Abstract
The functional trafficking steps used by soluble NSF attachment protein receptor (SNARE) proteins have been difficult to establish because of substantial overlap in subcellular localization and because in vitro SNARE-dependent binding and fusion reactions can be promiscuous. Therefore, to functionally identify the site of action of the vesicle-associated membrane protein (VAMP) family of R-SNAREs, we have taken advantage of the temporal requirements of adipocyte biosynthetic sorting of a dual-tagged GLUT4 reporter (myc-GLUT4-GFP) coupled with small interfering RNA gene silencing. Using this approach, we confirm the requirement of VAMP2 and VAMP7 for insulin and osmotic shock trafficking from the vesicle storage sites, respectively, and fusion with the plasma membrane. Moreover, we identify a requirement for VAMP4 for the initial biosynthetic entry of GLUT4 from the Golgi apparatus into the insulin-responsive vesicle compartment, VAMP8, for plasma membrane endocytosis and VAMP2 for sorting to the specialized insulin-responsive compartment after plasma membrane endocytosis.
Introduction
Trafficking between different intracellular membrane compartments involves transport vesicle intermediates that are generated at donor membranes and are then delivered to specific acceptor membranes (Boehm and Bonifacino, 2001; for reviews see Hong, 1998, 2005). The final step of this process is the physical fusion of vesicular trafficking intermediates with the target membrane compartment. This fusion step is mediated by the interactions of vesicle-associated SNARES and target SNARES (Sollner et al., 1993; Chen and Scheller, 2001). Specific interaction between a vesicle-SNARE and the cognate target-SNARE leads to the formation of a SNAREpin complex in which four SNARE motifs assemble into a twisted parallel four-helical bundle (for review see Hong, 2005). It is this helical structure that catalyzes the fusion of vesicles with their target compartments. SNARE proteins belong to a superfamily consisting of >35 proteins that share a common structural SNARE domain (Weimbs et al., 1997). Based on structural considerations required for the formation of the fusogenic SNAREpin complex, the SNARE proteins have been further classified into R-SNAREs and Q-SNAREs depending on whether the central amino acid in the SNARE domain is an arginine (R-SNARE) or a glutamine (Q-SNARE) residue, respectively (Bock et al., 2001).
The vesicle-associated membrane protein (VAMP) subfamily of R-SNARES consists of seven members: VAMP1 (synaptobrevin 1), VAMP2 (synaptobrevin 2), VAMP3 (cellubrevin), VAMP4, VAMP5 (myobrevin), VAMP7 (tetanus toxin insensitive [TI]–VAMP), and VAMP8 (endobrevin; for review see Hong, 2005). VAMP1 is primarily expressed in neurons, whereas the other VAMP isoforms are more ubiquitously expressed in secretory and nonsecretory cells (Lin and Scheller, 2000). However, the trafficking steps that use each of these isoforms have been more difficult to establish, as there is substantial overlap in subcellular localization, and a given isoform can be localized to multiple intracellular compartments in the same cell (for review see Hong, 2005). Moreover, the intracellular distribution of a given isoform is not necessarily the same in different cell types. For example, VAMP5 and 7 are found at the plasma membrane, but VAMP7 is also in late endosomes and lysosomes (Zeng et al., 1998; Lin and Scheller, 2000; for review see Hong, 2005). VAMP4 is found in the TGN but also in early endosome compartments (Advani et al., 1998; Steegmaier et al., 1999; Mallard et al., 2002). Similarly, VAMP3 has been observed in recycling endosomes, specialized regulatory secretory compartments, and in early and late endosomes (McMahon et al., 1993; Galli et al., 1994; Lin and Scheller, 2000).
One physiologically important hormone-regulated trafficking event is the insulin-stimulated translocation of the facilitative glucose transporter (GLUT4) from intracellular vesicle compartments to the plasma membrane in adipocytes and skeletal muscle (Watson and Pessin, 2001). In the basal state, ∼95–98% of the GLUT4 protein is intracellular localized, and, after insulin stimulation, there is a marked increase in the rate of exocytosis with a smaller inhibition of plasma membrane endocytosis (Pessin et al., 1999; Holman and Sandoval, 2001; Martin et al., 2006). This relative change in kinetic transport rates results in a redistribution such that ∼50% of the GLUT4 protein is now at the cell surface membrane that directly accounts for the insulin-stimulated increase in glucose uptake activity (Pessin et al., 1999; Holman and Sandoval, 2001). After the removal of insulin, the rate of exocytosis and endocytosis returns to the basal kinetic rates such that GLUT4 endocytosis now exceeds exocytosis, resulting in the internalization, endocytic recycling, and repopulation of the GLUT4 intracellular membrane storage compartments that are accessible for a second round of insulin stimulation (Martin et al., 2006).
Of the known 35 SNARE proteins, only a small subset have been examined for their role in GLUT4 trafficking. For example, syntaxin 4 and SNAP23 are thought to be the Q-SNAREs required for insulin-stimulated GLUT4 plasma membrane fusion, and syntaxin 6 and 16 are thought to be required for intracellular sorting (Rea et al., 1998; Perera et al., 2003; Shewan et al., 2003; Proctor et al., 2006). In the case of the VAMP family of R-SNAREs, only VAMP2 and 7 have been examined in terms of insulin- and osmotic shock–stimulated plasma membrane translocation, but their role in specific trafficking steps were not defined (Cheatham et al., 1996; Rea et al., 1998; Randhawa et al., 2004). Thus, to functionally identify the site of action of the VAMP family of R-SNAREs in the trafficking itinerary of GLUT4 in adipocytes, we have developed an assay approach that can readily distinguish four trafficking events responsible for (1) biosynthetic entry of GLUT4 into the insulin-responsive storage compartment, (2) trafficking from the storage sites and fusion with the plasma membrane, (3) plasma membrane endocytosis, and (4) endocytotic recycling back into the insulin-responsive intracellular storage location. Using siRNA to reduce R-SNARE expression coupled with the expression of a myc-GLUT4-GFP dual reporter, we have confirmed the requirement of VAMP2 and 7 for insulin and osmotic shock–stimulated translocation. Moreover, we successfully identified the functional sites of action for VAMP2, 4, 7, and 8 in distinct steps in the regulated trafficking of GLUT4 in adipocytes.
Results
Expression and subcellular localization of VAMP proteins in 3T3L1 adipocytes
To determine which of the VAMP family of R-SNARE proteins are expressed in adipocytes, we examined the levels of these proteins in predifferentiated adipocytes (day 0) and during their differentiation to full mature adipocytes (day 12). The conversion of 3T3L1 from fibroblast to adipocyte state was confirmed by the appearance of lipid droplets (unpublished data) and the induction of caveolin 1 protein that is highly induced during adipogenesis (Fig. 1 A; Scherer et al., 1994; Kandror et al., 1995). As expected, VAMP1 was expressed in mouse brain extracts, but it was essentially undetectable in either 3T3L1 preadipocytes or differentiated adipocytes (Fig. 1 A). In contrast to VAMP1, all of the other VAMP proteins were expressed, albeit with small changes occurring during adipogenesis. For example, the protein expression levels of VAMP2, 3, 5, and 7 were somewhat increased, whereas VAMP4 and 8 protein levels remained relatively unchanged during adipocyte differentiation.
Figure 1.
Expression and siRNA-mediated gene silencing of VAMP proteins in 3T3L1 adipocytes. (A) Total cell extracts were prepared from 3T3L1 cells before the initiation of differentiation and at 4, 8, and 12 d after differentiation. The cell extracts were then immunoblotted for VAMP proteins and caveolin-1 (Cav-1). (B) Fully differentiated adipocytes were transfected with random or VAMP-specific siRNA constructs. Total cell extracts were prepared 72 h later and immunoblotted for the presence of VAMP proteins. Representative immunoblots from three independent experiments are shown.
