC2 and pre-DC2 acquired mature DC morphology (data not shown). Moreover, flow cytometry analysis revealed that both cultured pro-DC2 and pre-DC2 expressed mature DC markers, such as high HLA-DR, CD80, CD86, CD40, and CD83 (Fig. 3). These mature DC2 strongly induced the proliferation of allogeneic naive CD4+ T cells (data not shown). These results suggest that pro-DC2 and pre-DC2 have an equal potential to develop into mature DCs.
Figure 3.
Flow cytometric analysis of pro-DC2 and pre-DC2 after 5 d of culture with IL-3 and CD40 ligand L cells. Harvested cells were stained using PE-conjugated antibodies against HLA-DR, IL-3Rα, CD80, CD86, CD40, and CD83. Open histograms represent expression after staining with an isotype control antibody. The shaded histograms represent expression of the indicated antigen.
Pro-DC2 Have a Limited Proliferative Capacity.
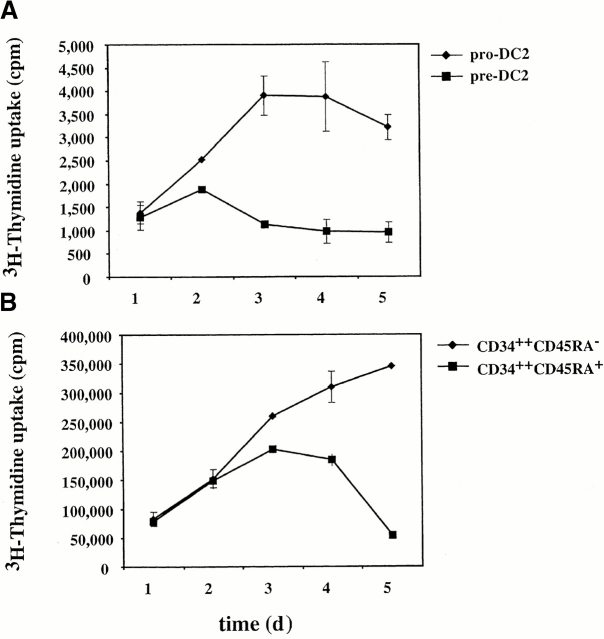
The proliferation potential of pro-DC2 in response to hematopoietic cytokines was analyzed, in parallel with that of pre-DC2, CD34++CD45RA− (population A), and CD34++CD45RA+ (population B) from fetal liver. Cells of each population were cultured for a total of 5 d in a cytokine cocktail consisting of GM-CSF, IL-3, SCF, and FLT3 ligand. [3H]Thymidine was added to the cultures 8 h before incorporation was analyzed. Pro-DC2 proliferated moderately better than pre-DC2 (Fig. 4 A), but 50–100 times less than CD34++CD45RA− (population A) and CD34+CD45RA+ (population B; Fig. 4 B). In addition, both pro-DC2 and pre-DC2 failed to increase cell numbers in these cultures (data not shown).
Figure 4.
Proliferative potential of pro-DC2, pre-DC2, and CD34++ progenitor cells. Freshly sorted CD34++CD45RA−, CD34++ CD45RA+, pro-DC2, and pre-DC2 were cultured for a total of 5 d with GM-CSF, IL-3, SCF, and FLT3 ligand. [3H]Thymidine was added to the cultures 8 h before incorporation was analyzed. (A) [3H]Thymidine uptake of pro-DC2 and pre-DC2. (B) [3H]Thymidine uptake of CD34++CD45RA− and CD34++CD45RA+ cells. Uptake is calculated as counts per minute.
These data suggest that although pro-DC2 still express the CD34 antigen and proliferate moderately better in response to cytokines than pre-DC2, the pro-DC2 may have lost the expansion potential of hematopoietic progenitor cells.
FLT3 Ligand Induces CD34++CD45RA− Early Hematopoietic Progenitor Cells to Differentiate into IFN-α–producing Cells.
