Abstract
Bcl-xL, a member of the Bcl-2 family, inhibits many pathways of apoptosis when overexpressed in the cell cytosol. We examined the capacity of Bcl-xL fusion proteins to bind cells from the outside and block apoptosis. Full-length Bcl-xL protein at micromolar concentrations did not affect apoptosis when added to cell media. To increase uptake by cells, Bcl-xL was fused to the receptor-binding domain of diphtheria toxin (DTR). The Bcl-xL–DTR fusion protein blocked apoptosis induced by staurosporine, γ-irradiation, and poliovirus in a variety of cell types when added to media. The potency of inhibition of poliovirus-induced apoptosis by Bcl-xL–DTR was greater than that of strong caspase inhibitors. Brefeldin A, an inhibitor of vesicular traffic between the endoplasmic reticulum and Golgi apparatus, prevented the Bcl-xL–DTR blockade of apoptosis induced by staurosporine, suggesting that Bcl-xL–DTR must be endocytosed and reach intracellular compartments for activity. Many diseases are caused by overexpression or underexpression of Bcl-xL homologues. Extracellular delivery of Bcl-2 family member proteins may have a wide range of uses in promoting or preventing cell death.
Keywords: diphtheria toxin, Bax, Bcl-2, immunotoxin, endocytosis
Bcl-xL and certain other members of the Bcl-2 family are powerful inhibitors of cell death. Although the mechanism of apoptosis inhibition by these proteins remains unknown, the discovery of a structural similarity between Bcl-xL and a domain of diphtheria toxin (DT) suggests that membrane permeability activity of Bcl-2 family members may be important (1).
DT three structurally and functionally distinct domains; a cell-surface receptor-binding domain, a translocation domain that allows passage of the A subunit across the cell membrane, and the A chain that ADP-ribosylates elongation factor 2 and inactivates translation. The structure of Bcl-xL resembles that of the DT translocation domain. The DT translocation domain contains several hydrophobic and amphipathic α-helices and, after insertion into cell membranes, creates voltage-dependent ion channels (2, 3). Bcl-xL (4), Bcl-2 (5, 6), and Bax (6, 7) also have been found to create voltage-dependent ion channels, and Bax can create large lipidic pores in membranes (8). Another similarity to the DT membrane translocation event (9) is that Bcl-xL and Bax change from soluble, cytosolic proteins to membrane-inserted forms during apoptosis (10–12).
The DT receptor-binding domain (DTR) can be replaced with other domains to alter the receptor-binding specificity of DT. Altering the receptor specificity can generate cell type-specific toxins and allows selective killing of cancer cells in vitro (13) and in man (14). Toxins also have been used to introduce exogenous proteins and peptides into the cell cytosol, with the binding and translocation activities of the protein toxins acting as transport systems. Peptides linked to DT (15) or to anthrax toxin (16, 17) have been delivered to the cytosol with this approach to stimulate the class I major histocompatibility complex immunity pathway. Diphtheria toxin fused to fibroblast growth factor allows cytosol delivery and stimulation of cell division (18), and a ribonuclease, barnase, was able to enter and kill cells when fused to the Pseudomonas exotoxin A-binding and -translocation domains (19).
We explored the potential to block cell death by deleting the toxic A chain from DT and substituting the DT translocation domain with the structurally similar Bcl-xL protein. Delivery of Bcl-xL protein to cells via the DT receptor-mediated uptake pathway inhibits apoptosis.
MATERIALS AND METHODS
Construction of Prokaryotic Expression Plasmids.