Reduction of VAMP protein expression by siRNA
Because the function of VAMP proteins cannot be inferred based on their intracellular distribution and expression of dominant-interfering mutants will likely affect several family members as a result of their structural similarity, we examined the efficiency and specificity of siRNA to reduce VAMP protein expression levels. Preliminary experiments demonstrated that after siRNA transfection, there was little effect of VAMP protein expression 24 h later, but that subsequently declined over the next 48 h and, with optimum reduction of protein expression, occurred by 72 h after transfection (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200709108/DC1). As shown in Fig. 1 B, siRNA treatment resulted in a significant reduction in the levels of each VAMP protein compared with cells treated with a control construct containing a random siRNA sequence. Quantification of the degree of siRNA-mediated knockdowns indicated that by 72 h, the extent of VAMP protein reduction ranged from 68 to 94% compared with random siRNA-treated cells (Fig. S2). Notably, knockdown of each protein was specific, as the siRNA constructs did not result in significant changes in the levels of the nontargeted family members. Therefore, in the 3T3L1 adipocyte cell system, siRNA-mediated silencing is an effective mechanism for reducing the expression of specific members of the VAMP protein family without affecting closely related proteins.
Effects of VAMP knockdowns on the translocation of GLUT4
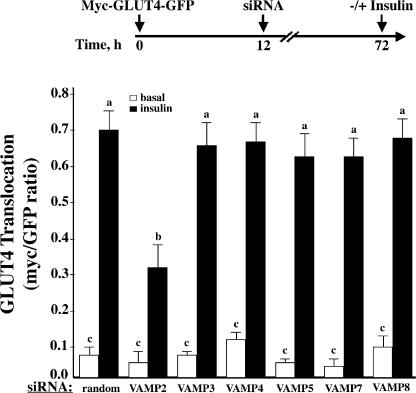
Previously, we have demonstrated that adipocytes transfected with various constitutive plasma membrane trafficking proteins (i.e., GLUT1 and vesicular stomatitis virus glycoprotein) undergo a rapid sorting out of the Golgi/TGN biosynthetic endomembrane system (Watson et al., 2004). On the other hand, GLUT4 undergoes a slow sorting process out of the endomembrane system that requires 9–12 h to populate the membrane vesicle compartments and acquire insulin-responsive translocation to the plasma membrane (Watson et al., 2004). Because introduction of the VAMP siRNA did not affect VAMP protein levels between 12 and 24 h and was maximal by 72 h, coexpression of the VAMP siRNA with the myc-GLUT4-GFP cDNA effectively allows the GLUT4 reporter to be expressed and to traffic into the insulin-responsive membrane compartments before any significant reduction in the VAMP protein. Thus, in this experimental paradigm, we cotransfected the specific VAMP siRNAs with myc-GLUT4-GFP for 72 h and quantified the insulin stimulation of GLUT4 reporter translocation (Fig. 2). As expected, for adipocytes cotransfected with the random siRNA and myc-GLUT4-GFP for 72 h and stimulated with 100 nM insulin for 30 min, there was an approximate 10-fold increase in the plasma membrane–localized GLUT4 compared with unstimulated cells. Reduction of VAMP2 expression had no significant effect on the basal state distribution of myc-GLUT4-GFP at the plasma membrane but attenuated the insulin-stimulated translocation by ∼60%. In contrast, siRNA-mediated knockdowns of the other VAMP proteins were without any significant effect.
Figure 2.
The effect of VAMP knockdowns on insulin-stimulated myc-GLUT4-GFP reporter translocation. Fully differentiated 3T3L1 adipocytes were cotransfected with a random siRNA or VAMP-specific siRNA plus the myc-GLUT4-GFP reporter cDNA. 72 h later, the cells were either left untreated or stimulated with 100 nM insulin for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of myc-GLUT4-GFP. These data represent the analysis of 15 cells/experiment from three to five independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD.
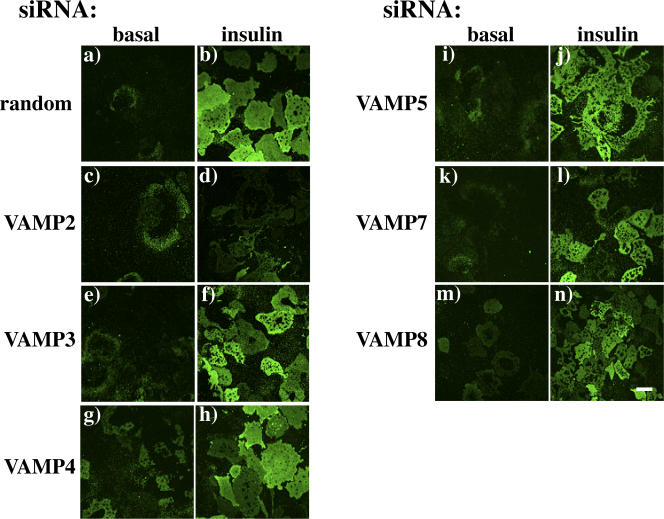
To confirm this effect on translocation of the endogenous GLUT4 protein, we also generated plasma membrane sheets from cells transfected for 72 h with random and VAMP-specific siRNAs (Fig. 3). Plasma membrane sheets isolated from random siRNA-treated control cells had a relatively low level of immunoreactive GLUT4 in the basal state (Fig. 3 a). Insulin stimulation resulted in translocation of the endogenous GLUT4 protein as depicted by the increase in plasma membrane sheet immunofluorescence (Fig. 3 b). In agreement with the results obtained using the myc-GLUT4-GFP reporter construct, the depletion of VAMP2 resulted in reduced plasma membrane sheet immunofluorescence after insulin stimulation (Fig. 3 d). Depletion of the other VAMP proteins resulted in a translocation profile that was similar to random siRNA-treated cells (Fig. 3, e–n). These data suggest that once GLUT4 is localized to the insulin-responsive compartment, only VAMP2 is required for insulin-stimulated translocation to the plasma membrane
Figure 3.
The effect of VAMP knockdowns on the translocation of endogenous GLUT4 protein. Fully differentiated 3T3L1 adipocytes were transfected with a random siRNA or a VAMP-specific siRNA. 72 h later, the cells were either left untreated or stimulated with 100 nM insulin for 30 min. Plasma membrane sheets were prepared and subjected to confocal fluorescent microscopy for the presence of the endogenous GLUT4 protein. Representative images taken from three independent experiments are shown. Bar, 20 μm.