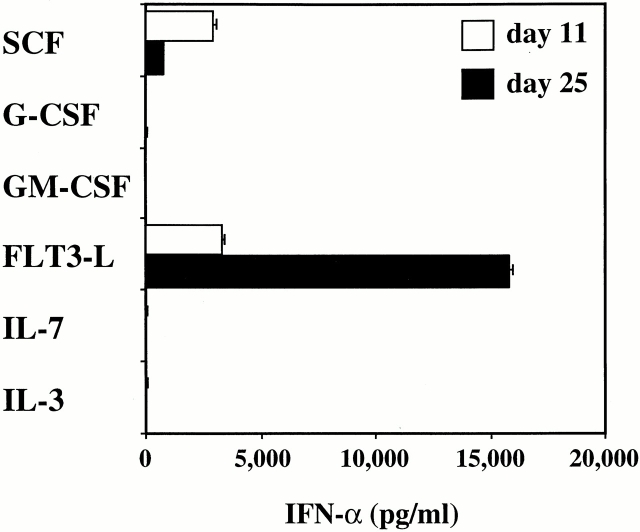
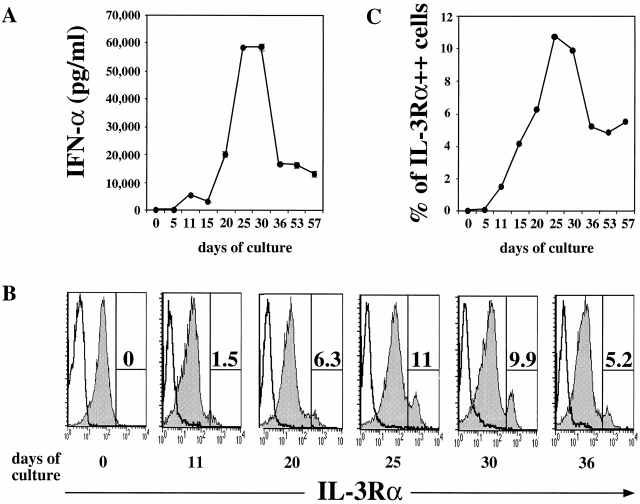
CD34++CD45RA− cells did not produce IFN-α after viral stimulation, but were found to have the best clonal expansion potential (Fig. 4 B). We investigated whether CD34++CD45RA− early progenitor cells can be induced to differentiate into pre-DC2/IPCs in vitro with one of the following cytokines: IL-3, IL-7, SCF, FLT3 ligand, GM-CSF, or G-CSF. After 11 and 25 d of culture, 20,000 cells were stimulated with HSV-1 for 24 h and the amount of IFN-α secreted in the supernatants was analyzed by ELISA. At day 11, cells cultured with FLT3 ligand or SCF produced moderate levels of IFN-α (FLT3 ligand, 3,323 pg/ml; SCF, 2,910 pg/ml; Fig. 5) after viral stimulation. Cells cultured in any of the other cytokines produced low to undetectable levels of IFN-α (<17–24 pg/ml). The cells expanded in number up to 35-fold with IL-3 and 24-fold with GM-CSF, whereas the cell number expansion with the other cytokines was <5-fold (data not shown). Strikingly, after 25 d of culture with FLT3 ligand, CD34++CD45RA− cells produced 15,794 pg/ml of IFN-α after viral stimulation (Fig. 5). Interestingly, the capacity of cells to produce IFN-α after culture in SCF had decreased to 767 pg/ml (Fig. 5). Cellular expansion in this experiment was 24-fold in FLT3 ligand and 6-fold in SCF (data not shown). None of the other cytokines had induced the CD34+CD45RA− cells to produce IFN-α upon viral challenge (Fig. 5).
Figure 5.
Generation of IFN-α–producing cells from CD34++ CD45RA− cells with FLT3 ligand or SCF. Sorted CD34++CD45RA− cells were cultured with one of the following cytokines: IL-3, IL-7, SCF, FLT3 ligand (FLT3-L), GM-CSF, or G-CSF. After 11 and 25 d of culture, equal numbers of cells were stimulated with HSV-1 for 24 h and IFN-α (pg/ml) in the supernatants analyzed by ELISA.
To analyze the detailed kinetics of the capacity of FLT3 ligand–cultured cells to produce IFN-α in response to HSV-1 stimulation, CD34++CD45RA− fetal liver cells were cultured in FLT3 ligand and harvested every 5 d. After 10–15 d of culture, CD34++CD45RA− early progenitor cells acquired the capacity to produce significant amounts of IFN-α (3,000–5,000 pg/ml; Fig. 6 A). This capacity was further increased at day 21 (20,000 pg/ml), peaked at days 25 to 30 (>58,000 pg/ml), and started to decrease after 50 d of culture. The cell number of CD34++CD45RA− cells cultured in FLT3 ligand in this experiment increased six- to sevenfold after 4 wk of culture (data not shown).
