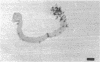
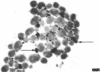
Abstract
Chitin synthesis was studied in both yeast and hyphae of the dimorphic fungus Candida albicans. Incorporation of N-acetyl-d-[1-3H]glucosamine ([3H]GluNAc) into an acid-alkali-insoluble fraction was 10 times greater in hyphal-phase cells. A crude preparation of chitin synthetase was obtained from sonically treated protoplasts of both forms of Candida. Enzyme activity, which was determined by using [14C]UDP-GLuNAc as a substrate, was exclusively associated with the 80,000 × g pellet from sonically treated protoplasts of both forms. It was determined that enzyme activity (nanomoles of [14C]UDP-GluNAc incorporated per milligram of protein) was approximately 2 times greater in hyphae versus yeast cells. Enzyme activity in both yeast and hyphae increased six- to sevenfold when the enzyme preparations were preincubated with trypsin. A vacuolar fraction, obtained from yeast cells but not from hyphae, stimulated enzyme activity when incubated with either yeast or hyphal enzyme preparations. Membrane fractions from protoplasts coated with [3H]concanavalin A before disruption were isolated by Renografin density gradient centrifugation. Chitin synthetase activity was preferentially associated with the concanavalin A-labeled fraction, suggesting that the enzyme was located on the plasma membrane. In addition, enzyme activity in protoplasts treated with cold glutaraldehyde before disruption was significantly greater than in protoplasts that were sonically disrupted and then treated with cold glutaraldehyde, indicating that the enzyme resides on the inner side of the plasma membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- Bacon J. S., Davidson E. D., Jones D., Taylor I. F. The location of chitin in the yeast cell wall. Biochem J. 1966 Nov;101(2):36C–38C. doi: 10.1042/bj1010036c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E. Molecular aspects of yeast morphogenesis. Annu Rev Microbiol. 1975;29:191–214. doi: 10.1146/annurev.mi.29.100175.001203. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marriott M. S. Isolation and chemical characterization of plasma membranes from the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1975 Jan;86(1):115–132. doi: 10.1099/00221287-86-1-115. [DOI] [PubMed] [Google Scholar]
- Partridge B. M., Drewe J. A. Candida protoplasts and their ultrastructure. Sabouraudia. 1974 Jul;12(2):166–178. [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Bartnicki-Garcia S. Proteolytic activation and inactivation of chitin synthetase from Mucor rouxii. J Gen Microbiol. 1976 Dec;97(2):241–249. doi: 10.1099/00221287-97-2-241. [DOI] [PubMed] [Google Scholar]
- Ulane R. E., Cabib E. The activating system of chitin synthetase from Saccharomyces cerevisiae. Purification and properties of the activating factor. J Biol Chem. 1976 Jun 10;251(11):3367–3374. [PubMed] [Google Scholar]