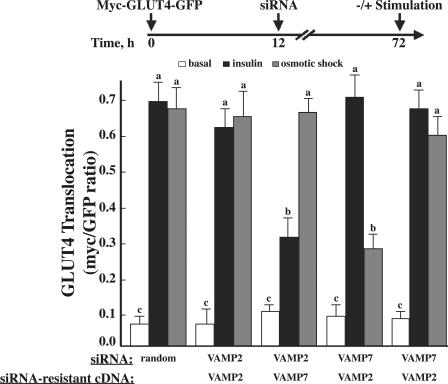
Alternatively, it is possible that levels of the siRNA-mediated protein knockdowns for the other VAMPs were insufficient to detect any phenotype. It was previously reported that VAMP7 (TI-VAMP) was necessary for osmotic shock–mediated GLUT4 translocation (Randhawa et al., 2004). Therefore, we cotransfected the VAMP siRNA with myc-GLUT4-GFP and, 72 h later, compared the basal and osmotic shock stimulation of GLUT4 translocation (Fig. 4). Adipocytes depleted of VAMP7 displayed a 70% decrease in osmotic shock–stimulated GLUT4 translocation, whereas siRNA-mediated knockdown of VAMP2, 3, 4, 5, and 8 were without effect. We next transfected the adipocytes with siRNA-resistant VAMP2 and 7 to confirm the specificity of the VAMP2 and 7 knockdowns. As shown in Fig. 5, expression of a VAMP2 siRNA-resistant cDNA restored insulin-stimulated but not osmotic shock–stimulated GLUT4 translocation in the VAMP2 knockdown cells. In contrast, expression of a VAMP7 siRNA-resistant cDNA restored osmotic shock–stimulated but not insulin-stimulated GLUT4 translocation in the VAMP7 knockdown adipocytes. Similarly, knockdown of VAMP2 inhibited insulin-stimulated glucose uptake without affecting osmotic shock–mediated glucose uptake (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200709108/DC1). Conversely, VAMP7 depletion inhibited osmotic shock–stimulated glucose uptake but had no effect on insulin-stimulated glucose uptake. Collectively, these data are consistent with VAMP2 being required for insulin-stimulated GLUT4 translocation, whereas VAMP7 is required for the osmotic shock–stimulated translocation from the GLUT4 storage compartment to the plasma membrane.
Figure 4.
The effect of VAMP knockdowns on osmotic shock–stimulated myc-GLUT4-GFP reporter translocation. Fully differentiated 3T3L1 adipocytes were cotransfected with a random siRNA or VAMP-specific siRNA plus the myc-GLUT4-GFP reporter cDNA. 72 h later, the cells were either left untreated or stimulated with 600 mM sorbitol for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of myc-GLUT4-GFP. These data represent the analysis of 15 cells/experiment from three to five independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD.
Figure 5.
VAMP isoform-specific rescue of the VAMP2 and 7 knockdowns in insulin- and osmotic shock-stimulated GLUT4 translocation. Fully differentiated 3T3L1 adipocytes were cotransfected with random siRNA, VAMP2- or 7-specific siRNA, the GLUT4 reporter construct, and a VAMP2- or 7-resistant cDNA. 72 h later, the cells were either left untreated or stimulated with 100 nM insulin or 600 mM sorbitol for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for localization of the GLUT4 reporter. These data represent the analysis of 15 cells/experiment from three independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD.
Effects of VAMP knockdowns on the biosynthetic sorting of GLUT4 into the insulin-responsive compartment
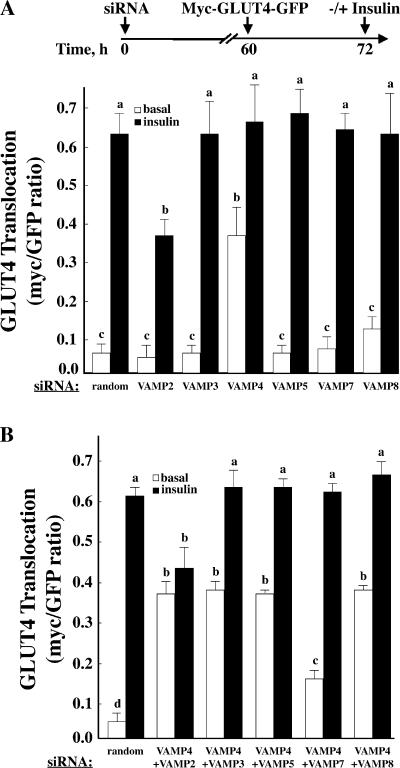
As indicated in the previous section, after biosynthesis and Golgi/TGN processing, GLUT4 is directly sorted into the insulin-responsive compartment without having to transit the plasma membrane (Watson et al., 2004). To identify the VAMP proteins involved in the initial biosynthetic sorting to the insulin-responsive compartment, we transfected adipocytes with the VAMP siRNAs and waited 60 h for the reduction of VAMP protein expression. We then transfected the cells with the myc-GLUT4-GFP reporter and, 12 h later, examined insulin-stimulated GLUT4 translocation. As shown in Fig. 6 A, random siRNA-treated cells showed the expected profile of intracellular localization in the basal state, which is indicative of sorting and sequestration into intracellular storage compartments. In response to insulin, the GLUT4 reporter construct redistributed to the plasma membrane, indicating that the GLUT4 in these intracellular compartments was in fact insulin responsive. Because VAMP2 is required for insulin-stimulated translocation out of the insulin-responsive compartment, siRNA-mediated knockdown of VAMP2 followed by myc-GLUT4-GFP expression also inhibited insulin-stimulated GLUT4 translocation. In contrast, the profile for VAMP3, 5, 7, and 8 knockdown cells was similar to that of random siRNA-treated control cells, indicating proper sorting and sequestration into the insulin-responsive compartment. Surprisingly, when our GLUT4 reporter construct was introduced in cells depleted of VAMP4, there was a high percentage of GLUT4 at the plasma membrane in the basal state. Despite the higher basal state plasma membrane distribution, the VAMP4 knockdown cells did show translocation in response to insulin stimulation, and the extent of translocation was similar to that of control cells. These data indicate that the loss of VAMP4 protein results in the biosynthetic sorting of GLUT4 to the constitutive trafficking pathway, although a substantial proportion can still sort to the insulin-responsive compartment.
Figure 6.
The effects of VAMP knockdowns on the sorting of newly synthesized GLUT4. (A) Fully differentiated 3T3L1 adipocytes were transfected with a random siRNA or VAMP-specific siRNA. (B) Fully differentiated adipocytes were cotransfected with a random siRNA or VAMP4-specific siRNA in combination with the indicated VAMP-specific siRNA. (A and B) 60 h later, the cells were transfected with the myc-GLUT4-GFP reporter construct. 12–16 h later, the cells were left untreated or stimulated with 100 nM insulin for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of myc-GLUT4-GFP. These data represent the analysis of 15 cells/experiment from three independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD.
If VAMP4 depletion results in the biosynthetic sorting of GLUT4 to the constitutive trafficking pathway, another VAMP must be required for basal state translocation to the plasma membrane. Therefore, we cotransfected adipocytes with the siRNA for VAMP4 plus VAMP2, 3, 5, 7, or 8 for 60 h followed by the expression of myc-GLUT4-GFP for 12 h (Fig. 6 B). As previously observed, siRNA knockdown of VAMP4 resulted in an increased basal state translocation of GLUT4 to the plasma membrane, but the translocation still displayed insulin responsiveness. Simultaneous siRNA-mediated reduction of both VAMP4 and 2 protein levels had no effect on the high basal state translocation of GLUT4 but, in this case, completely prevented the insulin-stimulated translocation. In contrast, siRNA knockdown of VAMP4 with VAMP3, 5, or 8 had no effect on the high basal state or insulin-stimulated translocation of GLUT4. In contrast, the coknockdown of both VAMP4 and 7 reduced the basal state trafficking of newly synthesized GLUT4 to the plasma membrane yet allowed the full extent of insulin-stimulated translocation. These data indicated that in the absence of VAMP4, approximately half of the newly synthesized GLUT4 protein sorts to a VAMP7-dependent compartment, whereas the other half sorts to a VAMP2-dependent compartment.
Effects of VAMP knockdowns on GLUT4 endocytosis
In addition to the biosynthetic sorting steps to the insulin-responsive compartment and regulated translocation to the plasma membrane, there are additional mechanisms to ensure that GLUT4 undergoes proper endocytosis and recycling back to the insulin-responsive storage sites for subsequent rounds of insulin-stimulated translocation. Therefore, we assayed whether the depletion of VAMP proteins would affect the ability of cells to efficiently retrieve GLUT4 from the plasma membrane.