The human Bcl-xL gene from codon 1–233 (generously provided by Craig Thompson, University of Chicago) and the diphtheria toxin gene from codon 384–535 (DTR), containing mutations in codons 508 and 525, were amplified by PCR so that the DT mutation at codon 525 was mutated to the wild-type by the PCR primer. The two PCR products, Bcl-xL1–233 and DT384–535 (DTR), were digested with NdeI–NotI and NotI–XhoI, respectively. Bcl-xL was fused to the 5′ end of the DTR gene with a linker (GCG TAT TCT GCG GCC GCG) to encode for Ala Tyr Ser Ala Ala Ala between the two peptide domains. The two digested fragments were ligated into the prokaryotic expression vector pET16b (Novagen) cut with NdeI and XhoI (Fig. 1A). Codon 508 of DTR was mutated to the wild-type form (Phe → Ser), and the first three nucleotides (CAT) of NdeI were deleted by double-stranded, site-directed mutagenesis. As controls, human Bcl-xL (codons 1–233) and DTR (codons 384–535 of DT) genes were separately subcloned into pET16b vectors through NdeI and XhoI sites. The histidine tag and factor Xa digestion site sequences from the expression vector were upstream of Bcl-xL, DTR, and Bcl-xL–DTR coding sequences. All three expression constructs were verified by sequencing.
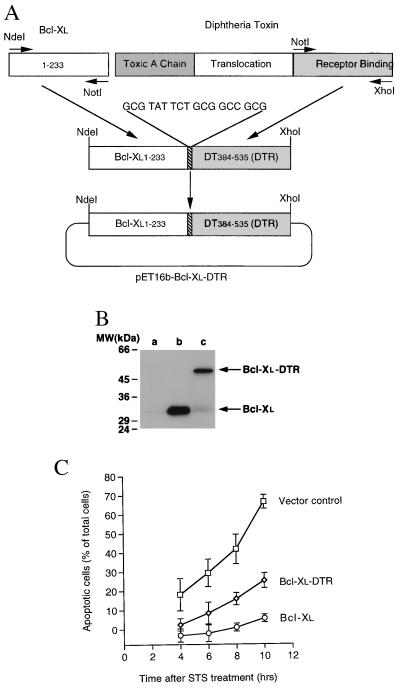
Figure 1.
Construction, Western blotting, and bioactivity of transfected Bcl-xL–DTR and Bcl-xL. (A) Schematic diagram of the chimera, Bcl-xL–DTR. The fusion gene, Bcl-xL–DTR, was inserted into the vector, pET16b yielding a histidine tag sequence at the N terminus of the Bcl-xL–DTR gene. An 18-bp linker between the hBcl-xL and DTR genes was introduced via the PCR primers (→). (B) Western blotting of the lysates of HeLa cells transiently transfected with Bcl-xL and Bcl-xL–DTR. At 20 hr after transfection with Bcl-xL–DTR or Bcl-xL genes, 106 HeLa cells were lysed in 1 ml of buffer containing 100 μg/ml leupeptin and centrifuged, and 15 μl of the supernatant was loaded onto SDS/PAGE gels, immunoblotted with anti-Bcl-xL antibody (2H12), and developed with enhanced chemiluminescence. Lane a, untransfected cells; lane b, cells transfected with Bcl-xL; lane c, cells transfected with Bcl-xL–DTR. A small amount of endogeneous Bcl-xL is present in lanes a and c. (C) Transient transfection of Bcl-xL (○) and Bcl-xL–DTR (⋄) genes into HeLa cells shows an inhibition of cell death induced by the addition of 0.8 μM STS compared with pcDNA3 vector transfected cells (□).
Construction of Eukaryotic Expression Plasmids, Transfection, and Western Blotting.
Bcl-xL–DTR and Bcl-xL genes were inserted in the eukaryotic vector pcDNA3 (Invitrogen), and the constructs were verified by sequencing. Transfection of HeLa cells with the constructs and assay of apoptosis inhibition after transient transfection were performed as reported (11). HeLa cells were harvested and lysed 20 hr after transfection, and aliquots were loaded onto SDS/10–20% PAGE gels. The plasmid-encoded proteins were visualized by immunoblotting with anti-Bcl-xL mAb (2H12, Trevigen, Gaithersburg, MD) and developed by using enhanced chemiluminescence (Amersham Pharmacia).
Protein Purification, Refolding, SDS/PAGE, and Western Blotting.
