Abstract
Potassium-selective leak channels control neuromuscular function through effects on membrane excitability. Nonetheless, their existence as independent molecular entities was established only recently with the cloning of KCNKØ from Drosophila melanogaster. Here, the operating mechanism of these 2 P domain leak channels is delineated. Single KCNKØ channels switch between two long-lived states (one open and one closed) in a tenaciously regulated fashion. Activation can increase the open probability to ∼1, and inhibition can reduce it to ∼0.05. Gating is dictated by a 700-residue carboxy-terminal tail that controls the closed state dwell time but does not form a channel gate; its deletion (to produce a 300-residue subunit with two P domains and four transmembrane segments) yields unregulated leak channels that enter, but do not maintain, the closed state. The tail integrates simultaneous input from multiple regulatory pathways acting via protein kinases C, A, and G.
Keywords: background conductance; 2 P domain; protein kinases C, A, and G; open rectifier ; ORK1
INTRODUCTION
Potassium currents that develop without delay in response to voltage steps and pass current across the physiological voltage-range are called leak (or background) conductances when recorded in native cells (Goldman 1943; Hodgkin and Katz 1949; Hodgkin et al. 1952; Hille 1975; Adams et al. 1980). Their existence as unique molecular transport entities, rather than accumulations of residual flux through known pathways has been questioned, even as they were thought to be key to the activity of sympathetic ganglia (Jones 1989; Koyano et al. 1992), invertebrate axons (Chang 1986), vertebrate myelinated axons (Schmidt and Stampfli 1966; Hille 1973; Baker et al. 1987; Koh et al. 1992; Wu et al. 1993), and cardiac myocytes (Apkon and Nerbonne 1988; Yue and Marban 1988; Boyle and Nerbonne 1992; Backx and Marban 1993; Wang et al. 1993; Van Wagoner et al. 1997). Native leak channels operate under the tight regulation of agents as disparate as molecular oxygen, cyclic ATP, noradrenaline, serotonin, and γ-aminobutyric acid, and serve to establish the resting membrane potential and modify the duration, frequency, and amplitude of action potentials (Siegelbaum et al. 1982; Shen et al. 1992; Buckler 1997; Wagner and Dekin 1997; Talley et al. 2000).
Cloning and expression of KCNKØ (previously ORK1) of Drosophila nerves and muscles revealed a leak-type channel that functions like an open, potassium-selective portal in an electric field (Goldstein et al. 1996; Ilan and Goldstein 2000). KCNKØ subunits are predicted to have 1,001 amino acids, two P domains, four membrane-spanning segments (2P/4TM) and an extensive carboxy-terminal tail, constituting approximately three fourths of the channel protein (Goldstein et al. 1999). Genes for 2P/4TM subunits were first recognized in the genome of a nematode (Ketchum et al. 1995; Wei et al. 1996) and now number >50; they are designated as KCNK genes encoding KCNK proteins (Goldstein et al. 1998). Mammalian relatives of KCNKØ are enumerated KCNK1-9 ; those isolates that function are leak channels like KCNKØ, i.e., they are open across the physiological voltage range and show open, outward or inward rectification (Fink et al. 1996a, Fink et al. 1998; Lesage et al. 1996; Duprat et al. 1997; Goldstein et al. 1998; Kim et al. 1998; Leonoudakis et al. 1998; Lopes et al. 1998; Reyes et al. 1998; Manjunath et al. 1999; Pountney et al. 1999; Salinas et al. 1999; Lopes et al. 2000; Bockenhauer et al. 2000).
Although single KCNKØ channel currents develop without delay in response to voltage steps (as expected for an open, nonvoltage-gated channel), constant field current formulations (Goldman 1943; Hodgkin et al. 1952) are inadequate to describe their function (Ilan and Goldstein 2000). Rather, single KCNKØ channels exhibit attributes like channels formed with single P loop subunits (Neyton and Miller 1988; Doyle et al. 1998) including the following: concentration-dependent unitary conductance; an Eisenman type IV relative permeability series; anomalous mole fraction behavior; and pore occlusion by barium (Ilan and Goldstein 2000). These findings are consistent with ion–ion and ion–channel interactions in a multi-ion pore, and indicate that ion permeation in 2 P domain leak channels and classical potassium channels (formed with one P domain subunits) proceeds by similar mechanisms.
Here, we show that the opening and closing of KCNKØ is strictly regulated. Single KCNKØ channels are seen to open in long-lived bursts lasting many minutes and to enter an equally long-lasting closed conformation (Clong) in a voltage-independent fashion (Ilan and Goldstein 2000); the basis for regulated activity of KCNKØ is found to be tight control over the frequency and duration of visits to Clong. KCNKØ subunits are shown to have two functional domains: an ∼300-residue amino-terminal segment that is pore-forming, and an ∼700 carboxy-terminal tail that mediates dwell time in Clong. The tail is found to be essential only for regulation: its deletion yields fully functional channels that enter Clong but cannot remain closed. Finally, regulated gating is seen to employ multiple second messenger pathways that utilize distinct carboxy-terminal KCNKØ residues and act separately or concurrently. Despite their functional and structural independence, pathways using PKC, PKA, and PKG all serve to control dwell time in the closed state Clong.
MATERIALS AND METHODS
Molecular Biology
The cloning and sequence of KCNKØ (previously ORK1) has been described (Goldstein et al. 1996, Goldstein et al. 1999). cRNA was synthesized using T7 polymerase and the mMESSAGE mMACHINE™ system (Ambion). Site-directed mutagenesis was performed with the QuickChange™ system (Stratagene). Mutations were verified by automated DNA sequencing. Deletion mutants were produced by creation of an in-frame stop signal at a native restriction site or by site-directed mutagenesis.
Electrophysiology
Xenopus laevis oocytes were isolated and injected with 46 nl containing 0.2–2 ng cRNA. Whole-cell currents were measured 1–3 d after injection by two-electrode voltage clamp (Warner Instruments Corp.). Data were filtered at 1 kHz and sampled at 4 kHz. The patch-clamp technique was used to record single channels in on-cell patches 2–4 d after cRNA injection using an EPC-9 amplifier (HEKA Elektronik) and stored on videocassettes. For analysis, records were sampled at 20 kHz or 940 Hz using ACQUIRE software (Bruxton Corporation, Inc.) and digitally filtered at 3 kHz or 100 Hz, respectively. Kinetic analyses were performed on patches judged to contain only one channel on the basis of the single current level. Closed and open durations were determined using a half-amplitude threshold-detected technique (Colquhoun and Sigworth 1995) implemented using TAC single-channel analysis software (Bruxton Corp.). Dwell-time distributions were plotted on a logarithmic time axis with a square-root vertical axis to allow better discernment of event populations (Sigworth and Sine 1987). The dwell-time histograms were fitted with TAC software to sums of exponential probability density functions using a maximum likelihood method with compensation to correct for missed events (Colquhoun and Sigworth 1995). Closures lasting >2 s were used to define the end of each burst (and entry into the long-lasting closed state). Means and standard errors are given where applicable.
Unless otherwise noted, the bath solution for two-electrode voltage clamp experiments contained (in mM): 20 KCl, 78 NaCl, 1 MgCl2, 0.3 CaCl2, 5 HEPES, pH 7.5, with NaOH. For patch-clamp experiments, both pipet and bath solutions contained (in mM): 140 KCl, 2 MgCl2, 5 EGTA, 5 HEPES, pH 7.4, with KOH. All kinase modulators were purchased from Calbiochem-Novabiochem. All experiments were conducted at room temperature.