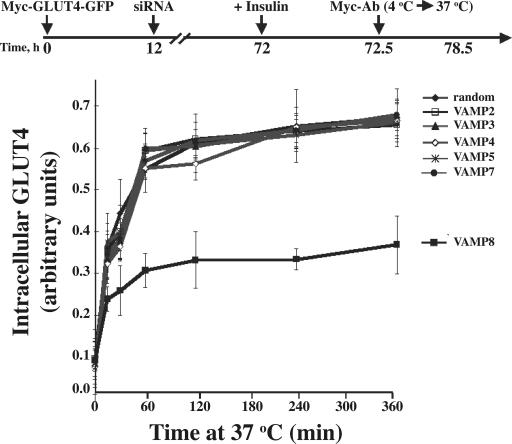
In the first set of experiments shown in Fig. 7, we addressed this issue by taking advantage of the exofacial myc epitope on our GLUT4 reporter protein. In this experimental paradigm, adipocytes were cotransfected with the siRNA and myc-GLUT4-GFP reporter for 72 h and were stimulated with insulin to translocate myc-GLUT4-GFP to the cell surface as described in Fig. 2. It should be noted that although there was a reduced amount of plasma membrane myc-GLUT4-GFP in the VAMP2 siRNA knockdown cells, the amount at the cell surface was sufficient to determine the rate and extent of endocytosis. In any case, the exofacial myc epitope was then labeled with the myc antibody at 4°C, and the cells were warmed to 37°C for various times in the absence of insulin. The time-dependent appearance of intracellular localized myc antibody was determined and quantified as a percentage of the total labeled myc epitope (Fig. 7). In control cells, the t 1/2 for endocytosis was ∼28 min, which is in agreement with previous studies reporting an approximate t 1/2 of 20 min (Satoh et al., 1993; Al-Hasani et al., 2002; Kanzaki et al., 2004; Karylowski et al., 2004), although somewhat slower than a t 1/2 of ∼4 min observed by Yang et al. (1996). In any case, the VAMP2, 3, 4, 5, and 7 siRNA knockdown cells showed a rate of endocytosis that was essentially identical to the control cells. In contrast, in VAMP8 knockdown cells, the extent of GLUT4 endocytosis was significantly reduced compared with the control and other VAMP knockdown cells. These data demonstrate that VAMP8 plays a functional role in the plasma membrane retrieval of GLUT4.
Figure 7.
The effects of VAMP knockdowns on GLUT4 endocytosis. Fully differentiated adipocytes were cotransfected with a random siRNA or VAMP-specific siRNA plus the myc-GLUT4-GFP cDNA reporter. 72 h later, the cells were stimulated with 100 nM insulin for 30 min and cooled to 4°C. The exofacial exposed myc epitope was labeled with a myc antibody, and the cells were washed to remove insulin and excess antibody and subsequently were warmed to 37°C. At various times, the cells were fixed, permeabilized, and incubated with an AlexaFluor594 anti–mouse secondary antibody, and the cells were scored for the presence of internalized GLUT4 as described in Materials and methods. These data represent the analysis of 15 cells/experiment from three independent experiments. Error bars represent SD.
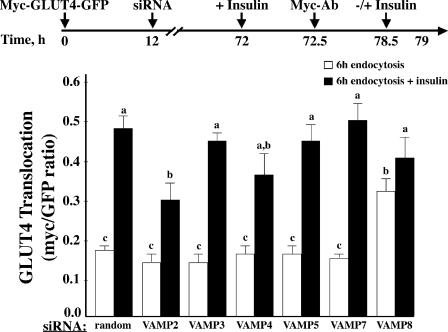
After plasma membrane endocytosis, GLUT4 undergoes recycling back to the insulin-responsive compartment to become available for a second round of insulin-stimulated translocation. To assess the VAMP protein responsible for recycling back to the insulin-responsive compartment, we allowed the exofacial myc-labeled GLUT4 reporter to internalize for 6 h, restimulated the cells with insulin, and assayed for insulin-dependent translocation (Fig. 8). The random siRNA-treated cells showed an approximate 2.5-fold increase in the amount of GLUT4 at the plasma membrane after insulin stimulation. This fold increase is ∼50% less than the translocation observed in the direct translocation experiments because we are only observing the population of GLUT4 that has been exofacially labeled and, once internalized, mixes with the unlabeled GLUT4 population. This mixing of the surface label GLUT4 with the intracellular unlabeled GLUT4 and subsequent reduction in the labeled GLUT4 that is translocated after a second round of insulin-stimulated translocation have been described previously (Satoh et al., 1993). The second round of insulin-stimulated GLUT4 translocation in the VAMP3, 4, 5, and 7 knockdown cells was similar to that of control cells, indicating that these VAMPs do not play a significant role in the recycling of internalized GLUT4 to the insulin-responsive compartment. Although the VAMP2 knockdown cells showed an inhibition of translocation in response to insulin compared with the control cells, this was most likely caused by the inhibition of translocation from the insulin-responsive compartment to the plasma membrane (Fig. 2). Consistent with the results in Fig. 7, VAMP8 knockdown cells showed high levels of GLUT4 at the plasma membrane after 6 h of internalization. Thus, upon insulin restimulation, there was only a small effect of insulin to stimulate translocation.
Figure 8.
The effects of VAMP knockdowns on insulin restimulation of GLUT4 translocation after endocytosis. Fully differentiated adipocytes were cotransfected with a random siRNA or VAMP-specific siRNA plus the myc-GLUT4-GFP cDNA reporter. 72 h later, endocytosis assays were performed as described in Materials and methods. Cells were warmed to 37°C for 6 h followed by restimulation with 100 nM insulin for 30 min. The cells were fixed and subjected to confocal fluorescent microscopy for the localization of myc-GLUT4-GFP. These data represent the analysis of 15 cells/experiment from three independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD.
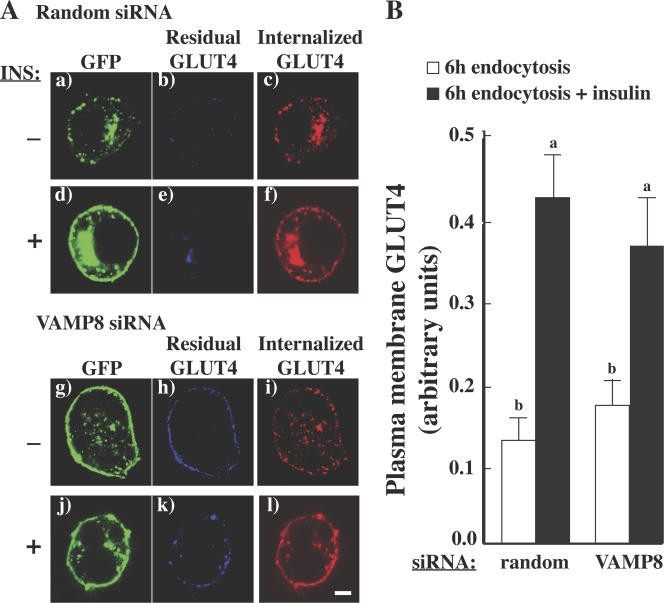
Therefore, to determine whether VAMP8 also plays a role in the endocytotic recycling back to the insulin-responsive compartment, we need to remove or ablate the high plasma membrane GLUT4 label after endocytosis in the VAMP8 knockout cells. This was accomplished by masking the residual myc label remaining at the plasma membrane. The endocytosis assay was performed as described in the previous paragraph on random or VAMP8-specific siRNA-treated cells. However, in this modified protocol, after 6 h at 37°C, the unpermeabilized cells were treated with AlexaFluor647 anti–mouse secondary antibody at 4°C to label the residual myc-GLUT4-GFP at the cell surface. Cells were then washed and treated with or without 100 nM insulin for 30 min followed by fixing and labeling with AlexaFluor594 anti–mouse secondary antibody. The percentage of internalized, unblocked GLUT4 (AlexaFluor594-labeled GLUT4) at the cell surface was then determined for each condition by confocal microscopy.
