Abstract
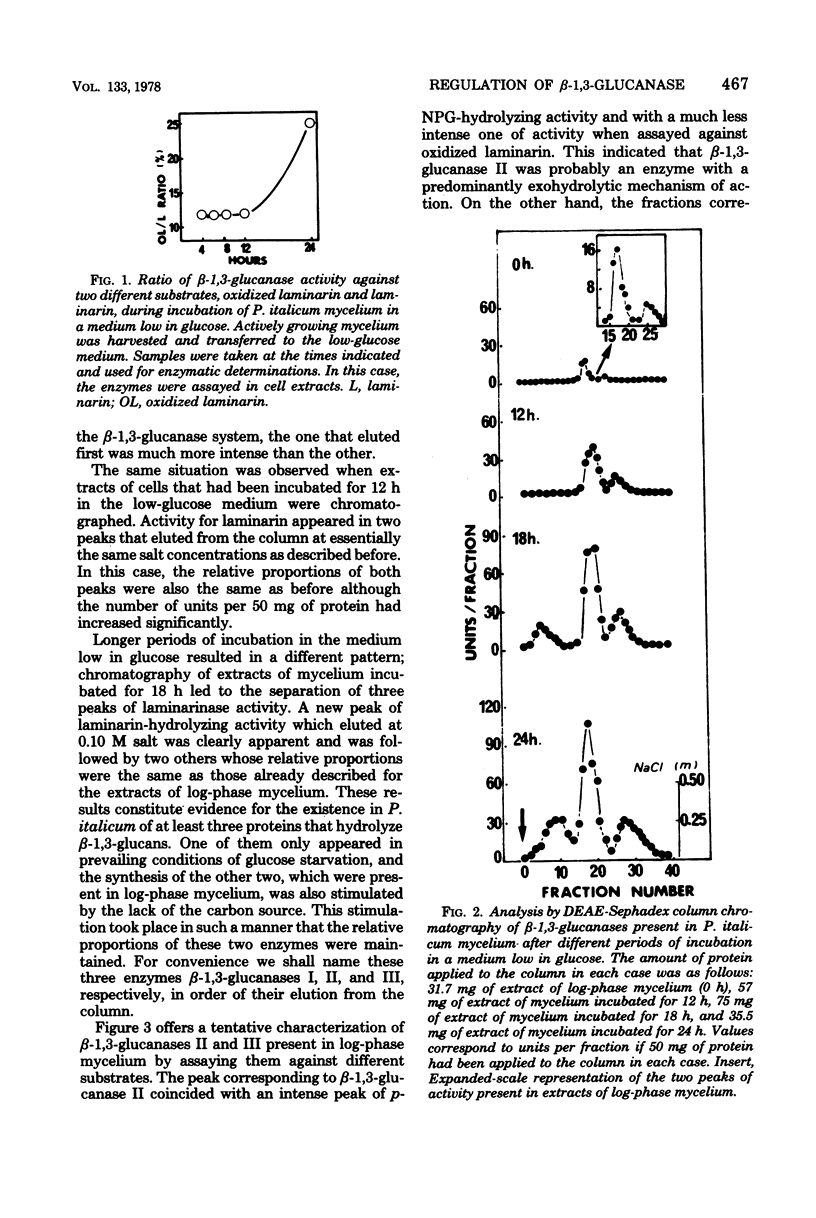
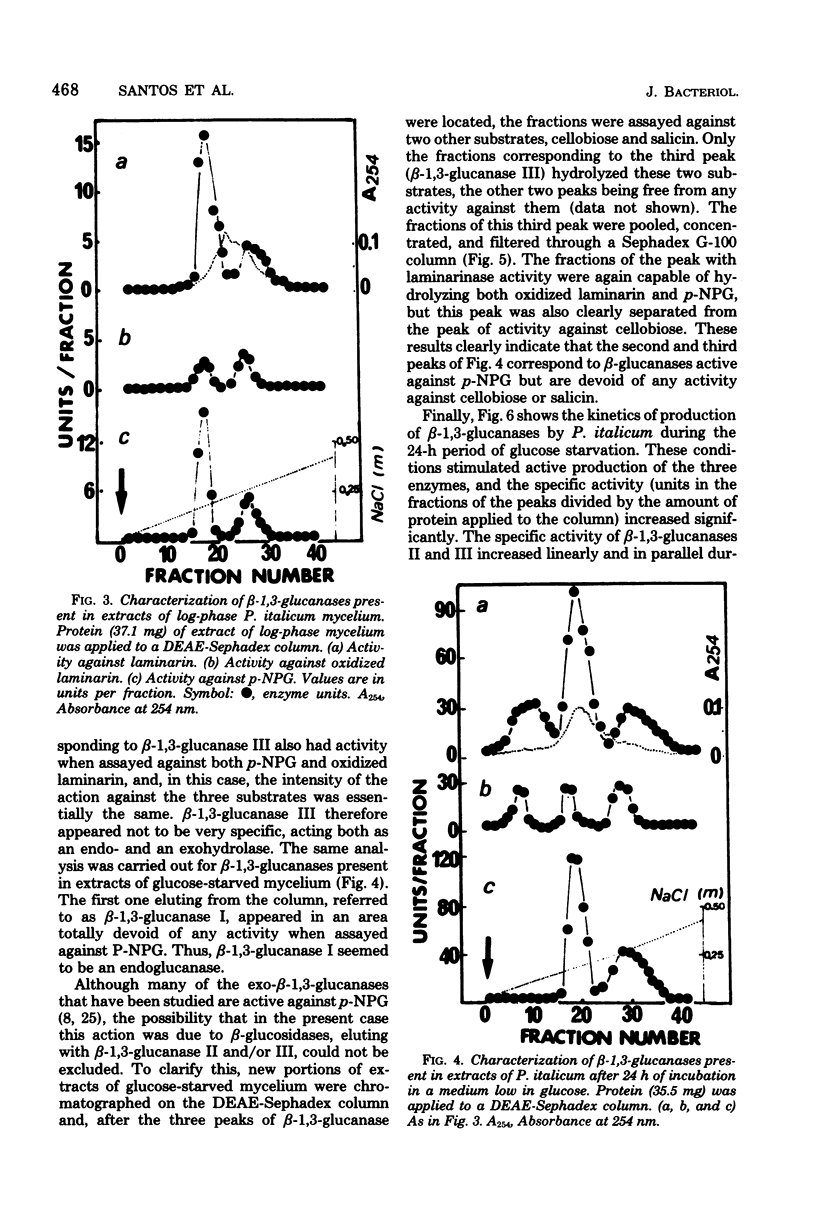
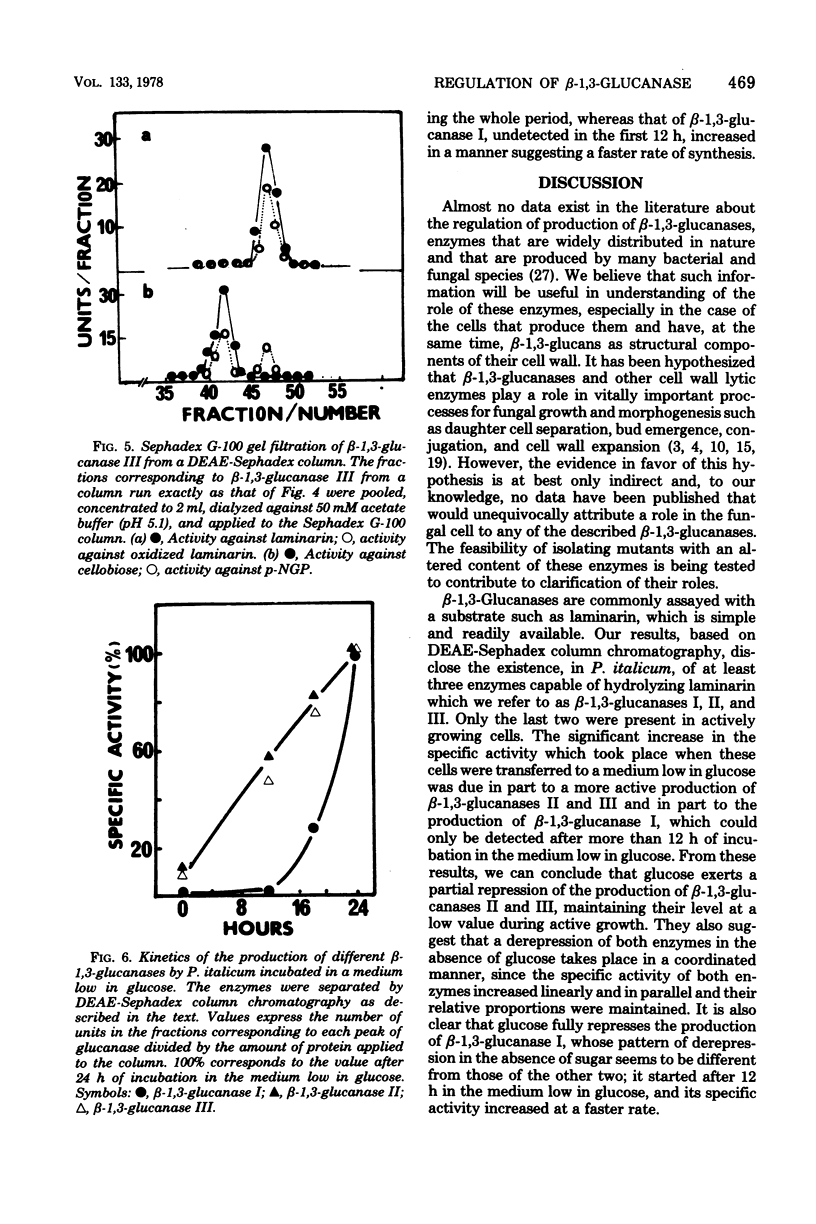
The microscopic fungus Penicillium italicum when grown in a synthetic liquid medium produced at least three enzymes with beta-1,3-glucanase activity which were separated by diethylaminoethyl-Sephadex column chromatography. These were named beta-1,3-glucanases I, II, and III respective to their order of elution from the column. A tentative characterization of these three enzymes indicated that they have different modes of action; the first one is an endoglucanase, the second is an exoglucanase, and the third probably has both mechanisms of action. Glucose had a repressive effect on all three enzymes. Only small amounts of beta-1,3-glucanases II and III were present in the cells when they were actively growing in the presence of this sugar. However, when the cells were transferred to a medium low in glucose, a significant increase in the specific activity of beta-1,3-glucanase took place; this was due in part to a much more active production of beta-1,3-glucanases II and III and in part to the appearance of beta-1,3-glucanase I, which could only be detected after more than 12 h of incubation in this medium. The results are discussed in the context of possible beta-1,3-glucanase functions in the fungal cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. The structure of the yeast cell wall. Solubilization of a marker enzyme, -fructofuranosidase, by the autolytic enzyme system. J Biol Chem. 1972 Feb 25;247(4):1161–1169. [PubMed] [Google Scholar]