Figure 6.
Kinetic analysis on the ability of FLT3 ligand to generate pre-DC2/IPCs from CD34++CD45RA− cells. Sorted CD34++CD45RA− cells were cultured for a period up till 57 d with FLT3 ligand. Every 5 d, cells were harvested and analyzed. (A) Equal numbers of cells were stimulated with HSV-1 (10 PFU/cell) for 24 h. The amount of IFN-α (pg/ml) produced in the supernatants was measured by ELISA. (B) Phenotypical analysis by flow cytometry after staining with CD11c-FITC, IL-3Rα–PE, and HLA-DR–CyChrome. Shaded histograms show the IL-3Rα expression after electronic gating on HLA-DR+CD11c− cells. Open histograms represent staining with an isotype control antibody. (C) Graphical representation of the percentages of IL-3Rα++ cells gated in B.
FLT3 Ligand Induces CD34++CD45RA− Early Progenitor Cells to Differentiate into CD4+HLA-DR+IL-3Ra++ CD45RA+CD11c− Pre-DC2.
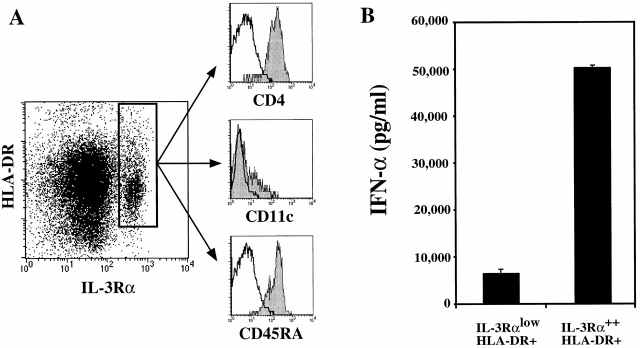
Next, we used four-color flow cytometry to determine whether the production of the vast amounts of IFN-α by FLT3 ligand–cultured cells was due to the generation of pre-DC2/IPCs. In normal human adult blood, the frequency of CD4+HLA-DR+IL-3Rα++CD45RA+CD11c− pre-DC2s is between 0.3 and 0.8% of total peripheral blood MNCs 30. The percentage of CD4+HLA-DR+IL-3Rα++CD45RA+CD11c− cells in freshly isolated CD34++CD45RA− early progenitor cells from fetal liver or in 5 d FLT3 ligand–cultured cells was <0.05% (Fig. 1 B and Fig. 6B and Fig. C). A progressive increase (from 1.5 to 6%) in the percentage of HLA-DR+IL-3Rα++ cells was observed from days 11 to 20 of culture (Fig. 6B and Fig. C). Between days 25 and 30, up to 10% of the cultured cells were HLA-DR+IL-3Rα++ cells (Fig. 6B and Fig. C). Detailed flow cytometric analysis of CD34++CD45RA− cells at day 30 of FLT3 ligand culture revealed that the HLA-DR+IL-3Rα++ cells expressed CD4, CD45RA, and low levels of CD11c (Fig. 7 A), a typical phenotype of pre-DC2/IPCs. Most interestingly, a clear correlation between the ability of cultured cells to produce large amounts of IFN-α in response to viral stimulation and the appearance of CD4+HLA-DR+IL-3Rα++ CD45RA+CD11c− cells in culture with FLT3 ligand was evidenced (Fig. 6A and Fig. C).
Figure 7.
Phenotype and IFN-α production of IL-3Rα++HLA-DR+ cells. (A) CD34++CD45RA− cells cultured for 30 d in FLT3 ligand were analyzed by flow cytometric analysis after staining with either CD11c-FITC, IL-3Rα–PE, and HLA-DR–CyChrome or HLA-DR–FITC, IL-3Rα–PE, and CD4-CyChrome or CD45RA-CyChrome. Shaded histograms show the CD4, CD11c, and CD45RA expression after electronic gating on IL-3Rα++HLA-DR+ cells. Open histograms represent staining with an isotype control antibody. (B) CD34++CD45RA− cells cultured for 30 d in FLT3 ligand were stained with HLA-DR–FITC and IL-3Rα–PE antibodies. IL-3Rα++HLA-DR+ and IL-3RαlowHLA-DR+ subsets were sorted and stimulated with HSV-1 for 24 h. IFN-α (pg/ml) was measured in the supernatants by ELISA.