Escherichia coli BL21(DE3) strain was used to express Bcl-xL–DTR, Bcl-xL, and DTR, with addition of 1 mM IPTG when the OD260 reached 0.5–0.7. After 2 hr of incubation and lysis by French press, the inclusion bodies were collected and dissolved in 6 M guanidine⋅HCl. Histidine tag-binding resin (Novagen) was used to purify Bcl-xL–DTR, Bcl-xL, or DTR. Proteins were refolded by dialysis against, or dilution into, 100 mM Tris⋅acetate (pH 8.0)/0.5 M arginine, concentrated with PEG 15,000–20,000, and dialyzed against PBS. The three proteins were subjected to SDS/10–20% PAGE, visualized by immunoblotting with either anti-Bcl-xL monoclonal (2H12) or horse anti-DT polyclonal antibodies (Centers for Disease Control, Atlanta, GA), and developed as above.
Receptor-Binding Analysis.
Protein binding to the DT receptor was performed as reported (20) with the following modifications. DT was radiolabeled with I125 by using iodobeads (Pierce) as described by the manufacturer. Cos-7 cells, grown to confluence in 12-well Costar plates, were analyzed for receptor binding and competition by incubation for 3 hr on ice.
Poly(ADP-Ribose) Polymerase (PARP) Cleavage Detection after Bcl-xL–DTR and Staurosporine (STS) Treatment.
HeLa cells were plated in Eagle’s minimal essential medium containing 10% FBS at 2 × 105 cells per ml and treated with two different batches of Bcl-xL–DTR at 1.48 μM or 1 μM. Fifteen hours later, cells were treated again with Bcl-xL–DTR at 1.48 μM or 1 μM. Immediately after the second treatment, 0.8 μM STS was added. Three hours later, cell lysates were made, and aliquots were loaded onto SDS/PAGE, immunoblotted with anti-PARP polyclonal antibody (Boehringer Mannheim), and developed with enhanced chemiluminescence.
RESULTS AND DISCUSSION
The gene for Bcl-xL was fused to the gene for DTR. The subunit organization of the chimera was designed to resemble DT lacking the toxic A subunit (Fig. 1A). The Bcl-xL gene, including the hydrophobic C-terminal tail, was fused in-frame to the 5′ end of the gene for DT from codon 384–535 that encodes DTR (21) with a Ala Tyr Ser Ala Ala Ala spacer between the domains. The genes encoding the fusion protein Bcl-xL–DTR and Bcl-xL were subcloned into the mammalian expression vector pcDNA3, and their expression in mammalian cells was confirmed by Western blotting with an antibody to Bcl-xL after transient transfection into HeLa cells (Fig. 1B). The Bcl-xL–DTR fusion gene blocked apoptosis after transient transfection into HeLa cells (Fig. 1C) to an extent similar to that of the Bcl-xL gene after C-terminal tail truncation (11). To produce proteins for extracellular addition to cells, the Bcl-xL gene, the DTR gene, and the Bcl-xL–DTR fusion gene were cloned into pET16b. The proteins were expressed in E. coli and purified to >90% homogeneity. They were of the expected molecular weight on SDS/PAGE analysis and of the expected immunoreactivity to antibodies against Bcl-xL or DT on Western blots (data not shown).
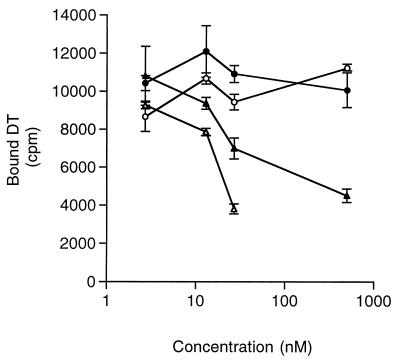
Binding of the refolded proteins to DT receptors on Cos-7 cells was examined. DT and the Bcl-xL–DTR fusion protein competed for I125-DT binding to its receptor to a similar extent, although the affinity of the chimera was one-third that of native DT (Fig. 2). Neither the Bcl-xL domain alone nor the DTR alone was able to compete for DT receptor binding. It is interesting that the more complete protein (Bcl-xL–DTR), where Bcl-xL is substituted for the DT translocation domain, folded such that DT receptor-binding activity was retained, whereas the isolated DTR did not retain binding. Addition of the DT A chain domain to the N terminus of Bcl-xL–DT receptor further increased the affinity of the chimera to the DT receptor (X.-H.L., R. Prill, and R. Youle, unpublished data).