- Biely P., Kovarik J., Bauer S. Lysis of Saccharomyces cerevisiae with 2-deoxy-2-fluoro-D-glucose, an inhibitor of the cell wall glucan synthesis. J Bacteriol. 1973 Sep;115(3):1108–1120. doi: 10.1128/jb.115.3.1108-1120.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Beta-glucanase of yeast. Biochem Biophys Res Commun. 1965 May 18;19(5):623–629. doi: 10.1016/0006-291x(65)90385-2. [DOI] [PubMed] [Google Scholar]
- CHESTERS C. G., BULL A. T. The enzymic degradation of laminarin. 2. The multicomponent nature of fungal laminarinases. Biochem J. 1963 Jan;86:31–38. doi: 10.1042/bj0860031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Biely P., Bauer S. Extracellular beta-glucanases of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Sep 15;321(1):246–255. doi: 10.1016/0005-2744(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- Gooday B. W. Biosynthesis of the fungal wall - mechanisms and implications. The first Fleming Lecture. J Gen Microbiol. 1977 Mar;99(1):1–11. doi: 10.1099/00221287-99-1-1. [DOI] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Holten V. Z., Bartnicki-Garcia S. Intracellular beta-glucanase activity of Phytophthora palmivora. Biochim Biophys Acta. 1972 Jul 13;276(1):221–227. doi: 10.1016/0005-2744(72)90023-x. [DOI] [PubMed] [Google Scholar]
- Huotari F. I., Nelson T. E., Smith F., Kirkwood S. Purification of an exo-beta-D-(1 bonded to 3)-glucanase from Basidiomycete species QM 806. J Biol Chem. 1968 Mar 10;243(5):952–956. [PubMed] [Google Scholar]