To directly prove that the IL-3Rα++HLA-DR+ cells generated from CD34++CD45RA− early progenitor cells with FLT3 ligand were pre-DC2/IPCs, FLT3 ligand–cultured cells were separated into IL-3Rα++HLA-DR+ and IL-3RαlowHLA-DR+ populations by cell sorting. Stimulation of sorted cells with HSV-1 for 24 h revealed that the IL-3Rα++HLA-DR+ produced the larger amount of IFN-α (50,238 pg/ml), which was seven times more than produced by the IL-3RαlowHLA-DR+ (7,125 pg/ml; Fig. 7). Notably, the amount of IFN-α produced by these in vitro generated cells is comparable to that produced by freshly isolated IPCs from fetal liver (Table ).
These data indicate that pre-DC2/IPCs were generated from CD34++CD45RA− early hematopoietic progenitor cells in culture with FLT3 ligand.
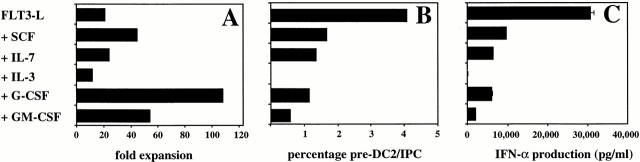
Other Hematopoietic Cytokines Do Not Promote the FLT3 Ligand–induced Generation of Pre-DC2/IPCs from CD34++CD45RA− Early Progenitor Cells.
To determine if other cytokines could enhance the activity of FLT3 ligand to induce the generation of pre-DC2/IPCs, CD34++CD45RA− early hematopoietic progenitor cells were cultured with FLT3 ligand with each of the following cytokines: GM-CSF, G-CSF, IL-3, IL-7, and SCF. Although G-CSF, and to a lesser extend GM-CSF and SCF, enhanced the cellular expansion of CD34++CD45RA− cells after 25 d of culture (Fig. 8 A), none of the cytokines tested promoted the generation of pre-DC2/IPCs induced by FLT3 ligand as determined by the capacity of IFN-α/β production or by the pre-DC2/IPC phenotype (CD4+ HLA-DR+IL-3Rα++CD45RA+CD11c−; Fig. 8B and Fig. C). Moreover, the tested cytokines, in particular IL-3, inhibited the generation of pre-DC2/IPCs induced by FLT3 ligand.
Figure 8.
Proliferation, IFN-α–producing capacity, and phenotype of CD34++CD45RA− cells cultured with FLT3 ligand (FLT3-L) together with other cytokines. CD34++CD45RA− cells were cultured for 28 d and analyzed. (A) The cell number expansion of the different cultures was calculated after counting the live cells by trypan blue exclusion. (B) Percentages of IL-3Rα++HLA-DR+CD11c− cells. Cultured cells were analyzed by flow cytometric analysis after staining with a combination of antibodies including CD11c-FITC, IL-3Rα–PE, and HLA-DR–CyChrome. (C) 20,000 cells of each culture condition were stimulated with HSV-1 for 24 h and the amount of IFN-α measured by ELISA.
Discussion
The evolutionary pressure to fight numerous types of microorganisms has endowed us with the development of not only the sophisticated adaptive immune system, including T and B lymphocytes, but also the divine innate immune system. Whereas neutrophils and macrophages are dedicated to eat and kill various bacterias, the eosinophils, basophils, and mast cells have evolved to kill parasites. The robust production of IFN-α/β of pre-DC2/IPCs in response to viral stimulation suggests that pre-DC2/IPCs represent a new member cell type of the hematopoietic family and an effector cell type of the innate immune system against antiviral infection.