Figure 2.
Binding the DT receptor. Competition for radiolabeled DT receptor binding. Cold competitor proteins, native DT (▵), Bcl-xL–DTR (▴), Bcl-xL (○), and DTR (●) were used to displace I125-labeled DT tracer. Native DT and Bcl-xL–DTR compete for DT receptor binding in the nanomolar concentration range.
We determined whether extracellular delivery of Bcl-xL or the Bcl-xL–DTR fusion protein could inhibit the rate of cell death by apoptosis. STS was used to induce apoptosis in Cos-7, Jurkat, U251, WEHI-7.1, 9L, and HeLa cell lines. Addition of Bcl-xL–DTR minutes before the addition of STS blocked >70% of Cos-7 cell death after 6 hr and >50% of cell death after 12 hr of STS exposure (Fig. 3A). Jurkat, HeLa, and U251 cells were also protected from STS-induced apoptosis by Bcl-xL–DTR (Table 1). Bcl-xL protein added to Cos-7 cells, however, did not alter the extent of cell death induced by STS (data not shown). A nontoxic DT mutant able to bind the DT receptor, CRM197, also had no effect on apoptosis induced by STS (data not shown). To further test the role of DT receptor binding in apoptosis inhibition, cells expressing DT receptors were compared with cells lacking DT receptors. Mouse and rat cells are thousands of times less sensitive to DT than human or monkey cell lines because of a lack of the DT receptor (22). Comparing human, monkey, mouse, and rat cell lines revealed that those cells lacking the DT receptor, WEHI-7.1 and 9L, were insensitive to apoptosis protection by Bcl-xL–DTR (Table 1). The sensitivity of the six cell lines to DT toxicity, thought to reflect DT receptor levels, correlated with sensitivity to apoptosis prevention by Bcl-xL–DTR (Table 1).
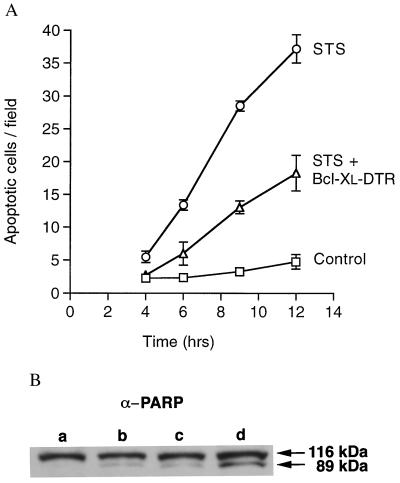
Figure 3.
Inhibition of apoptosis. (A) The time course of apoptosis induced by STS in Cos-7 cells with and without Bcl-xL–DTR protein. Cos-7 cells at 3 × 104 cells per cm2 in 100 μl of DMEM with 10% FBS were incubated with 0.1 μM STS (○), 0.1 μM STS plus 4.8 μM Bcl-xL–DTR protein added to the medium (▵), or 20 μl of PBS (□). Apoptotic cells were quantified by staining with Hoechst dye no. 33342. Results are presented as the average number of cells per field (magnification ×160). For each point, at least five fields were counted in each of at least three wells. Bcl-xL–DTR dramatically decreased the rate of apoptosis in Cos-7 cells. Six different batches of Bcl-xL–DTR were found to have activity, and the apoptosis prevention activity was stable for at least 5 months when Bcl-xL–DTR was stored at 4°C. (B) Prevention of PARP cleavage by Bcl-xL–DTR. HeLa cells were treated with two different batches of Bcl-xL–DTR at 1.48 μM or 1 μM. Fifteen hours later, cells were treated again with Bcl-xL–DTR at 1.48 μM or 1 μM. Immediately after the second treatment, 0.8 μM STS was added. Three hours later, cell lysates were made and loaded onto SDS/PAGE gels, immunoblotted with anti-PARP polyclonal antibody, and developed with enhanced chemiluminescence. Lane a, HeLa cells not incubated with STS; lane b, HeLa cells treated with STS plus 1 μM Bcl-xL–DTR protein; lane c, HeLa cells treated with STS plus 1.48 μM Bcl-xL–DTR protein; lane d, HeLa cells treated with STS.