RESULTS
Modulators of Protein Kinase C Regulate Activity of KCNKØ Channels
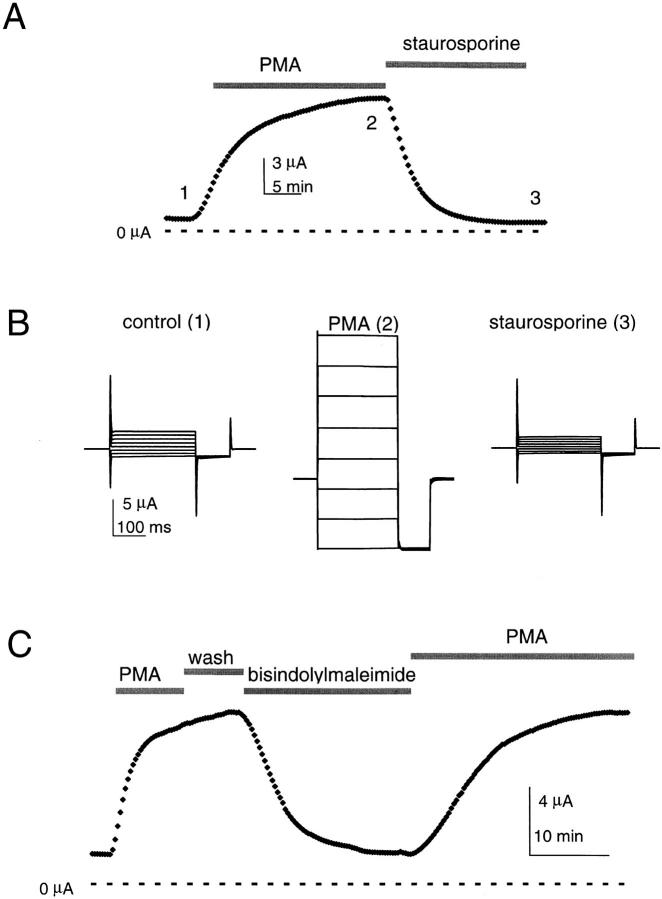
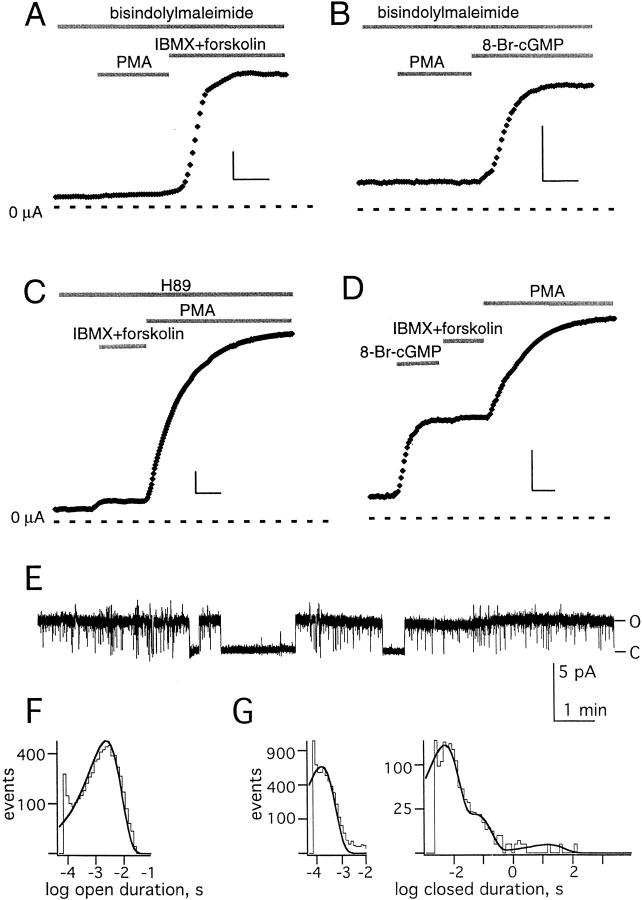
KCNKØ subunits contain 42 canonical consensus sequences for phosphorylation in the 700-residue carboxy-terminal tail region, which is predicted to be intracellular; as 11 sites are for PKC, the PKC activator PMA was evaluated. When KCNKØ was expressed in Xenopus laevis oocytes, exposure to 50 nM PMA had a dramatic effect on channel activity, increasing mean whole-cell currents up to 11-fold (Fig. 1A and Fig. B). Similar results were produced by steady exposure to 5 or 100 nM PMA for 10 or 20 min (not shown, n = 8–10). Conversely, the inactive PMA analogue 4α-phorbol-12,13-didecanoate had no effect (500 nM, n = 10; not shown). When PMA-treated cells were bathed in a nonspecific protein kinase inhibitor (2 μM staurosporine), the current augmentation was reversed (Fig. 1A and Fig. B). Similarly, bisindolylmaleimide I (4 μM), a specific PKC inhibitor, reversed the effect of PMA treatment (Fig. 1 C). After staurosporine or bisindolylmaleimide I, reexposure to PMA again increased KCNKØ channel currents (Fig. 1 C).
Figure 1.
Modulators of PKC determine KCNKØ current magnitude. Macroscopic KCNKØ channels currents measured by two-electrode voltage clamp in 20 mM potassium solution with or without 50 nM PMA or 2 μM staurosporine (see materials and methods). (A) Currents measured during the application of PMA and then staurosporine; the oocyte was held at −40 mV and stepped to 25 mV for 250 ms with a 20-s interpulse interval. The response to PMA varied among batches of oocytes from 3–11-fold; this appeared to reflect different levels of prior activation as a consistent 10 ± 1-fold increase in currents was observed when PMA and staurosporine treatments were compared (mean ± SEM, n = 10). Zero current is indicated. (B) Raw current traces for the oocyte in A at various times as follows: (1) control solution; (2) after 20 min in PMA; and (3) after 20 min in staurosporine. The cell was held at −80 mV and studied at these times by 250-ms steps from−150 to 60 mV in 30-mV increments, and then studied for 75 ms at −150 mV with a 2-s interpulse interval. (C) Currents measured at 25 mV by the protocol in A during 10-min activation by 50 nM PMA, 6-min wash with control solution, 2-min inhibition by 4 μM bisindolylmaleimide I, and then a 20-min reactivation by 50 nM PMA. Zero current is indicated.
Upregulation did not change the attributes of macroscopic KCNKØ currents; they continued to develop instantaneously in response to changes in membrane voltage and were noninactivating (Fig. 1 B). Activated KCNKØ channels were also unchanged in their selectivity for potassium over sodium; a 10-fold increase in bath potassium concentration (achieved by isotonic substitution for sodium) altered whole-cell reversal potentials by 52 ± 1 mV in PMA or control solution (n = 4).