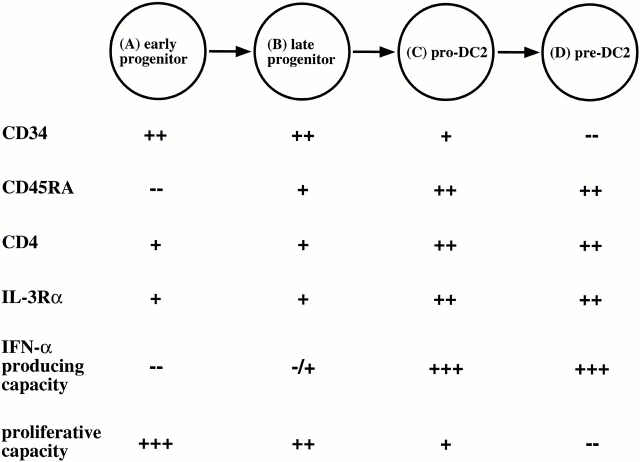
Hematopoietic stem cells develop into committed effector cells depending on their microenvironment, which provides them with the necessary stimuli. This development is a multistep process that requires the orchestrated availability of cytokines and chemokines in order for the cells to proliferate, differentiate, and migrate accurately. This study suggests a developmental pathway of pre-DC2/IPCs from CD34++CD45RA− early progenitors, to CD34++CD45RA+ late progenitors, to CD34+ CD45RA++IL-3Rα++CD4+HLA-DR+CD11c− pro-DC2, and finally to CD34−CD45RA+IL-3Rα++CD4+ HLA-DR+CD11c− pre-DC2/IPCs (Fig. 9). This differentiation pathway may provide a basis to study further the function and development of pre-DC2 in normal and disease states. For example, it was recently reported that both G-CSF and FLT3 ligand treatment of healthy donors increased pre-DC2 numbers in the peripheral blood. This study provides a model to study the effects of both cytokines on the different developmental stages of pre-DC2 in vivo. Furthermore, it has been reported that there is a progressive loss of the IFN-α/β–producing capacity of peripheral blood cells in response to viral stimulation in patients with hairy cell leukemia, steroid treatment, idiopathic CD4 lymphopenia, and HIV (for a review, see reference 51). It will be interesting to determine the stage where pre-DC2 development and function is disturbed in these patients in order to anticipate clinical treatment.
Figure 9.
Schematic representation of pre-DC2/IPC development from CD34++ early progenitor cells.
This study also demonstrated the generation of pre-DC2/IPCs in vitro from CD34++CD45RA− early hematopoietic progenitor cells with FLT3 ligand. The generated cells not only had the capacity to produce huge amounts of IFN-α/β in response to viral stimulation, but also displayed a typical pre-DC2/IPC phenotype of CD4+IL-3Rα++CD45RA+HLADR+CD11c−. Notably, the finding that neither pro-DC2 nor pre-DC2 could survive and proliferate in culture with FLT3 ligand over a 2-wk culture period indicates that the presence of pre-DC2/IPCs was due to their differentiation from CD34++CD45RA− early progenitor cells, and not the selective expansion of contaminating pro-DC2s or pre-DC2s. Injection of FLT3 ligand was previously shown to dramatically increase the numbers of both myeloid and lymphoid DCs in blood and lymphoid tissues of mice 46 52. A recent study by Pulendran et al. 45 showed that FLT3 ligand treatment also increased the number of both CD11c+ myeloid DCs and CD4+IL-3Rα++CD11c− pre-DC2/IPCs in the peripheral blood of human donors. These findings, together with this study, suggest that FLT3 ligand directly induces the differentiation of a proportion of CD34++CD45RA− early progenitor cells into pre-DC2/IPCs. Whether FLT3 ligand promotes the migration of pre-DC2 generated in bone marrow into the blood circulation remains unknown. Two recent studies showed that in G-CSF–treated patients or stem cell donors, the number of pre-DC2 in blood was increased fivefold, whereas the number of CD11c+ DCs was not affected 10 45. Our current finding that G-CSF was unable to induce CD34++CD45RA− early progenitor cells to differentiate into pre-DC2/IPCs suggests that unlike FLT3 ligand, G-CSF did not support differentiation of early hematopoietic progenitor cells into pre-DC2/IPCs, but may promote the migration of pre-DC2 into peripheral blood.
IFN-α/β has been widely used in treating patients with viral hepatitis 53. Potentially, combination therapy of IFN-α/β and FLT3 ligand or G-CSF can be used to increase the number of pre-DC2/IPCs to treat patients with chronic viral infection. Several studies suggest that in HIV-infected patients, there is a progressive loss of the ability of blood leukocytes to produce IFN-α/β in response to viral infections 54 55 56. Pre-DC2/IPCs express CD4 and chemokine receptors CXCR4 and CCR5 (Kanzler, H., V. Soumelis, and Y.-J. Liu, unpublished observation), which are the receptors allowing HIV entry 57 58. We have recently observed that there is a dramatic decrease in pre-DC2/IPC numbers in HIV-infected patients with AIDS, in particular in AIDS patients with complication of infections and of Kaposi sarcoma (Soumelis, V., and Y.-J. Liu, unpublished observation). Although it remains to be established whether pre-DC2/IPCs are infected by HIV viruses, FLT3 ligand together with the therapies preventing HIV infection may be beneficial for patients to ultimately eliminate viruses.