Table 1.
Inhibition of apoptosis by Bcl-xL–DTR
| Cell line | STS concentration, μM | Concentration of Bcl-xL–DTR, μM | Time of STS treatment, hr | Apoptosis prevention, %* | DT IC50, M |
|---|---|---|---|---|---|
| Cos-7 (monkey kidney) | 0.1 | 4.8 | 12 | 58.4 | 10−12–10−11 |
| U251 (human glioma) | 0.1 | 4.68 | 16 | 57.5 | 10−12–10−11 |
| HeLa (human cervical Ca) | 0.2 | 2.17 | 10 | 32.4 | 10−12–10−11 |
| Jurkat (human T leukemia) | 0.1 | 4.68 | 12 | 21.2 | 10−9 |
| 9L (rat gliosarcoma) | 0.1 | 4.68 | 12 | −5.4 | >10−7 |
| WEHI7.1 (mouse T lymphoma) | 0.1 | 4.68 | 12 | 0.5 | >10−7 |
Apoptotic cells were counted with Hoechst dye no. 33342, and the percentage prevention from apoptosis was calculated as 1 − (number of apoptotic cells with STS and Bcl-xL–DTR − number of apoptotic cells without STS and Bcl-xL–DTR)/(number of apoptotic cells with STS − number of apoptotic cells without STS and Bcl-xL–DTR) except for the nonadherent Jurkat and WEHI7.1 cells, which were counted by using trypan blue dye exclusion and % apoptosis prevention calculated as (number of living cells with STS and Bcl-xL–DTR − number of living cells with STS)/(number of living cells without STS and Bcl-xL–DTR).
The magnitude of apoptosis inhibition by extracellular Bcl-xL–DTR (Fig. 3A, Table 1) was similar to that found by transfection of the fusion gene into cells (Fig. 1C). Although fusion to the C terminus of Bcl-xL inhibited bioactivity relative to native Bcl-xL after transfection (Fig. 1C), a very substantial prevention of cell death was obtained at both the gene level and the protein level (Fig. 3A). Thus, the delivery of Bcl-xL–DTR is efficient, and apoptosis can be prevented by delivery of Bcl-xL from the outside of cells. To confirm the results of cell death measurements by Hoechst staining and trypan blue dye exclusion, we examined caspase-induced cleavage of PARP. HeLa cells incubated with Bcl-xL–DTR showed significantly less cleavage of PARP after apoptosis induction with STS (Fig. 3B).
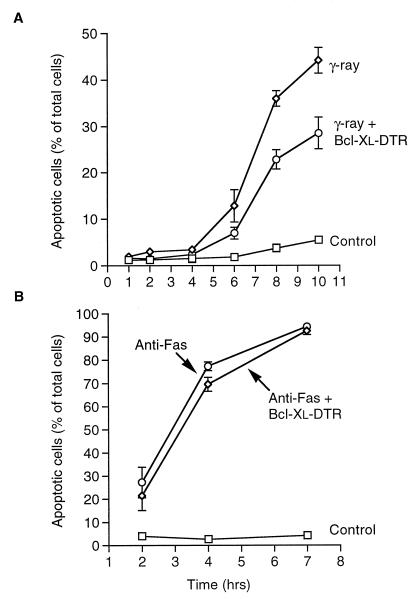
Radiation is a potent inducer of apoptosis in many hematopoietic cell types. We examined the ability of Bcl-xL–DTR to prevent radiation-induced apoptosis in the human T cell line, Jurkat. When added to the media of Jurkat cells a few minutes before induction of apoptosis by 10-gray γ-radiation, Bcl-xL–DTR blocked almost half of the ensuing cell death (Fig. 4A). In a clonogenic assay measuring long-term survival, Jurkat cells showed more than a 3-fold greater survival when Bcl-xL–DTR was added to the media immediately before 5-gray γ-radiation (data not shown). Jurkat cells are also sensitive to apoptosis induced by antibody binding to the Fas/APO-1/CD95 receptor. The Fas pathway of apoptosis is one of the few pathways shown to be less sensitive or insensitive to apoptosis protection by Bcl-2 and Bcl-xL (23–25) and contrasts with radiation-induced apoptosis in this regard. Fas antigen-induced apoptosis in Jurkat cells showed very little inhibition of apoptosis by Bcl-xL–DTR, although there was a statistically significant decrease in apoptosis between 2 and 4 hr in some experiments (Fig. 4B). The degree of protection of different apoptosis pathways by extracellular Bcl-xL–DTR corresponded with that seen by transfection with the Bcl-xL gene.