Single KCNKØ Channels Occupy Long-lived Open Burst and Closed States
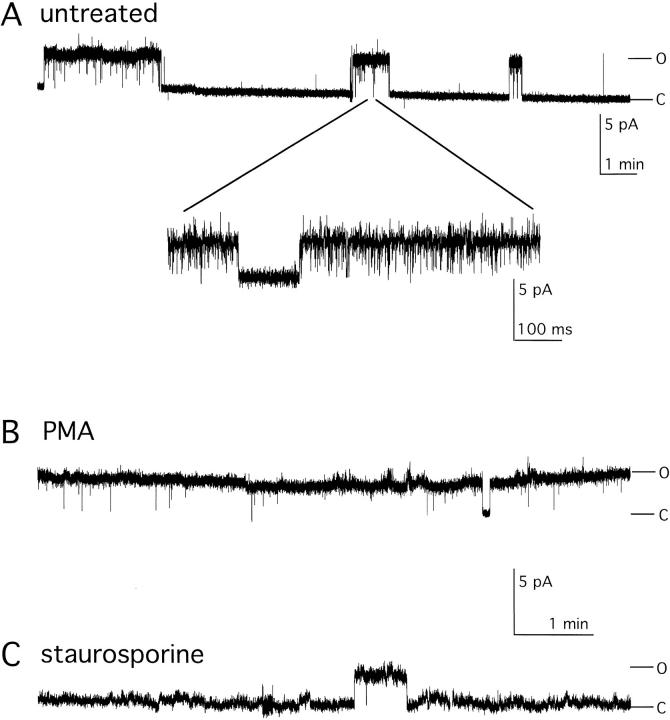
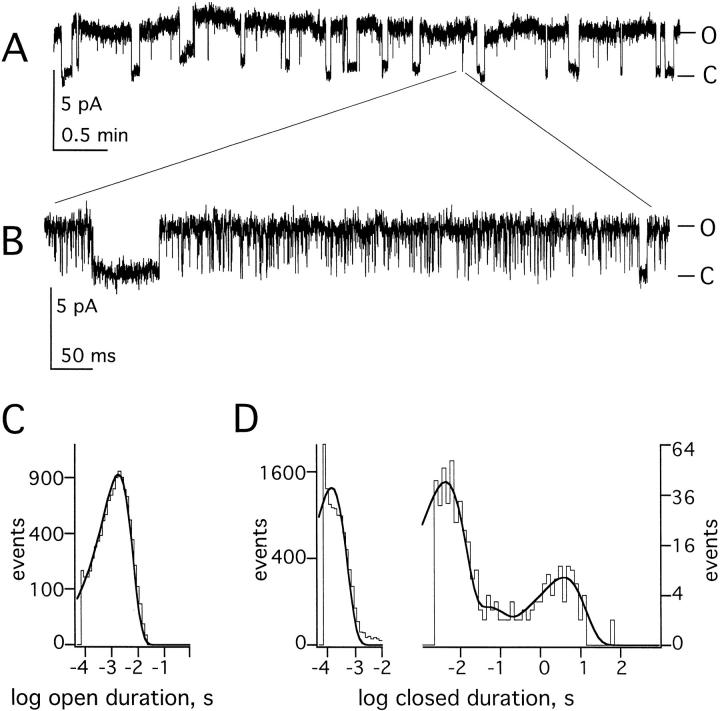
To assess the mechanism by which PKC activators and inhibitors altered macroscopic KCNKØ currents, single channels were studied. Channel behavior was first evaluated in untreated cells. Fig. 2 A shows a single KCNKØ channel in an on-cell patch held at 60 mV for 17 min. The record reveals transitions of the channel between long-lived open burst and closed conformations that last for many minutes. Expanding a portion of the record during an open burst (Fig. 2 A, inset) reveals the presence of short intraburst closures that are quantified below. The mean duration of open bursts (Οburst) and long-lived closures (Clong) were ∼50 and ∼70 s, respectively (Table ). KCNKØ channels in untreated cells were open approximately one third of the time (open probability, Po, Table ).
Figure 2.
Regulation of single KCNKØ channels. Single KCNKØ channels were studied in on-cell patches held at 60 mV with 140 mM potassium solution in the absence or presence of PMA or staurosporine (see materials and methods). Open (O) and closed (C) state levels are indicated. Unitary current amplitudes of wild-type KCNKØ channels studied in control, PMA, and staurosporine solutions were indistinguishable (3.23 ± 0.04, 3.22 ± 0.07, and 3.24 ± 0.06 pA, respectively, mean ± SEM, filtered at 2 kHz, n = 3). (A) Single KCNKØ channel in control solution filtered at 20 Hz. (Inset) Expanded trace from the indicated region filtered at 2 kHz. (B) Single KCNKØ channel after 20-min incubation with 50 nM PMA filtered at 20 Hz. (C) Single KCNKØ channel after 20-min incubation with 2 μM staurosporine filtered at 20 Hz.
Table 1.
Dwell Times for Open and Closed States of Single KCNKØ Channels
| Channel, condition | Total | Po | Oburst | Otime | Clong | Cshort1 | Cshort2 | Cshort3 |
|---|---|---|---|---|---|---|---|---|
| min | s | ms | s | ms | ms | ms | ||
| Wild type, Control | 41 | 0.320 ± 0.080 | 50 ± 17 | 2.29 ± 0.03 | 71.40 ± 17.50 | 101 ± 22 | 3.9 ± 0.4 | 0.147 ± 0.006 |
| (5 ± 3%) | (10 ± 3%) | (85 ± 6%) | ||||||
| Wild type, PMA | 54 | 0.993 ± 0.003 | >100 | 2.47 ± 0.03 | 2.39 ± 0.26 | 80 ± 11 | 3.8 ± 0.3 | 0.138 ± 0.003 |
| (1 ± 0.1%) | (10 ± 2%) | (89 ± 2%) | ||||||
| Wild type, Staurosporine | 29 | < 0.05* | 9 ± 4* | 2.30 ± 0.30 | 45 to >150* | 99 ± 50* | 3.5 ± 0.3 | 0.160 ± 0.010 |
| Wild type, IBMX + forskolin | 45 | 0.750 ± 0.060 | 100+ | 2.42 ± 0.05 | 16.80 ± 0.25 | 64 ± 10 | 5.0 ± 0.7 | 0.164 ± 0.005 |
| (1 ± 0.2%) | (11 ± 1%) | (88 ± 5%) | ||||||
| Δ299-1001, Control | 23 | 0.770 ± 0.020 | 16 ± 2 | 2.00 ± 0.04 | 2.80 ± 0.40 | 54 ± 6 | 4.2 ± 0.6 | 0.142 ± 0.004 |
| (15 ± 3%) | (8 ± 1%) | (77 ± 4%) | ||||||
| S270LΔ872-1001, Control | 64 | 0.040 ± 0.010 | 10 ± 3 | 2.40 ± 0.05 | 60.00 ± 11.00 | 200 ± 40 | 4.7 ± 0.5 | 0.160 ± 0.050 |
| (30 ± 5%) | (6 ± 2%) | (64 ± 6%) |
*As channels in the presence of staurosporine revealed few transitions (nine closures and five bursts with four channels over 29 min) values are coarse approximations. Kinetics parameters were obtained by fitting dwell-time distributions from four or five single channels. Duration was determined by a half-amplitude threshold criterion, and τ values were determined using a binned-maximum likelihood method. Po, Oburst, Clong, Cshort1, and Cshort2 were analyzed from long records filtered at 100 Hz. Otime and Cshort3 were obtained by analysis of short portions of open burst records filtered at 3 kHz. The relative frequency of the three longest closed times are reported in parentheses. Closures longer than 2 s were used to define the end of an open burst. The absolute frequency of closures lasting >2 s was 0.6, 0.07, and 2.1 min−1 for wild type/control, wild type/PMA, and Δ299-1001/control recordings, respectively.
Residence of Single KCNKØ Channels in the Long-lived Closed State Is Regulated
Single KCNKØ channels behaved quite differently when treated with 50 nM PMA (Fig. 2 B). The PKC activator virtually eliminated residence in the long-lived closed state. The open probability for single KCNKØ channels during exposure to PMA was 0.993 ± 0.003 (Table ). Conversely, application of staurosporine depressed the open probability to <0.05 by increasing the duration of long closures and decreasing the frequency and duration of open channel bursts (Table ).