- Johnson B. F. Lysis of yeast cell walls induced by 2-deoxyglucose at their sites of glucan synthesis. J Bacteriol. 1968 Mar;95(3):1169–1172. doi: 10.1128/jb.95.3.1169-1172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Gordon A. H., Bacon J. S. Co-operative action by endo- and exo-beta-(1 leads to 3)-glucanases from parasitic fungi in the degradation of cell-wall glucans of Sclerotinia sclerotiorum (Lib.) de Bary. Biochem J. 1974 Apr;140(1):47–55. doi: 10.1042/bj1400047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindsay B., Glaser L. Characterization of the N-acetylmuramic acid L-alanine amidase from Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):803–811. doi: 10.1128/jb.127.2.803-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Mahadkar U. R. Role of enzymes in growth and morphology of Neurospora crassa: cell-wall-bound enzymes and their possible role in branching. J Bacteriol. 1970 Mar;101(3):941–947. doi: 10.1128/jb.101.3.941-947.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 3)-glucanases from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):121–130. doi: 10.1111/j.1432-1033.1976.tb10214.x. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Production and catabolite repression of Penicillium italicum beta-glucanases. J Bacteriol. 1977 Jan;129(1):52–58. doi: 10.1128/jb.129.1.52-58.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Halvorson H. O. A comparison of -glucanase and -glucosidase in Saccharomyces lactis. Biochim Biophys Acta. 1971 Oct;250(1):165–171. doi: 10.1016/0005-2744(71)90130-6. [DOI] [PubMed] [Google Scholar]
- Villa T. G., Notario V., Villanueva J. R. Beta-glucanases of the yeast Pichia polymorpha. Arch Microbiol. 1975 Jun 22;104(2):201–206. doi: 10.1007/BF00447325. [DOI] [PubMed] [Google Scholar]