Figure 4.
Bcl-xL–DTR inhibition of apoptosis induced by γ-radiation and α-Fas antibody. At various times after induction of apoptosis by γ-radiation or α-Fas antibody, viable and apoptotic cells were counted by using Hoechst dye no. 33342. (A) Jurkat cells were plated at 105 cells per ml in serum-free RPMI-1640 medium with insulin and transferrin and γ-irradiated at 10 grays a few minutes after addition of Bcl-xL–DTR to a concentration of 4.68 μM. Control cells were not irradiated and not treated with Bcl-xL–DTR. (B) Jurkat cells were plated at 105 cells per ml in serum-free RPMI-1640 medium with insulin and transferrin, and treated with 100 ng/ml anti-Fas antibody (CH11, Upstate Biotechnology, Lake Placid, NY) minutes after addition of Bcl-xL–DTR to a concentration 4.68 μM. In contrast to irradiation-induced apoptosis of Jurkat cells, Bcl-xL–DTR had little inhibitory effect on apoptosis induced by anti-Fas antibody. Control cells were treated with PBS and no anti-Fas antibody.
Viruses induce a powerful apoptosis response in certain cells, and prevention of this apoptosis may have therapeutic utility (26). We examined poliovirus-induced apoptosis of HeLa cells for sensitivity to extracellular Bcl-xL–DTR, a system where inhibition of cell death by transfection with the Bcl-xL gene has been demonstrated (27). Adding Bcl-xL–DTR 30 min after infection of cells with low titers (multiplicity of infection of 1 plaque-forming unit per cell) of poliovirus (Fig. 5) or with moderately high titers (multiplicity of infection of 20 plaque-forming unit per cell) of poliovirus (data not shown) prevented more than half of the cell death for up to 24 hr. Addition of extracellular Bcl-xL or the DTR proteins alone had no affect on poliovirus-induced apoptosis (data not shown).
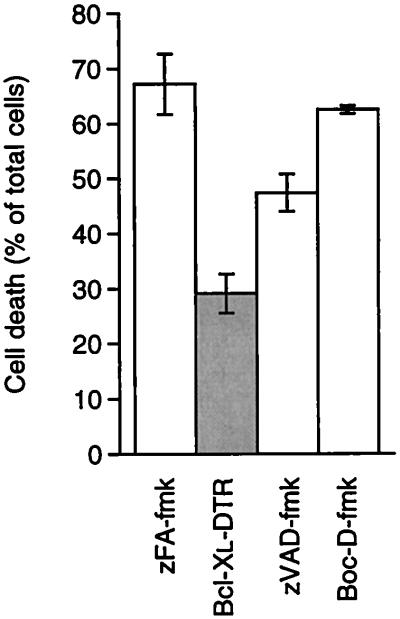
Figure 5.
Bcl-xL–DTR inhibition of apoptosis induced by poliovirus. HeLa cells were plated at a density of 1 × 105 cells per well in Eagle’s minimal essential medium containing 10% FBS and antibiotics, infected with poliovirus at a multiplicity of infection of 1 plaque-forming unit per cell as reported previously (27), and immediately treated with negative control peptide zFA-fmk at 20 μM, Bcl-xL–DTR at 0.48 μM, or peptides zVAD-fmk or Boc-D-fmk at 20 μM. Cell viability was assessed by trypan blue dye exclusion 24 hr after addition of virus. zFA-fmk, zVAD-fmk, and Boc-D-fmk were purchased from Enzyme Systems Products, Livermore, CA.