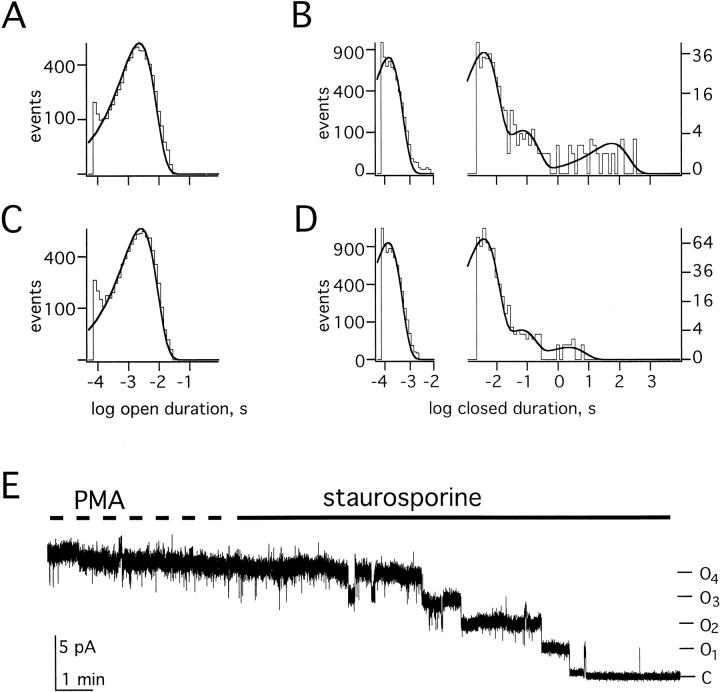
To further explore the influence of PMA on channel state, recordings of single KCNKØ channels were first analyzed at a bandwidth of 100 Hz, and then reanalyzed at 3 kHz to assess brief closed times. Closed time distributions in the absence of the PMA were best fit by four time constants (Fig. 3 B); these represented three brief closed states present in open bursts and the long-lived closed states (Clong) that separated open bursts. Channels in untreated cells showed mean dwell times for closures within bursts of 0.14, 4, and 100 ms while the time constant for the long-lived interburst closed state was 71.4 s (Table ). Dwell times for openings within bursts were well-fit by a single time constant of 2 ms (Fig. 3 A) and multichannel patches showed no evidence for additional open states. The mean open burst duration for KCNKØ channels in untreated cells was 50 s (Table ). After PMA treatment, closed times were also well-fit by four exponential components (Fig. 3 D). PMA did not change the mean duration of the three intraburst closed states or alter the mean open time (Table ). However, PMA reduced the frequency of long-lived closures 8-fold and their duration 30-fold (Table ).
Figure 3.
Regulation alters occupancy of the long-lived closed state. Single KCNKØ channels were studied as in Fig. 2. (A) Open time histogram obtained from 17 s of single KCNKØ channel recordings filtered at 3 kHz under control conditions. (B) Closed time histograms under control conditions; (left) obtained from 17 s of single KCNKØ channel recordings filtered at 3 kHz; (right) obtained from 41 min of recordings of single KCNKØ channels filtered at 100 Hz. Four channel records were combined for this analysis. (C) Open time histogram obtained from 22 s of single KCNKØ channel recordings filtered at 3 kHz in the presence of PMA. (D) Closed time histograms in the presence of PMA: (left) obtained from 22 s of single KCNKØ channel recordings filtered at 3 kHz; and (right) obtained from 54 min of recordings of single KCNKØ channels filtered at 100 Hz. Four channel records were combined for this analysis. (E) Four KCNKØ channels were recorded in an on-cell patch at 60 mV with PMA in the bath and then in staurosporine as indicated. Data were sampled at 940 Hz and filtered at 20 Hz.
To assess the effect of staurosporine, patches were first treated with PMA to confirm that only a single KCNKØ channel was present. 1–4 min after PMA was replaced by staurosporine, profound current suppression was observed. Four single KCNKØ channels, studied for a total of 29 min, demonstrated just five open bursts (that were approximately fivefold shorter than open bursts under control conditions) and nine long closures that lasted no less than 45 s with four longer than 150 s (the time when the experiment was concluded by discarding the patch or reexposure to PMA). This offered a rough estimate for open probability of <0.05 (Table ).
No change in the single channel current amplitude was observed in >100 min of the recording of single KCNKØ channels in control, PMA-exposed, or staurosporine-treated cells (Fig. 2). This argued that changes in macroscopic currents were due to altered gating of KCNKØ channels. Consistent with this conclusion was the uniform stepwise decrease in the current observed when four KCNKØ channels in one patch were first fully activated by PMA and subsequently driven into the long-lived closed state by exposure to staurosporine (Fig. 3 E).
The Carboxy-terminal Portion of KCNKØ Is Required to Regulate Long Closures
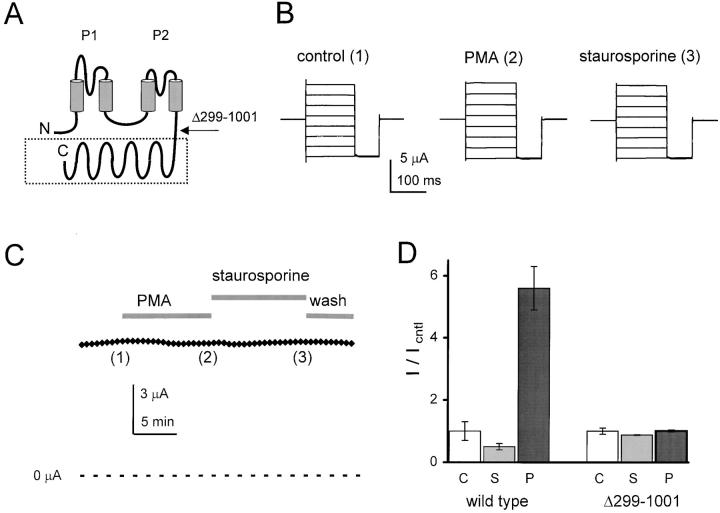
The pore-forming portion of KCNKØ subunits has two P domains and four predicted transmembrane segments extending from amino acid 1 to ∼264; the residues that follow are hydrophilic in nature and predicted to be cytoplasmic (Fig. 4 A). To assess the role of the carboxy-terminal region in regulation, subunits were produced that contained only residues 1–298 (KCNKΔ299-1001). The truncated subunits lacked most of the carboxy terminus and 10/11 consensus sites for PKC-mediated phosphorylation. The channels formed with KCNKΔ299-1001 subunits were fully functional except that they showed no regulation by PKC modulators: the subunits were unaffected by exposure to either PMA or staurosporine (Fig. 4B and Fig. C). Thus, oocytes expressing wild-type KCNKØ channels showed an ∼11-fold increase in macroscopic currents when treated with PMA for 20 min, whereas KCNKΔ299-1001 channels showed no change (Fig. 4 D).
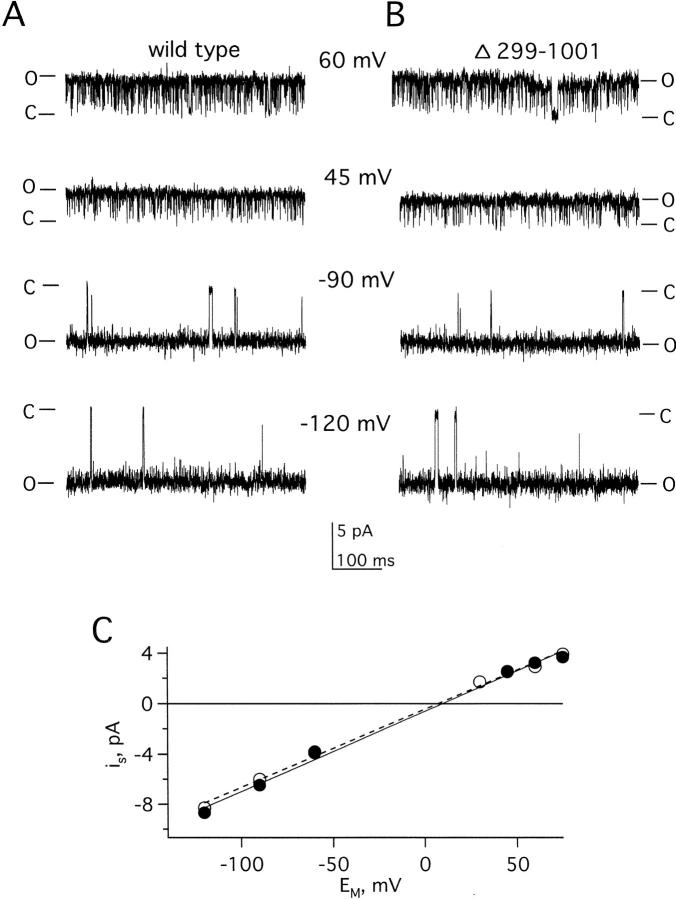
Figure 4.