In conclusion, this study has mapped the pathways and identified the cytokine regulation of pre-DC2/IPC development from early hematopoietic stem cells in humans. The pre-DC2/IPC developmental pathway may provide a basis for monitoring pre-DC2/IPC numbers and development in patients with tumors, autoimmune diseases, and infectious diseases. FLT3 ligand and G-CSF may have the potential to be used to stimulate the generation and mobilization of pre-DC2/IPCs in patients who are fighting against cancers and infectious diseases.
Acknowledgments
We thank J. Cupp for cell sorting, Drs. R. Chase for HSV-1, S. Menon for FLT3 ligand, and H. Kanzler and V. Soumelis for critical reading of the manuscript.
DNAX Research Institute of Molecular and Cellular Biology is supported by the Schering-Plough Corporation.
Footnotes
Abbreviations used in this paper: APC, allophycocyanin; DC, dendritic cell; FSC, forward scatter; HS, human serum; IPC, natural type 1 interferon-producing cell; LC, Langerhans cell; MNC, mononuclear cell; SCF, stem cell factor; SSC, side scatter.
References
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P., Hilkens C.M., Wierenga E.A., Kapsenberg M.L. T-cell priming by type-1 and type-2 polarized dendritic cellsthe concept of a third signal. Immunol. Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- d'Ostiani C.F., Del Sero G., Bacci A., Montagnoli C., Spreca A., Mencacci A., Ricciardi-Castagnoli P., Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C., Sher A., Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α1 and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpinati M., Green C.L., Heimfeld S., Heuser J.E., Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- Liu Y.J., Blom B. IntroductionTH2-inducing DC2 for immunotherapy. Blood. 2000;95:2482–2483. [PubMed] [Google Scholar]
- Shortman K. Burnet orationdendritic cells: multiple subtypes, multiple origins, multiple functions. Immunol. Cell. Biol. 2000;78:161–165. doi: 10.1046/j.1440-1711.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- Ardavin C., Wu L., Li C.L., Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Shortman K., Vremec D., Corcoran L.M., Georgopoulos K., Lucas K., Wu L. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol. Rev. 1998;165:39–46. doi: 10.1111/j.1600-065x.1998.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Deguchi M., Hagi K., Yasumizu R., Ikehara S., Muramatsu S., Steinman R.M. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci. USA. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.W., Steinman R.M. The hematopoietic development of dendritic cellsa distinct pathway for myeloid differentiation. Stem Cells. 1996;14:376–387. doi: 10.1002/stem.140376. [DOI] [PubMed] [Google Scholar]
- Suss G., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand– induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronin V., Vremec D., Winkel K., Classon B.J., Miller R.G., Mak T.W., Shortman K., Suss G. Are CD8+ dendritic cells (DC) veto cells? The role of CD8 on DC in DC development and in the regulation of CD4 and CD8 T cell responses. Int. Immunol. 1997;9:1061–1064. doi: 10.1093/intimm/9.7.1061. [DOI] [PubMed] [Google Scholar]
- Stumbles P.A., Thomas J.A., Pimm C.L., Lee P.T., Venaille T.J., Proksch S., Holt P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Fukao T., Suzue K., Maki C., Ito M., Nakamura M., Koyasu S. Interleukin 12-dependent interferon gamma production by CD8α1 lymphoid dendritic cells. J. Exp. Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A.W., Drakes M.L., Zahorchak A.F., O'Connell P.J., Steptoe R.J., Qian S., Lu L. Hepatic dendritic cellsimmunobiology and role in liver transplantation. J. Leukoc. Biol. 1999;66:322–330. doi: 10.1002/jlb.66.2.322. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Kelsall B.L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A., Morelli A.E., Zhong C., Takayama T., Lu L., Thomson A.W. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J. Immunol. 2000;164:1346–1354. doi: 10.4049/jimmunol.164.3.1346. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kampgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P.O., Steinman R.M., Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan M.C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.C., Filgueira L., Durand I., Banchereau J., Liu Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchetti F., Candiago E., Vermi W. Plasmacytoid monocytes express IL3-receptor alpha and differentiate into dendritic cells. Histopathology. 1999;35:88–89. doi: 10.1046/j.1365-2559.1999.0728d.x. [DOI] [PubMed] [Google Scholar]