Caspase inhibitors block many pathways of apoptosis and are being explored for pharmacologic potential to inhibit cell death (28). zVAD-fmk and Boc-D-fmk are powerful, low-specificity caspase inhibitors that block many apoptosis pathways (29). We compared the apoptosis-inhibition activity of zVAD-fmk and Boc-D-fmk with that of Bcl-xL–DTR. Bcl-xL–DTR at 0.48 μM blocked cell death to a greater extent than either zVAD-fmk or Boc-D-fmk at 20 μM (Fig. 5). Bcl-xL–DTR showed a strong inhibition of a potent and pathologically important apoptosis pathway.
DT is endocytosed by cells and reaches low-pH intracellular compartments. The low pH triggers a conformational change in the translocation domain that allows this domain to insert into membranes and form channels. The toxicity of DT is blocked by lysosomotropic agents such as chloroquine, which increase the pH of intracellular compartments. Chloroquine at a concentration that blocks DT toxicity (10 μM) did not block the activity of Bcl-xL–DTR to inhibit poliovirus-induced cell death (data not shown). Thus, the mechanism of membrane interaction of Bcl-xL–DTR differs to some extent from that of DT. This was consistent with the observation that Bcl-2 family member insertion into membranes (10) was not triggered by low pH (Y.-T. Hsu and R.Y., unpublished data). However, brefeldin A, an inhibitor of vesicle traffic between the endoplasmic reticulum and the Golgi apparatus (30, 31), does block the anti-apoptosis activity of Bcl-xL–DTR (Table 2). These results indicate that Bcl-xL–DTR must be endocytosed and suggest that Bcl-xL–DTR must reach the Golgi apparatus or the endoplasmic reticulum to prevent cell death. The subcellular location from which native Bcl-2 family members regulate apoptosis is currently under scrutiny (11). Several intracellular membrane locations, including the endoplasmic reticulum, appear able to mediate Bcl-2-family regulation of cell death (32). Bcl-xL may reach the endoplasmic reticulum to translocate into the cell cytosol, or perhaps Bcl-xL, when bound closely to a membrane, can insert into that membrane and inhibit apoptosis in the membrane-intercalated form.
Table 2.
Brefeldin A prevents the Bcl-xL–DTR blockade of apoptosis
| Compound | Cell death, % | Protection, % |
|---|---|---|
| PBS (control) | 1 | |
| STS | 24 | |
| STS + Bcl-xL–DTR | 11 | |
| Bcl-xL–DTR | — | 56 |
| Brefeldin A | 2 | |
| STS + brefeldin A | 35 | |
| STS + brefeldin A + Bcl-xL–DTR | 32 | |
| Bcl-xL–DTR + brefeldin A | — | 9 |
Apoptotic cells were counted with Hoechst dye no. 33342 14 hr after addition of STS and/or brefeldin A minutes after Bcl-xL–DTR was added to Cos-7 cells. STS was used at 0.1 μM; Bcl-xL–DTR was used at 2.24 μM; brefeldin A was used at 2 μM.
Genetic overexpression of Bcl-2 has been shown to block apoptosis in the nervous system of transgenic mice and would have potential for disease therapy if delivery to target tissues was possible (28, 33). We find that a Bcl-xL fusion protein can be directed to the cell surface and will block apoptosis induced by STS, radiation, and poliovirus. Proteins can be delivered effectively throughout the body and targeted to select tissues and cells. Fusing various binding domains to Bcl-xL may allow targeting of specific subsets of cells in vivo. Interestingly, Bcl-xL appears to act at an early step in the cell death pathway when intervention can permit long-term viability of cells, whereas caspase inhibitors appear to work relatively more downstream in the apoptosis pathway (34–36). Bcl-xL has the potential to block apoptosis before cell damage becomes detectable. The delivery of other Bcl-2 homologues to the cell surface has intriguing potential to regulate cell viability either positively, as shown here, or negatively, by the use of a proapoptotic member of the Bcl-2 family such as Bax.