Truncated (KCNKΔ299-1001) channels are not subject to regulation. Macroscopic KCNKØ currents were measured as in Fig. 1. (A) Predicted membrane topology of KCNKØ indicating two P loop domains (P1 and P2), four transmembrane segments and the ∼700-residue segment that is deleted in KCNKΔ299-1001 subunits (boxed). (B) Raw current traces for an oocyte expressing KCNKΔ299-1001 channels under various conditions by the protocol in Fig. 1 B: (1) control solution; (2) after 10-min perfusion with PMA; and (3) after 10-min perfusion with staurosporine. (C) Currents measured during application of control, PMA, and staurosporine solutions. The oocyte was held at −40 mV and stepped to 25 mV for 250 ms with a 20-s interpulse interval. Data for B was collected at the indicated times. (D) Normalized mean currents measured in groups of 17 oocytes at 25 mV after 20- min incubation in the following: (C) 20 mM potassium solution (open bars); (S) 2 μM staurosporine (gray bars); or (P) 50 nM PMA (black bars). Bars are mean ± SEM for current in experimental compared with control solution.
The Carboxy Terminus Does Not Affect Ion Selectivity or Unitary Current Amplitude
Although KCNKΔ299-1001 channels were unresponsive to PMA and staurosporine, their other functional attributes were well-preserved. Like the wild type, KCNKΔ299-1001 channels showed macroscopic currents that developed instantaneously with changes in transmembrane voltage and were noninactivating (Fig. 4 B). Channels formed with truncated subunits showed the same selectivity for potassium over sodium as wild-type channels (a 10-fold change in bath potassium produced the same shift in reversal potential of 52 ± 2 mV, n = 4). Further, wild-type and KCNKΔ299-1001 channels displayed the same relative permeability for monovalent cations (K+ > Rb+ > Cs+ ≫ Na+ and Li+) based on whole-cell reversal potential measurements (Table ). This indicated that carboxy-terminal residues were not essential for normal selective ion permeation through KCNKØ channels. Moreover, single wild-type and KCNKΔ299-1001 channels exhibited the same unitary conductance: 64 ± 2 pS for truncated channels, and 63 ± 1 pS for wild type (Fig. 5).
Table 2.
Ionic Selectivity of Wild-type KCNKØ and KCNKΔ299-1001 Channels
| Erev, mV(PK/PX) | ||
|---|---|---|
| Wild-type KCNKØ | KCNKΔ299-1001 | |
| Li+ | −118 ± 9 | −126 ± 3 |
| (148 ± 23) | (160 ± 12) | |
| Na+ | −118 ± 10 | −122 ± 6 |
| (149 ± 15) | (150 ± 2) | |
| Cs+ | −72 ± 8 | −74 ± 5 |
| (23 ± 4) | (22 ± 2) | |
| Rb+ | −36 ± 5 | −32 ± 2 |
| (5 ± 1) | (3.7 ± 0.1) | |
| K+ | −12 ± 1 | −7 ± 1 |
| (1.5 ± 0.1) | (1.3 ± 0.1) | |
Whole-cell macroscopic reversal potentials in millivolts (Erev) were measured by two-electrode voltage clamp under nearly bi-ionic conditions with 100 mM of the indicated cation in the external solution. To measure wild-type KCNKØ channel currents, oocytes were fully activated by 20-min preincubation in 50 nM PMA. Values represent the mean ± SEM of four oocytes. Permeability ratios (shown in parentheses) were approximated according to: PK/PX = exp(−FErev/RT), where Pk and Px represent the permeability of potassium and the test cation, respectively.
Figure 5.
Unitary conductance of wild-type and KCNKΔ299-1001 channels is the same. Single channels were studied during open bursts in on-cell patches with 140 mM KCl solution at the indicated voltages. Open (O) and closed (C) state levels are indicated. Data were filtered at 2 kHz and sampled at 20 kHz. (A) Sample recording of a single wild-type KCNKØ channel at four indicated voltages. (B) Sample recording of a single KCNKΔ299-1001 channel as in A. (C) Current–voltage relationships of the channels in A (•) and B (○). The line is a linear fit to the data; slope conductances were indistinguishable (see results for values).
The Carboxy Terminus Is Not a Channel Gate
Single channel recordings supported the idea that deletion of the carboxy terminus did not change the states visited by KCNKØ channels, but rather it appeared primarily to alter the stability of the long-lived closed conformation, Clong. Thus, single wild-type and KCNKΔ299-1001 channels were almost indistinguishable within bursts from −120 to 60 mV (Fig. 5A and Fig. B). Like the wild type, mutant channels revealed one open and three brief closed states within open bursts and one long-lived closed state (Fig. 6, Table ). Furthermore, time constants for the four closed states were similar for KCNKΔ299-1001 and wild-type channels activated by PMA; although, mutant channels visited their longest closed state more frequently, producing a shorter mean burst duration (Table ). These findings suggested that residues 299–1,001 were critical to sustaining the long-lived closed state, whereas residues 1–298 were sufficient to form the ion conduction pathway and closing gates.
Figure 6.
Closed and open states of KCNKΔ299-1001 channels are similar to wild type. Single KCNKΔ299-1001 channels were studied as in Fig. 2 and Fig. 3. Open (O) and closed (C) state levels are indicated. (A) Sample 5-min recording of a single channel at 60 mV filtered at 20 Hz. (B) Expansion of the indicated portion of A, filtered at 3 kHz, showing interburst behavior. (C) Open time histogram from 25 s of recording during open bursts filtered at 3 kHz. (D) Closed time histograms: (left) obtained from 25 s of recording during open bursts filtered at 3 kHz; and (right) obtained from 23 min of single KCNKØ channel recording filtered at 100 Hz. Five single channel records were combined for this analysis.
Multiple Regulatory Pathways Act Independently via the KCNKØ Carboxy Terminus
In addition to 11 classical consensus sites for PKC-mediated phosphorylation, the carboxy terminus of KCNKØ has all of the following: 1 site for tyrosine kinase (TK); 2 for protein kinase B; 14 for casein kinase II; 8 for protein kinase A (PKA) or G; and 5 for PKG. Baseline whole-cell KCNKØ currents changed <20% when exposed to agents that inhibit TK (100 μM tyrphostin A25, n = 8; 100 μM genistein, n = 8) or PKB (100 nM wortmannin, n = 5). These agents were also without effect when applied after PMA activation (n = 5–6).