As shown in Fig. 9 A, using this approach, there was more residual GLUT4 at the plasma membrane after warm-up in VAMP8-deficient cells compared with control cells. However, the population of internalized GLUT4 showed a similar fold increase upon insulin stimulation in control and VAMP8 siRNA-treated cells (Fig. 9 B). Collectively, these data demonstrate that in the absence of VAMP8, GLUT4 is not effectively internalized from the plasma membrane. However, the population of GLUT4 that is in fact endocytosed in VAMP8-deficient cells is effectively resorted to the insulin-responsive compartment and, thus, is able to respond to a second round of insulin stimulation.
Figure 9.
The effects of VAMP8 knockdown on insulin restimulation of the internalized GLUT4 population. Fully differentiated adipocytes were cotransfected with a random siRNA or VAMP8-specific siRNA plus the myc-GLUT4-GFP cDNA reporter. 72 h later, endocytosis assays were performed as described in Materials and methods. Cells were warmed to 37°C for 6 h followed by treatment with AlexaFluor647 anti–mouse secondary antibody at 4°C (to label the residual myc-GLUT4-GFP at the cell surface). Cells were washed and treated with or without 100 nM insulin for 30 min. The cells were fixed, labeled with AlexaFluor594 anti–mouse secondary antibody, and subjected to confocal fluorescent microscopy for the localization of myc-GLUT4-GFP. (A) Panels a–l depict representative images from each treatment condition. (B) The percentage of internalized, unblocked GLUT4 (AlexaFluor594-labeled GLUT4) at the cell surface was determined by confocal microscopy. These data represent the analysis of 15 cells/experiment from three independent experiments. Statistical analysis was performed using a one-way ANOVA followed by the Student-Newman-Keuls multiple range test. Identical letters indicate bars that are not statistically different from each other (P < 0.05). Error bars represent SD. Bar, 5 μm.
Discussion
One of the key features of intracellular membrane trafficking is the dependence of vesicular fusion on cognate SNARE proteins to facilitate the transfer of membrane proteins and luminal cargoes (for review see Rothman, 1994). Currently, there are nine R-SNARE and 26 Q-SNARE genes in mammalians with various splice variants that display complex and overlapping intracellular compartmentalization. Part of this complexity is based on the fact that after SNAREpin assembly, trans-SNAREs are transferred to the opposite recipient membrane, resulting in localization to the cis-membrane. Moreover, although a subset of possible SNAREpin complexes have been shown to display specificity in vitro, most SNAREpin complexes appear to be promiscuous in promoting in vitro fusion reactions (Tsui and Banfield, 2000; Wendler and Tooze, 2001; Bajohrs et al., 2005). In this regard, many laboratories have investigated the cellular trafficking functions of various SNARE proteins, and typically this has been done using multiple different cell types and cargo/reporter proteins (Bose et al., 2005; Pocard et al., 2007; Tran et al., 2007).
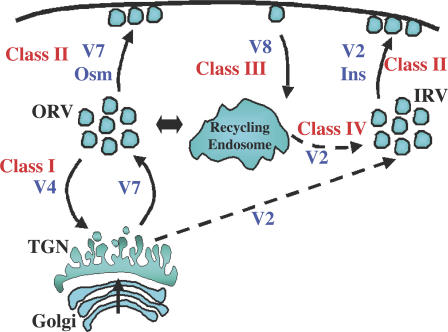
Thus, we undertook a systematic approach to identify the functional sites of action of the VAMP family of R-SNAREs in the trafficking of GLUT4 in adipocytes, a critical physiological cell system that is one of only a few that display hormone-regulated membrane protein trafficking (Mora and Pessin, 2002; Watson et al., 2004). To accomplish this, we took advantage of the biosynthetic and trafficking properties of insulin-regulated cargo in adipocytes that allow us to categorize four general sites of action. Previous studies have demonstrated that after biosynthesis, GLUT4 and another insulin-regulated membrane cargo protein, insulin-responsive aminopeptidase, undergo a 9–12-h Golgi/TGN sorting step before entering the insulin-responsive compartment without transiting the plasma membrane (Watson et al., 2004; Liu et al., 2005). Therefore, we propose that proteins affecting the direct sorting of GLUT4 after Golgi/TGN processing into the insulin-responsive compartment be considered as class I proteins (Fig. 10). Subsequently, once in the insulin-responsive compartment, proteins that affect the ability of insulin or other stimuli to stimulate GLUT4 translocation to the plasma membrane can be categorized as class II proteins.
Figure 10.
Schematic model for the function of VAMP proteins in distinct intracellular trafficking steps for GLUT4. During biosynthetic sorting, VAMP4 functions to ensure the proper sorting of GLUT4 into specialized insulin-responsive compartments (class I protein). Translocation from intracellular storage compartments to the plasma membrane is achieved through the function of class II proteins (VAMP2 [V2] for insulin-stimulated translocation and VAMP7 [V7] for osmotic shock–stimulated GLUT4 translocation). Plasma membrane endocytosis requires class III protein function (VAMP8 [V8]). Recycling back to the insulin-responsive compartment after endocytosis requires class IV protein function. This function is probably mediated by VAMP2. In addition, VAMP2 appears to function in the biosynthetic sorting out of the TGN into the insulin-responsive vesicle compartment. OSV, osmotic shock–responsive vesicle compartment; IRV, insulin-responsive vesicle compartment.
With these definitions, how can we experimentally distinguish between these two events? This was accomplished by alternating the temporal expression of GLUT4 versus the ablation of the protein of interest. In the case of the R-SNAREs reported here, we coexpressed GLUT4 with the VAMP siRNAs. Because newly synthesized GLUT4 requires 9–12 h to display insulin responsiveness and significant VAMP protein reduction requires a minimum of 48 h, this effectively allowed GLUT4 to be expressed first and equilibrate in all of its appropriate compartments before loss of a VAMP protein. Thus, any knockdown that affected GLUT4 translocation under these conditions would involve a class II protein, as the GLUT4 is already localized to the insulin-responsive compartment. In contrast, if we first treat cells with siRNA and reduce VAMP protein levels and then transfect with the GLUT4 reporter, alteration in the intracellular localization or insulin-stimulated translocation would define a class I protein. Using these criteria, we have defined VAMP4 as a class I protein, whereas VAMP2 and VAMP7 act as class II proteins for insulin-stimulated and osmotic shock–stimulated GLUT4 translocation, respectively.
The identification of VAMP2 as a class II protein is consistent with earlier studies in which GLUT4 translocation was inhibited by the injection of VAMP2 neurotoxins and the overexpression of dominant-interfering VAMP2 proteins and peptides (Cheatham et al., 1996; Olson et al., 1997; Martin et al., 1998). Similarly, in L6 myotubes, VAMP7 (TI-VAMP) was reported to be required for osmotic shock–stimulated GLUT4 translocation (Randhawa et al., 2004), and our siRNA classification paradigm has confirmed this finding in adipocytes. Although the physiological significance of osmotic shock–induced GLUT4 translocation is not known, previous studies have demonstrated that this pathway occurs in a phosphoinositol-3-kinase– and Akt-independent pathway (Chen et al., 1997; Sajan et al., 2002). Thus, the distinct requirement of VAMP2 as a class II protein for insulin-stimulated translocation and VAMP7 for osmotic shock–stimulated plasma membrane translocation is consistent with two distinct vesicle populations that are independently mobilized via different signaling pathways.