Acknowledgments
We thank Dr. Yi-Te Hsu for valuable discussions, Drs. Motoshi Suzuki, Xu-Guang Xi, and Amotz Nechushtan for technical assistance, and Drs. Pierre Henkart, Josh Zimmerberg, and John Heiss for critical reading of the manuscript.
ABBREVIATIONS
- DT
diphtheria toxin
- DTR
DT receptor-binding domain
- PARP
poly(ADP-ribose) polymerase
- STS
staurosporine
References
- 1.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, et al. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 2.Kagan B L, Finkelstein A, Colombini M. Proc Natl Acad Sci USA. 1981;78:4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donovan J J, Simon M I, Draper R K, Montal M. Proc Natl Acad Sci USA. 1981;78:172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 5.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlesinger P H, Gross A, Yin X M, Yamamoto K, Saito M, Waksman G, Korsmeyer S J. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, et al. Science. 1997;227:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 8.Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood K A, Hsu Y, Zimmerberg J, Youle R J. Proc Natl Acad Sci USA. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan H, OH, K J, Shin Y K, Hubbell W L, Collier R J. Biochemistry. 1995;34:4856–4863. doi: 10.1021/bi00014a043. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y-T, Wolter K G, Youle R J. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolter K G, Hsu Y-T, Smith C L, Nechushtan A, Xi X-G, Youle R J. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross A, Jockel J, Wei M C, Korsmeyer S J. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorpe P E, Ross W C, Cumber A J, Hinson C A, Edwards D C, Davies A J. Nature (London) 1978;271:752–755. doi: 10.1038/271752a0. [DOI] [PubMed] [Google Scholar]
- 14.Laske D W, Youle R J, Oldfield E H. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 15.Stenmark H, Moskaug J O, Madshus I H, Sandvig K, Olsnes S. J Cell Biol. 1991;113:1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballard J D, Doling A M, Beauregard K, Collier R J, Starnbach M N. Infect Immun. 1998;66:615–619. doi: 10.1128/iai.66.2.615-619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanke S R, Milne J C, Benson E L, Collier R J. Proc Natl Acad Sci USA. 1996;93:8437–8442. doi: 10.1073/pnas.93.16.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedlocha A, Falnes P O, Madshus I H, Sandvig K, Olsnes S. Cell. 1994;76:1039–1051. doi: 10.1016/0092-8674(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 19.Prior T I, FitzGerald D J, Pastan I. Biochemistry. 1992;31:3555–3559. doi: 10.1021/bi00129a001. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield L, Johnson V G, Youle R J. Science. 1987;238:536–539. doi: 10.1126/science.3498987. [DOI] [PubMed] [Google Scholar]
- 21.Greenfield L, Bjorn M J, Horn G, Fong D, Buck G A, Collier R J, Kaplan D A. Proc Natl Acad Sci USA. 1983;80:6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pappenheimer A M., Jr Harvey Lect. 1982;76:45–73. [PubMed] [Google Scholar]
- 23.Boise L H, Thompson C B. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu V K, Walsh C M, Liu C C, Reed J C, Clark W R. J Immunol. 1995;154:2023–2032. [PubMed] [Google Scholar]
- 25.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 26.Hardwick J M. Adv Pharmacol. 1997;41:295–336. doi: 10.1016/s1054-3589(08)61063-7. [DOI] [PubMed] [Google Scholar]
- 27.Castelli J C, Hassel B A, Wood K A, Li X-L, Amemiya K, Dalakas M C, Torrence P F, Youle R J. J Exp Med. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D-F, Schneider G E, Martinou J-C, Tonegawa S. Nature (London) 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 29.Henkart P A. Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 30.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner R D. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 31.Hunziker W, Whitney J A, Mellman I. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- 32.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 33.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 34.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 35.Xiang J, Chao D T, Korsmeyer S J. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller T M, Moulder K L, Knudson C M, Creedon D J, Deshmukh M, Korsmeyer S J, Johnson E M., Jr J Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]