In contrast, activation of PKA by a mixture of 3-isobutyl-1-methylxanthine (IBMX, 1 mM) and forskolin (20 μM) or cytoplasmic microinjection of 8-Br-cAMP (to an internal concentration of ∼450 μM) produced an approximately fourfold increase in KCNKØ current (not shown, n = 6). Similarly, the PKG activator 8-Br-GMP (microinjected to ∼450 μM) also produced an approximately fourfold increase in the KCNKØ current (not shown, n = 6). Upregulation by these agents was not due to surreptitious activation of PKC or stimulation of a PKC-dependent pathway. Although the PKC inhibitor bisindolylmaleimide I blocked activation by PMA (Fig. 7A and Fig. B), it did not modify stimulation by IBMX and forskolin (Fig. 7 A) or 8-Br-cGMP (Fig. 7 B). Reciprocally, it was seen that PKC activation was not via a PKA-dependent mechanism, whereas the PKA inhibitor H89 (5 μM) suppressed the response to IBMX and forskolin, it did not ablate PMA-induced activation (Fig. 7 C). As for PKC, PKA and PKG did not alter the function of truncated KCNKΔ299-1001 channels that lacked the carboxy terminus (not shown).
Figure 7.
Multiple regulatory pathways act via the KCNKØ carboxy terminus. Macroscopic KCNKØ currents measured as in Fig. 1 after equilibration for 10 min in the initial bath solution with scale bars that represent 5 min and 0.5 μA. Single channels were studied as in Fig. 2. (A) Macroscopic currents measured in the constant presence of the PKC inhibitor bisindolylmaleimide I (4 μM) during application of 50 nM PMA and then a combination of IBMX (1 mM) and forskolin (20 μM) to activate PKA. The average increase in current under those conditions was 4.5 ± 1 (n = 5). (B) Macroscopic currents measured in the constant presence of the PKC inhibitor bisindolylmaleimide I (4 μM) during the application of 50 nM PMA and then microinjection of 8-Br-cGMP (9.2 nl of 30 mM solution) to activate PKG. The average increase in current under those conditions was 4.4 ± 1 (n = 5). (C) Macroscopic currents measured in the constant presence of the PKA inhibitor H89 (5 μM) during application of a combination of IBMX (1 mM) and forskolin (20 μM) and then 50 nM PMA to activate PKC. The average increase in current under those conditions was 6 ± 1 (n = 5). (D) Macroscopic currents measured during microinjection of 8-Br-cGMP (9.2 nl of 30 mM solution) to activate PKG, followed by application of IBMX (1 mM) and forskolin (20 μM) to activate PKA and 50 nM PMA to activate PKC. The average increase in current under those conditions was 9.5 ± 0.8 (n = 5). (E) Sample single channel trace at 60 mV (filtered at 20 Hz) during concurrent exposure to the PKC inhibitor bisindolylmaleimide I (4 μM) and of IBMX (1 mM) and forskolin (20 μM) to activate PKA. Open (O) and closed (C) state levels are indicated. (F) Open time histogram for single KCNKØ channels exposed concurrently to a PKC inhibitor and PKA activators as in E, as determined from 18 s of single channel recording filtered at 3 kHz. (G) Closed time histograms for single KCNKØ channels exposed concurrently to a PKC inhibitor and PKA activators as in E: (left) determined from 18 s of single channel recording, filtered at 3 kHz; and (right) determined from 45 min of single channel recording, filtered at 50 Hz.
That channel activation by PKA or PKG proceeds despite concurrent inhibition of PKC (Fig. 7A and Fig. B) and that activation of PKC proceeds despite PKA inhibition (Fig. 7 C) indicates that the regulatory pathways function independently. That PKC activation further increases currents previously upregulated by PKG and PKA (Fig. 7 D) indicates that the pathways can act concurrently. Activation of PKA after PKG-induced upregulation yielded no significant additional increase in current (Fig. 7 D); this suggested PKA and PKG alter channel function by a similar mechanism. Like activation mediated by PKC, upregulation by PKA increased the open probability of KCNKØ channels by decreasing the frequency and duration of visits to the long-lived closed state, Clong (Fig. 7, E–G, and Table ).
KCNKØ Residues that Mediate PKC and PKA Regulation Are Distinct
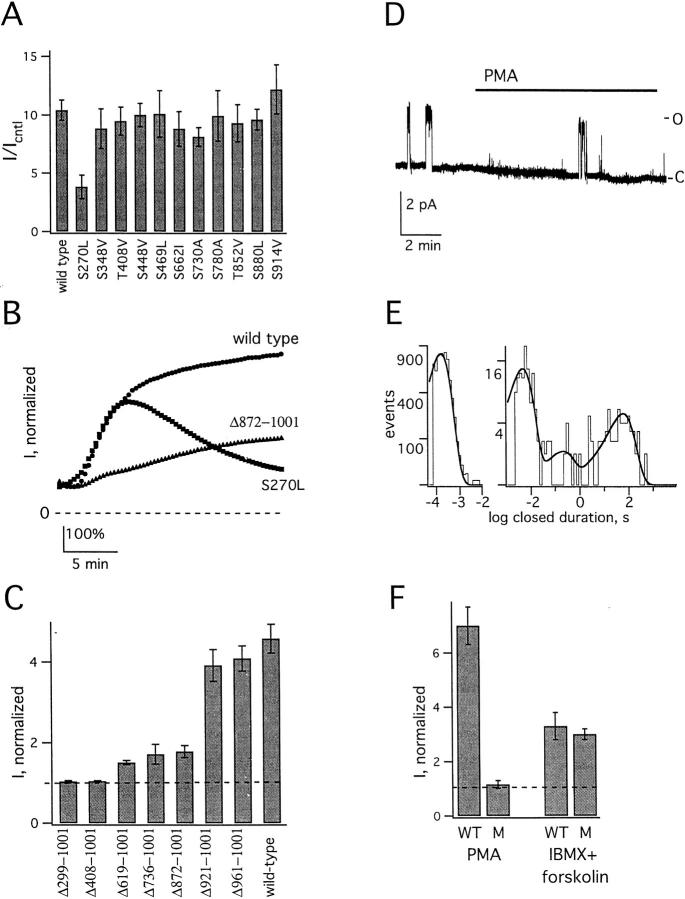
To identify residues involved in PMA-induced upregulation, each serine or threonine in the 11 consensus sites for PKC-mediated phosphorylation was mutated individually to a nonpolar residue; 10 point mutants behaved like wild type when exposed to PMA (Fig. 8 A). Conversely, the subunit mutated to leucine at position 270 (KCNKØ-S270L) formed channels that activated abnormally. Thus, wild-type KCNKØ currents rose in response to PMA in two phases: first, readily, and then more slowly (Fig. 8 B). In contrast, KCNKØ-S270L channels showed rapid development of peak currents like wild-type channels (4.4 ± 0.2 and 4.6 ± 0.3-fold increase at 5 min, n = 8–12, respectively) but, thereafter, returned slowly toward baseline without evidence for a slow phase of current activation.
Figure 8.
KCNKØ residues that mediate PKC and PKA effects are distinct. Macroscopic currents measured as in Fig. 1; single channels were studied as in Fig. 2. (A) Each of the 11 consensus sites for PKC-mediated phosphorylation was mutated individually to a nonpolar residue. Normalized mean currents were measured in groups of eight oocytes expressing the indicated channel after 20-min incubation with 50 nM PMA. Bars are mean ± SEM for current in experimental compared with staurosporine solution. (B) Normalized mean current in groups of 8–10 oocytes expressing the indicated KCNKØ channel type during application of 50 nM PMA; the currents in each cell were normalized to untreated current level before averaging. (C) After truncation at various sites, the normalized mean currents were measured in groups of 5–10 oocytes expressing the indicated channel after 5 min exposure to 50 nM PMA. Bars represent mean ± SEM for the ratio of current at 5 min to initial current. (D) Sample trace for a single KCNKØ-S270LΔ872-1001 channel at 60 mV (filtered at 20 Hz) under control conditions and in the presence of 50 nM PMA. Open (O) and closed (C) levels are indicated. (E) Closed time histograms for single KCNKØ-S270LΔ872 channels: (left) determined from 18 s of single channel open burst recording filtered at 3 kHz; τshort3 = 0.16 ms; and (right) determined from 64 min of single-channel recording filtered at 100 Hz. The mean duration in milliseconds (and relative frequency) of Clong, Cshort1, and Cshort2 were 60,000 (0.3), 200 (0.06), and 4.7 (0.64), respectively. Open time histogram for single KCNKØ-S270LΔ872-1001 channels were determined from 18 s of single channel open burst recording, filtered at 3 kHz, τo = 2.4 s (not shown). (F) Normalized wild-type (WT) and KCNKØ-S270LΔ872-1001 (M) channel currents in groups of five to eight oocytes after 20-min incubation with 50 nM PMA or 10-min exposure to IBMX (1 mM) and forskolin (20 μM). Bars are mean ± SEM for experimental compared with initial current level.