The identification of VAMP4 as a class I protein is also consistent with studies in fibroblasts suggesting that VAMP4 traffics from endosomes to the TGN and is involved in the retrograde transport of immature insulin secretory granule proteins to the TGN (Steegmaier et al., 1999; Kakhlon et al., 2006; Tran et al., 2007). Thus, although the partial basal state missorting of GLUT4 to the plasma membrane could be the result of the incomplete knockdown of VAMP4, it is more likely that there are two GLUT4 trafficking pathways out of the TGN (Fig. 10). One pathway is VAMP2 dependent and accounts for the sorting to the insulin-responsive compartment. The other utilizes VAMP7 to sort into an osmotic shock–sensitive compartment. This compartment may overlap with more general trafficking compartments such as recycling endosomes. VAMP4 functions to retrieve these wayward sorted GLUT4 proteins back to the TGN for resorting to the insulin-responsive compartment. This model also accounts for the dynamic retention of GLUT4 as has been proposed based on kinetic arguments (Zeigerer et al., 2002; Bogan et al., 2003; Karylowski et al., 2004; Muretta et al., 2007).
In any case, we can then expand this scheme to determine which proteins affect plasma membrane endocytosis (class III). This is readily determined using a GLUT4 reporter that expresses an exofacial epitope that is accessible to a high affinity antibody to only tag those molecules on the cell surface. Once internalized, those proteins that alter recycling to the insulin-responsive compartment after plasma membrane endocytosis are defined as class IV. In this case, class IV proteins would be defined as those that are not class II but affect the ability of internalized GLUT4 to undergo a second round of insulin-stimulated translocation. Within this framework, we identified VAMP8 functioning as a class III protein.
Recently, VAMP8 knockout mice have been shown to display defects in pancreatic acinar cell and platelet secretion (Wang et al., 2004; Ren et al., 2007). However, in adipocytes, our data clearly demonstrated that in the absence of VAMP8, GLUT4 is not properly retrieved from the plasma membrane. However, the population of GLUT4 that is internalized in the absence of VAMP8 was effectively sorted to the insulin-responsive storage compartment, where it underwent a second round of insulin-stimulated translocation to the plasma membrane. Although Sec22b and Ykt6 are two additional R-SNARE proteins, Sec22b is clearly localized in transport intermediates between the endoplasmic reticulum and the cis-Golgi, whereas Ykt6 is localized in the cis-Golgi and the Golgi stacks (Hay et al., 1997; McNew et al., 1997; Zhang et al., 1999; Zhang and Hong, 2001). Thus, because the levels of VAMP protein reduction were sufficient to block other specific trafficking steps, the most likely conclusion is that VAMP2 functions as both a class II and IV protein.
In summary, we have established an experimental paradigm whereby we can identify the trafficking of GLUT4 into four functional classes. Using this approach in combination with siRNA, we have defined the functional sites of action for the VAMP family of R-SNAREs in adipocytes: that is, VAMP4 functions as a class I protein, VAMP2 and 7 function as class II proteins, and VAMP8 functions as a class III protein. Because only a small subset of syntaxin and SNAP proteins have been studied in a limited context, this paradigm can now be used to place the entire Q-SNARE families as well as other trafficking protein effectors (Rabs, etc.) into distinct complementation groups that will allow for a more precise determination of functional specificity.
Materials and methods
Materials
Insulin, dexamethasone, isobutyl-1-methylxanthine, BSA, and donkey serum were purchased from Sigma-Aldrich. Vectashield was obtained from Vector Laboratories, and Supersignal ECL reagents were purchased from Thermo Fisher Scientific and used according to the manufacturer's instructions. The polyclonal antibody against rat GLUT4 (IAO2) was obtained by immunizing rabbits with a GLUT4 C-terminal peptide as described previously (Kao et al., 1998). The VAMP2 and GLUT4 antibodies were prepared as previously described (Thurmond et al., 1998). VAMP1, 3, 4, and 7 polyclonal antibodies were purchased from Novus Biologicals. Polyclonal antibodies to VAMP5 and 8 were gifts from W. Hong (Institute of Molecular and Cell Biology, Singapore). The caveolin-1 antibody was obtained from Transduction Laboratories. The c-myc antibody was obtained from Santa Cruz Biotechnology, Inc. Anti–rabbit and anti–mouse IgG AlexaFluor secondary antibodies were purchased from Invitrogen, and HRP-conjugated secondary antibodies were purchased from Thermo Fisher Scientific. The exofacial myc-tagged GLUT4-EGFP reporter was constructed as previously described (Thurmond et al., 1998; Kanzaki et al., 2004).
Double-stranded siRNA molecules were purchased from Ambion and were generated using the following target sequences: GGTTCGACTGAAAACTTTC and GGAAGGATCTAATCTTTTT (VAMP2), GCTCATGCTCTTATGTTAG (VAMP3), CGTACGTTTGAGCTTATAA (VAMP4), GGGAAGGCTGAATGACTGC (VAMP5), GGAAATTCATGTGACTATG (VAMP7), and GCCACGTCTGAACACTTCA (VAMP8). The random control sequence was CCCAUGGACGACAACACAA.
Cell culture and transient transfection of 3T3L1 adipocytes
Murine 3T3L1 preadipocytes were purchased from the American Type Culture Collection repository. Cells were cultured in DME supplemented with 25 mM glucose and 10% calf serum at 37°C with 8% carbon dioxide. Cells were differentiated into adipocytes with the addition of 1 μg/ml insulin, 1 mM dexamethasone, and 0.5 mM isobutyl-1-methylxanthine. Adipocytes were used in experiments 6–9 d after the initiation of differentiation. Cells were electroporated using the Gene Pulser II (Bio-Rad Laboratories) with settings of 0.16 kV and 960 microfarads. For all experiments, 50 μg DNA and 2–5 nmol siRNA were used for electroporation. After electroporation, cells were plated on glass coverslips and allowed to recover in complete medium.
Immunofluorescence and image analysis
Differentiated 3T3L1 adipocytes transfected with the exofacially tagged myc-GLUT4-GFP cDNA and relevant siRNA constructs were grown on coverslips and serum starved in DME for 2 h before each experiment. Cells were then incubated with 100 nM insulin for 30 min or left untreated. Shortly after stimulation, cells were fixed using 4% PFA for 10 min at room temperature. Cells were then blocked using 1% BSA solution with 5% donkey serum for 1 h at room temperature. Coverslips were then incubated in primary antibody solution for 1 h at 37°C. After incubation, the coverslips were washed three times with PBS solution followed by incubation in secondary antibody for 1 h at 37°C. Coverslips were again washed three times with PBS, mounted with Vectashield medium, and evaluated by confocal microcopy. Cells were imaged using a confocal fluorescence microscope (LSM510; Carl Zeiss, Inc.). Images of 15 representative cells per condition were processed using LSM510 software (Carl Zeiss, Inc.) to determine the ratio of plasma membrane GLUT4 (represented by the myc signal) over the total GLUT4 (the GFP signal). The data presented were collated from three to five independent experiments. The results represent the mean ± SD for three to five independent experiments. Images were imported into Photoshop (Adobe) for processing.
Statistical analysis
The GLUT4 reporter translocation data are presented as means ± SD. The data were analyzed by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls multiple range test. Statistical analyses were made at a significance level of P < 0.05 using SPSS program version 13.0 (SPSS, Inc.). Identical letters indicate bars that are not statistically significant from each other.
Immunoblotting
Samples were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. The samples were immunoblotted with monoclonal- or polyclonal-specific antibody as indicated in figure legends. The primary antibodies were detected with HRP-conjugated anti–mouse or anti–rabbit IgG antibodies. Specific signals were visualized, scanned, and analyzed with an imaging system (Molecular Imager FX; Bio-Rad Laboratories).