- Res P.C., Couwenberg F., Vyth-Dreese F.A., Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- Bruno L., Res P., Dessing M., Cella M., Spits H. Identification of a committed T cell precursor population in adult human peripheral blood. J. Exp. Med. 1997;185:875–884. doi: 10.1084/jem.185.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Couwengerg F., Bakker A.Q., Weijer K., Uittenbogaart C.H. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but Not into Pre-DC1evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 2000;192:1775–1783. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Peng M., Gezelter S., Swiggard W.J., Betjes M., Bhardwaj N., Steinman R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Olweus J., BitMansour A., Warnke R., Thompson P.A., Carballido J., Picker L.J., Lund-Johansen F. Dendritic cell ontogenya human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N., Antonenko S., Yiu-Nam Lau J., Liu Y.-J. Natural interferon α/β–producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Durand I., Cella M., Lanzavecchia A., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alphaII. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF α. J. Exp. Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy A., Travis M., Cen D., Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Zeigler F.C., Bennett B.D., Jordan C.T., Spencer S.D., Baumhueter S., Carroll K.J., Hooley J., Bauer K., Matthews W. Cellular and molecular characterization of the role of the flk-2/flt-3 receptor tyrosine kinase in hematopoietic stem cells. Blood. 1994;84:2422–2430. [PubMed] [Google Scholar]
- Shurin M.R., Esche C., Lotze M.T. FLT3receptor and ligand. Biology and potential clinical application. Cytokine Growth Factor Rev. 1998;9:37–48. doi: 10.1016/s1359-6101(97)00035-x. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Banchereau J., Burkeholder S., Kraus E., Guinet E., Chalouni C., Caron D., Maliszewski C., Davoust J., Fay J., Palucka K. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Brasel K., Teepe M., Roux E.R., Lyman S.D., Shortman K., McKenna H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated micemultiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Lingappa J., Kennedy M.K., Smith J., Teepe M., Rudensky A., Maliszewski C.R., Maraskovsky E. Developmental pathways of dendritic cells in vivodistinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- Yssel H., De Vries J.E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J. Immunol. Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- Fritsch G., Stimpfl M., Kurz M., Leitner A., Printz D., Buchinger P., Hoecker P., Gadner H. Characterization of hematopoietic stem cells. Ann. NY Acad. Sci. 1995;770:42–52. doi: 10.1111/j.1749-6632.1995.tb31042.x. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Chang C., Phillips J.H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol. Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin M.R., Pandharipande P.P., Zorina T.D., Haluszczak C., Subbotin V.M., Hunter O., Brumfield A., Storkus W.J., Maraskovsky E., Lotze M.T. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell. Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- Reusser P. Antiviral therapycurrent options and challenges. Schweiz. Med. Wochenschr. 2000;130:101–112. [PubMed] [Google Scholar]
- Siegal F.P., Lopez C., Fitzgerald P.A., Shah K., Baron P., Leiderman I.Z., Imperato D., Landesman S. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J. Clin. Invest. 1986;78:115–123. doi: 10.1172/JCI112539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Feldman M., Howell D., Kloser P. Deficient interferon-alpha production but normal natural killer cell activity in an AIDS patient with HIV-2 infection. J. Infect. Dis. 1989;160:1084–1085. doi: 10.1093/infdis/160.6.1084. [DOI] [PubMed] [Google Scholar]
- Rossol S., Voth R., Laubenstein H.P., Muller W.E., Schroder H.C., Meyer zum Buschenfelde K.H., Hess G. Interferon production in patients infected with HIV-1. J. Infect. Dis. 1989;159:815–821. doi: 10.1093/infdis/159.5.815. [DOI] [PubMed] [Google Scholar]
- Balter M. A second coreceptor for HIV in early stages of infection. Science. 1996;272:1740. doi: 10.1126/science.272.5269.1740. [DOI] [PubMed] [Google Scholar]
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R.E., Hill C.M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]