Six additional truncation mutants were also studied (Fig. 8 C). Deletion of 40 or 80 residues from the carboxy terminus yielded channels that responded to 50 nM PMA like wild type (Δ961-1001 and Δ921-1001). Conversely, larger deletions produced channels that showed minimal (Δ872-1001, Δ736-1001, and Δ619-1001) or no response to PMA (Δ407-1001 and Δ299-1001). Studies of these mutants suggested a role for residues between 872 and 921 in the rapid phase of current activation as Δ872-1001 channels responded only slowly to PMA (Fig. 8 B). Further changes were not seen with mutation of both local PKC consensus sites (S880L,S914V), motifs like those that bind synapsins (PPPPP889,890,894,895,896AAAAA), proteins with SH3 domains (P794A), or with WW domains (PP848,849AA; not shown).
Combining mutations that removed fast activation (Δ872-1001) and the slow second phase response (S270L) was sufficient to eliminate upregulation by PMA at both the macroscopic and microscopic levels (Fig. 8 D). Single KCNKØ-S270LΔ872-1001 channels under control conditions revealed a low open probability (0.04 ± 0.01, 64 min, n = 4) similar to wild-type channels in the presence of staurosporine (Table ); this was due to an increased frequency of long-lived, interburst closures (Clong), without a significant change in their duration, for the mutant channels compared to wild type (Table ). Conversely, the combined mutations did not alter the frequency or duration of brief closures, mean open time, or single-channel conductance (Fig. 8 E). Whereas KCNKØ-S270LΔ872-1001 channels were unresponsive to activators of PKC, they retained their sensitivity to PKA modulators. Thus, PMA did not alter activity of the mutant, whereas IBMX and forskolin activated both mutant and wild-type KCNKØ channels similarly (Fig. 8 F). This indicated that PKC and PKA regulators acted at distinct sites in the KCNKØ carboxy terminus to alter open probability despite their common effector mechanism: control over dwell time in the long-lived closed state.
DISCUSSION
KCNKØ Leak Channel Gating: Tightly Regulated Opening and Closing
Strictly regulated, potassium-selective leak conductances appear fundamental to excitability, synaptic transmission and neural plasticity (Siegelbaum et al. 1982; Yue and Marban 1988; Pellegrini et al. 1989; Premkumar et al. 1990a,Premkumar et al. 1990b; Koh et al. 1992; Koyano et al. 1992; Shen et al. 1992; Backx and Marban 1993; Wu et al. 1993; Enyeart et al. 1996; Theander et al. 1996; Buckler 1997; Wagner and Dekin 1997; Talley et al. 2000). In this report, we consider the first molecular example of a leak conductance channel, KCNKØ of Drosophila melanogaster nerves and muscles (Goldstein et al. 1996, Goldstein et al. 1998, Goldstein et al. 1999), and find it to be aggressively regulated by a simple mechanism. Single KCNKØ channels move between two long-lived states: one open and one closed. Protein kinase modulators open and close the channels by altering dwell time in the long-lived closed state, Clong (Fig. 3 and Table ). The effect on KCNKØ channel function is striking. Activation of single KCNKØ channels increases the open probability nearly to unity, producing potassium-selective holes in the membrane, whereas inhibition suppresses channel activity almost completely (Fig. 1 and Fig. 2 and Table ). Thus, KCNKØ channels are unlike voltage-gated potassium channels that show moderate (approximately twofold) changes in activity with PMA (Huang et al. 1994; Zhu et al. 1999), and more like ligand-gated channels that open infrequently without activation (Horrigan et al. 1999; Sunderman and Zagotta 1999). In native cells, regulated activation of KCNKØ channels is expected to diminish neuromuscular excitability by stabilizing the membrane near the equilibrium reversal potential for potassium and decreasing the amplitude, duration, and frequency of action potentials. Conversely, KCNKØ downregulation is expected to enhance receptiveness to excitation.
Two Functional Domains: Pore-forming and Regulatory
KCNKØ subunits were found to have two functional segments: one with a pore and gates and one with channel regulatory apparatus. KCNKØ residues 1–298 contain two P domains and four predicted transmembrane segments. On its own, this segment can form an ion conduction pathway and attain open and closed conformations like those observed with complete KCNKØ channels (Fig. 4 and Fig. 6). Thus, KCNKΔ299-1001 channels show single channel conductance (Fig. 5), ion selectivity (Table ), and gating kinetics like PMA-activated wild-type channels (Table ). The large carboxy terminus is essential for regulation of channel function. Channels without residues 299–1,001 cannot maintain the long-lived closed state (Table ) and are unaffected by activators or inhibitors of PKC (Fig. 4), PKA, or PKG (not shown). Moreover, upregulation of wild-type KCNKØ channels does not alter unitary conductance (Fig. 2), intraburst gating kinetics (Table ), or ion selectivity (Table ). These findings support the idea that the carboxy terminus does not contribute physically to channel gates or act as a blocking particle (Hoshi et al. 1990; Zagotta et al. 1990), but acts to modify the stability of states achievable by the pore-forming segment alone.
PKC, PKA, and PKG: Independent Collaborators in Regulation of KCNKØ Closed State Dwell Time
Regulation of KCNKØ involves at least two regulatory pathways (PKC and PKA/G) that can act independently or concurrently (Fig. 7) through distinct carboxy-terminal residues (Fig. 8). Despite their functional and structural independence, both pathways control the frequency and dwell time of KCNKØ channels in a single conformation, the long closed state (Table ). Indeed, the response to PMA or IBMX and forskolin is similar at the single-channel level at steady-state (Table ). Conversely, macroscopic current development is greater with PMA than IBMX and forskolin (Fig. 8 F) and fails to saturate (Fig. 7, A–D). This suggests PMA might also act by other mechanisms to increase the current. As PMA treatment does not alter membrane capacitance significantly (not shown), we speculate that quiescent KCNKØ channels already in the membrane may emerge from an even more deeply closed state. This mechanism has been seen with upregulation of other channels by PKC (Margiotta et al. 1987; Blumenthal and Kaczmarek 1994), and may underlie the slow phase of current development apparent in macroscopic recordings.
Potassium channels are well recognized targets for protein kinases and phosphatases (Levitan 1994; Jonas and Kaczmarek 1996). In some cases, these enzymes are known to associate intimately with ion channel proteins (Swope and Huganir 1994; Holmes et al. 1996; Wilson et al. 1998) in large assemblies that have multiple protein components (Schopperle et al. 1998; Zhou et al. 1999). Regulation in this manner has been shown to modify channel gating, ligand sensitivity, pharmacology, unitary conductance, and life span. Particularly relevant to our work are studies of a native cation channel that favors a long-lived closed state after tyrosine phosphorylation (Wilson and Kaczmarek 1993) but reduces closed state dwell time upon serine/threonine phosphorylation (Wilson et al. 1998), and those on a calcium and voltage-gated channel that, like KCNKØ, has an extended carboxy-terminal domain that has numerous sites for phosphorylation and receives multiple inputs, binding TK and PKA simultaneously (Wang et al. 1999; Zhou et al. 1999).