GLUT4 endocytosis assay
Insulin-stimulated transfected adipocytes were chilled to 4°C and incubated with the myc monoclonal antibody for 1 h to label GLUT4 at the plasma membrane. Cells were then washed to remove insulin and excess myc antibody as described previously (Kao et al., 1998). The cells were placed at 37°C and incubated for various times to allow the myc antibody–bound GLUT4 to internalize. For restimulation experiments, after 6 h of warm-up at 37°C, cells were treated with or without 100 nM insulin for 30 min. The reactions were stopped by washing once with ice-cold PBS and fixing in 4% PFA and 0.2% Triton X-100 in PBS at room temperature for 10 min. The cells were incubated with AlexaFluor584 mouse secondary antibody at 37°C for 1 h. Translocation of myc antibody–bound GLUT4 from the plasma membrane to the intracellular pool was examined by immunofluorescence microscopy.
Plasma membrane sheet assay
Plasma membrane sheets were prepared from adipocytes as previously described (Elmendorf et al., 1998). In brief, after the appropriate treatment (as described in the figure legend), coverslips were rinsed once in ice-cold PBS and incubated with 0.5 mg/ml poly-l-lysine in PBS for 30 s. Cells were then swollen in a hypotonic buffer (23 mM KCl, 10 mM Hepes, pH 7.5, 2 mM MgCl2, and 1 mM EGTA) by three successive rinses. The swollen cells were sonicated for 3 s in sonication buffer (70 mM KCl, 30 mM Hepes, pH 7.5, 5 mM MgCl2, 3 mM EGTA, 1 mM DTT, and 0.1 mM PMSF). The bound plasma membrane sheets were washed twice with sonication buffer and used for indirect immunofluorescence as described in the Immunofluorescence and image analysis section.
2-Deoxyglucose assay
3T3L1 adipocytes were seeded in 12-well plates, and 2-deoxyglucose uptake was determined as described previously (Kanzaki and Pessin, 2001). In brief, cells were serum starved for 3 h before the assay. Cells were then washed twice with KRPH buffer (5 mM Na2HPO4, 20 mM Hepes, pH 7.4, 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, 4.7 mM KCl, and 0.1% [wt/vol] BSA) and left untreated or stimulated with 100 nM insulin or 600 mM sorbitol for 30 min. Glucose uptake was measured by incubation with 0.1 mM 2-deoxyglucose containing 1 μCi/ml 2-deoxy-d-glucose [1,2-3H] at 4°C for 5 min. Transport was terminated by washing the cells three times with ice-cold PBS. Cells were solubilized with 1% Triton X-100, and radioactivity was detected by scintillation counting. Nonspecific deoxyglucose uptake was measured in the presence of 20 μM cytochalasin B and was subtracted from each determination to obtain specific uptake. Each value was corrected for protein content.
Online supplemental material
Fig. S1 shows the time course of VAMP protein expression after siRNA-mediated gene silencing in 3T3L1 adipocytes. Fig. S2 shows quantification of the levels of siRNA-mediated VAMP protein knockdown in 3T3L1 adipocytes. Fig. S3 shows the effects of VAMP2 and 7 protein knockdowns on insulin- and osmotic shock–stimulated glucose uptake. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200709108/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Robert Watson for helpful discussions during the course of this study.
This study was supported by research grants DK33823 and DK55811 from the National Institutes of Health.
Abbreviations used in this paper: ANOVA, analysis of variance; VAMP, vesicle-associated membrane protein.
References
- Advani, R.J., H.R. Bae, J.B. Bock, D.S. Chao, Y.C. Doung, R. Prekeris, J.S. Yoo, and R.H. Scheller. 1998. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273:10317–10324. [DOI] [PubMed] [Google Scholar]
- Al-Hasani, H., R.K. Kunamneni, K. Dawson, C.S. Hinck, D. Muller-Wieland, and S.W. Cushman. 2002. Roles of the N- and C-termini of GLUT4 in endocytosis. J. Cell Sci. 115:131–140. [DOI] [PubMed] [Google Scholar]
- Bajohrs, M., F. Darios, S.Y. Peak-Chew, and B. Davletov. 2005. Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell. Biochem. J. 392:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, J.B., H.T. Matern, A.A. Peden, and R.H. Scheller. 2001. A genomic perspective on membrane compartment organization. Nature. 409:839–841. [DOI] [PubMed] [Google Scholar]
- Boehm, M., and J.S. Bonifacino. 2001. Adaptins: the final recount. Mol. Biol. Cell. 12:2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan, J.S., N. Hendon, A.E. McKee, T.S. Tsao, and H.F. Lodish. 2003. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 425:727–733. [DOI] [PubMed] [Google Scholar]
- Bose, A., A. Guilherme, S. Huang, A.C. Hubbard, C.R. Lane, N.A. Soriano, and M.P. Czech. 2005. The v-SNARE Vti1a regulates insulin-stimulated glucose transport and Acrp30 secretion in 3T3-L1 adipocytes. J. Biol. Chem. 280:36946–36951. [DOI] [PubMed] [Google Scholar]
- Cheatham, B., A. Volchuk, C.R. Kahn, L. Wang, C.J. Rhodes, and A. Klip. 1996. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc. Natl. Acad. Sci. USA. 93:15169–15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., J.S. Elmendorf, A.L. Olson, X. Li, H.S. Earp, and J.E. Pessin. 1997. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J. Biol. Chem. 272:27401–27410. [DOI] [PubMed] [Google Scholar]
- Chen, Y.A., and R.H. Scheller. 2001. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2:98–106. [DOI] [PubMed] [Google Scholar]
- Elmendorf, J.S., D. Chen, and J.E. Pessin. 1998. Guanosine 5′-O-(3-thiotriphosphate) (GTPgammaS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J. Biol. Chem. 273:13289–13296. [DOI] [PubMed] [Google Scholar]
- Galli, T., T. Chilcote, O. Mundigl, T. Binz, H. Niemann, and P. De Camilli. 1994. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J. Cell Biol. 125:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C., D.S. Chao, C.S. Kuo, and R.H. Scheller. 1997. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 89:149–158. [DOI] [PubMed] [Google Scholar]
- Holman, G.D., and I.V. Sandoval. 2001. Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol. 11:173–179. [DOI] [PubMed] [Google Scholar]
- Hong, W. 1998. Protein transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Sci. 111:2831–2839. [DOI] [PubMed] [Google Scholar]
- Hong, W. 2005. SNAREs and traffic. Biochim. Biophys. Acta. 1744:493–517. [PubMed] [Google Scholar]
- Kakhlon, O., P. Sakya, B. Larijani, R. Watson, and S.A. Tooze. 2006. GGA function is required for maturation of neuroendocrine secretory granules. EMBO J. 25:1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror, K.V., J.M. Stephens, and P.F. Pilch. 1995. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J. Cell Biol. 129:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki, M., and J.E. Pessin. 2001. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276:42436–42444. [DOI] [PubMed] [Google Scholar]
- Kanzaki, M., M. Furukawa, W. Raab, and J.E. Pessin. 2004. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 279:30622–30633. [DOI] [PubMed] [Google Scholar]
- Kao, A.W., B.P. Ceresa, S.R. Santeler, and J.E. Pessin. 1998. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 273:25450–25457. [DOI] [PubMed] [Google Scholar]
- Karylowski, O., A. Zeigerer, A. Cohen, and T.E. McGraw. 2004. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell. 15:870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, R.C., and R.H. Scheller. 2000. Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 16:19–49. [DOI] [PubMed] [Google Scholar]
- Liu, G., J.C. Hou, R.T. Watson, and J.E. Pessin. 2005. Initial entry of IRAP into the insulin-responsive storage compartment occurs prior to basal or insulin-stimulated plasma membrane recycling. Am. J. Physiol. Endocrinol. Metab. 289:E746–E752. [DOI] [PubMed] [Google Scholar]
- Mallard, F., B.L. Tang, T. Galli, D. Tenza, A. Saint-Pol, X. Yue, C. Antony, W. Hong, B. Goud, and L. Johannes. 2002. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L.B., A. Shewan, C.A. Millar, G.W. Gould, and D.E. James. 1998. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 273:1444–1452. [DOI] [PubMed] [Google Scholar]
- Martin, O.J., A. Lee, and T.E. McGraw. 2006. GLUT4 distribution between the plasma membrane and the intracellular compartments is maintained by an insulin-modulated bipartite dynamic mechanism. J. Biol. Chem. 281:484–490. [DOI] [PubMed] [Google Scholar]
- McMahon, H.T., Y.A. Ushkaryov, L. Edelmann, E. Link, T. Binz, H. Niemann, R. Jahn, and T.C. Sudhof. 1993. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 364:346–349. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., M. Sogaard, N.M. Lampen, S. Machida, R.R. Ye, L. Lacomis, P. Tempst, J.E. Rothman, and T.H. Sollner. 1997. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J. Biol. Chem. 272:17776–17783. [DOI] [PubMed] [Google Scholar]
- Mora, S., and J.E. Pessin. 2002. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 18:345–356. [DOI] [PubMed] [Google Scholar]
- Muretta, J.M., I. Romenskaia, and C.C. Mastick. 2007. Insulin releases glut4 from static storage compartments into cycling endosomes and increases the rate constant for glut4 exocytosis. J. Biol. Chem. 283:311–323. [DOI] [PubMed] [Google Scholar]
- Olson, A.L., J.B. Knight, and J.E. Pessin. 1997. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 17:2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, H.K., M. Clarke, N.J. Morris, W. Hong, L.H. Chamberlain, and G.W. Gould. 2003. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell. 14:2946–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin, J.E., D.C. Thurmond, J.S. Elmendorf, K.J. Coker, and S. Okada. 1999. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 274:2593–2596. [DOI] [PubMed] [Google Scholar]
- Pocard, T., A. Le Bivic, T. Galli, and C. Zurzolo. 2007. Distinct v-SNAREs regulate direct and indirect apical delivery in polarized epithelial cells. J. Cell Sci. 120:3309–3320. [DOI] [PubMed] [Google Scholar]
- Proctor, K.M., S.C. Miller, N.J. Bryant, and G.W. Gould. 2006. Syntaxin 16 controls the intracellular sequestration of GLUT4 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 347:433–438. [DOI] [PubMed] [Google Scholar]
- Randhawa, V.K., F.S. Thong, D.Y. Lim, D. Li, R.R. Garg, R. Rudge, T. Galli, A. Rudich, and A. Klip. 2004. Insulin and hypertonicity recruit GLUT4 to the plasma membrane of muscle cells by using N-ethylmaleimide-sensitive factor-dependent SNARE mechanisms but different v-SNAREs: role of TI-VAMP. Mol. Biol. Cell. 15:5565–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea, S., L.B. Martin, S. McIntosh, S.L. Macaulay, T. Ramsdale, G. Baldini, and D.E. James. 1998. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J. Biol. Chem. 273:18784–18792. [DOI] [PubMed] [Google Scholar]
- Ren, Q., H.K. Barber, G.L. Crawford, Z.A. Karim, C. Zhao, W. Choi, C.C. Wang, W. Hong, and S.W. Whiteheart. 2007. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol. Biol. Cell. 18:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J.E. 1994. Intracellular membrane fusion. Adv. Second Messenger Phosphoprotein Res. 29:81–96. [DOI] [PubMed] [Google Scholar]
- Sajan, M.P., G. Bandyopadhyay, Y. Kanoh, M.L. Standaert, M.J. Quon, B.C. Reed, I. Dikic, and R.V. Farese. 2002. Sorbitol activates atypical protein kinase C and GLUT4 glucose transporter translocation/glucose transport through proline-rich tyrosine kinase-2, the extracellular signal-regulated kinase pathway and phospholipase D. Biochem. J. 362:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, S., H. Nishimura, A.E. Clark, I.J. Kozka, S.J. Vannucci, I.A. Simpson, M.J. Quon, S.W. Cushman, and G.D. Holman. 1993. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 268:17820–17829. [PubMed] [Google Scholar]
- Scherer, P.E., M.P. Lisanti, G. Baldini, M. Sargiacomo, C.C. Mastick, and H.F. Lodish. 1994. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 127:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan, A.M., E.M. van Dam, S. Martin, T.B. Luen, W. Hong, N.J. Bryant, and D.E. James. 2003. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol. Biol. Cell. 14:973–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner, T., S.W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J.E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. [DOI] [PubMed] [Google Scholar]
- Steegmaier, M., J. Klumperman, D.L. Foletti, J.S. Yoo, and R.H. Scheller. 1999. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 10:1957–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond, D.C., B.P. Ceresa, S. Okada, J.S. Elmendorf, K. Coker, and J.E. Pessin. 1998. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 273:33876–33883. [DOI] [PubMed] [Google Scholar]
- Tran, T.H., Q. Zeng, and W. Hong. 2007. VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes. J. Cell Sci. 120:1028–1041. [DOI] [PubMed] [Google Scholar]
- Tsui, M.M., and D.K. Banfield. 2000. Yeast Golgi SNARE interactions are promiscuous. J. Cell Sci. 113:145–152. [DOI] [PubMed] [Google Scholar]
- Wang, C.C., C.P. Ng, L. Lu, V. Atlashkin, W. Zhang, L.F. Seet, and W. Hong. 2004. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell. 7:359–371. [DOI] [PubMed] [Google Scholar]
- Watson, R.T., and J.E. Pessin. 2001. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 56:175–193. [DOI] [PubMed] [Google Scholar]
- Watson, R.T., A.H. Khan, M. Furukawa, J.C. Hou, L. Li, M. Kanzaki, S. Okada, K.V. Kandror, and J.E. Pessin. 2004. Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 23:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs, T., S.H. Low, S.J. Chapin, K.E. Mostov, P. Bucher, and K. Hofmann. 1997. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl. Acad. Sci. USA. 94:3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler, F., and S. Tooze. 2001. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic. 2:606–611. [DOI] [PubMed] [Google Scholar]
- Yang, J., J.F. Clarke, C.J. Ester, P.W. Young, M. Kasuga, and G.D. Holman. 1996. Phosphatidylinositol 3-kinase acts at an intracellular membrane site to enhance GLUT4 exocytosis in 3T3-L1 cells. Biochem. J. 313:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer, A., M.A. Lampson, O. Karylowski, D.D. Sabatini, M. Adesnik, M. Ren, and T.E. McGraw. 2002. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell. 13:2421–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q., V.N. Subramaniam, S.H. Wong, B.L. Tang, R.G. Parton, S. Rea, D.E. James, and W. Hong. 1998. A novel synaptobrevin/VAMP homologous protein (VAMP5) is increased during in vitro myogenesis and present in the plasma membrane. Mol. Biol. Cell. 9:2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., and W. Hong. 2001. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport. J. Biol. Chem. 276:27480–27487. [DOI] [PubMed] [Google Scholar]
- Zhang, T., S.H. Wong, B.L. Tang, Y. Xu, and W. Hong. 1999. Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment. Mol. Biol. Cell. 10:435–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.