The KCNK Superfamily: Regulated Leak Channels
The 2 P domain potassium channel superfamily has grown rapidly since isolation of TOK1, the nonvoltage-dependent outward rectifier of Saccharomyces cerevisiae with a predicted 2P/8TM topology (Ketchum et al. 1995) to include isolates from nematodes, plants, and mammals (Goldstein et al. 1998). Thus far all subunits with a predicted 2P/4TM topology that function have a nonzero Po across the physiological voltage range and are open rectifiers, KCNKØ, KCNK3 (Duprat et al. 1997; Kim et al. 1998; Leonoudakis et al. 1998; Manjunath et al. 1999; Lopes et al. 2000) and KCNK4 (Fink et al. 1998); outward rectifiers, TOK1 (Ketchum et al. 1995) and KCO1 (Czempinski et al. 1997); weak outward rectifiers, KCNK2 (Fink et al. 1996b; Goldstein et al. 1998) and KCNK5 (Reyes et al. 1998); or inward rectifiers, KCNK9 (Kim et al. 2000).
Another attribute shared by KCNK leak channels is regulated activity. Opening and closing of KCNKØ channels was shown here to depend quite strictly on activation (or inhibition) of PKC, PKA, or PKG. Regulation of other KCNK channels is notable if somewhat less aggressive. Activity of KCNK2 channels was moderately depressed by activators of PKC and PKA (Fink et al. 1996b) and increased by arachadonic acid, mechanical stretch, and lowered intracellular pH (Patel et al. 1998, Patel et al. 1999; Maingret et al. 1999). KCNK3 channel activity can be suppressed both by low external pH (Duprat et al. 1997; Kim et al. 1998; Leonoudakis et al. 1998; Lopes et al. 1998; Manjunath et al. 1999) in a potassium-dependent fashion (Lopes et al. 2000) and by neurotransmitters when studied in native rat hypoglossal motoneurons (Talley et al. 2000). KCNK4 channel activity was moderately increased by unsaturated fatty acids (Fink et al. 1998) and membrane stretch (Maingret et al. 1999). KCNK5 was inhibited by external acidification (Reyes et al. 1998). Other KCNK genes that are transcribed in vivo have not yet shown reproducible function in experimental cells; examples include KCNK6-8 (Pountney et al. 1999; Salinas et al. 1999; Bockenhauer et al. 2000) and KCNK1 (Goldstein et al. 1998; Pountney et al. 1999), which is an isolate originally suggested to encode an inwardly rectifying channel TWIK (Lesage et al. 1996). These KCNK channel subunits may require as-yet unidentified accessory subunits or activators.
Acknowledgments
We are grateful to F. Sigworth and N. Goldstein for thoughtful advice during the course of these studies.
This work was supported by grants from the National Institutes of Health (to S.A.N. Goldstein), the Human Frontier Science Program (to N. Zilberberg), and the Bi-national Agricultural Research and Development Fund (to N. Ilan).
Footnotes
N. Zilberberg and N. Ilan contributed equally to this work.
Abbreviations used in this paper: Clong, long-lasting closed conformation; IBMX, 3-isobutyl-1-methylxanthine; Οburst, open burst; Po, open probability; TK, tyrosine kinase.
References
- Adams D.J., Smith S.J., Thompson S.H. Ionic currents in molluscan soma. Annu. Rev. Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Apkon, M., and J.M. Nerbonne. 1988. Are there multiple types of depolarization-activated K+ channels in adult ventricular myocytes? Biophys. J. 53:458a. (Abstr.)
- Backx P.H., Marban E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ. Res. 1993;72:890–900. doi: 10.1161/01.res.72.4.890. [DOI] [PubMed] [Google Scholar]
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J. Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal E.M., Kaczmarek L.K. The mink potassium channel exists in functional and nonfunctional forms when expressed in the plasma membrane of Xenopus oocytes. J. Neurosci. 1994;14:3097–3105. doi: 10.1523/JNEUROSCI.14-05-03097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhauer D., Nimmakayalu M.A., Ward D.C., Goldstein S.A.N., Gallagher P.G. Genomic structure and chromosomal localization of the 2 P domain potassium channel gene KCNK8conservation of gene structure in 2 P domain potassium channels. Gene. 2000;In press doi: 10.1016/s0378-1119(00)00492-3. [DOI] [PubMed] [Google Scholar]
- Boyle W.A., Nerbonne J.M. Two functionally distinct 4-aminopyridine-sensitive outward K+ currents in rat atrial myocytes. J. Gen. Physiol. 1992;100:1041–1067. doi: 10.1085/jgp.100.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K.J. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.C. Is the K+ permeability of the resting membrane controlled by the excitable K+ channel? Biophys. J. 1986;50:1095–1100. doi: 10.1016/S0006-3495(86)83553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sigworth F.J. Fitting and statistical analysis of single-channel records. In: Sakmann B., Neher E., editors. Single-channel Recording. Plenum Press; New York: 1995. pp. 483–588. [Google Scholar]
- Czempinski K., Zimmermann S., Ehrhardt T., Muller-Rober B. New structure and function in plant K+ channelsKCO1, an outward rectifier with a steep Ca2+ dependency. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channelmolecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart J.J., Mlinar B., Enyeart J.A. Adrenocorticotropic hormone and cAMP inhibit noninactivating K+ current in adrenocortical cells by an A-kinase-independent mechanism requiring ATP hydrolysis. J. Gen. Physiol. 1996;108:251–264. doi: 10.1085/jgp.108.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M., Duprat F., Lesage F., Heurteaux C., Romey G., Barhanin J., Lazdunski M. A new K+ channel beta subunit to specifically enhance Kv2.2 (Cdrk) expression J. Biol. Chem. 271 1996. 26341 26348a [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat F., Lesage F., Reyes R., Romey G., Heurteaux C., Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel EMBO (Eur. Mol. Biol. Organ.) J. 15 1996. 6854 6862b [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage F., Duprat F., Heurteaux C., Reyes R., Fosset M., Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D.E. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S.A.N., Price L.A., Rosenthal D.N., Pausch M.H. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA. 1996;93:13256–13261. doi: 10.1073/pnas.93.23.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S.A.N., Wang K.W., Ilan N., Pausch M. Sequence and function of the two P domain potassium channelsimplications of an emerging superfamily. J. Mol. Med. 1998;76:13–20. doi: 10.1007/s001090050186. [DOI] [PubMed] [Google Scholar]
- Goldstein S.A.N., Price L.A., Rosenthal D.N., Pausch M.H. Sequence correction forORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster . Proc. Natl. Acad. Sci. 1999;USA. 96:318. doi: 10.1073/pnas.93.23.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J. Gen. Physiol. 1973;61:669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hodgkin A.L., Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., Huxley A.F., Katz B. Measurements of current-voltage relations in the membrane of the giant axon of Loigo. J. Physiol. 1952;116:424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T.C., Fadool D.A., Ren R., Levitan I.B. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- Horrigan F.T., Cui J., Aldrich R.W. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca(2+) . J. Gen. Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Huang X.Y., Morielli A.D., Peralta E.G. Molecular basis of cardiac potassium channel stimulation by protein kinase A. Proc. Natl. Acad. Sci. USA. 1994;91:624–628. doi: 10.1073/pnas.91.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N., Goldstein S.A.N. KCNKØsingle, cloned potassium leak channels are multi-ion pores. Biophys. J. 2000;In press doi: 10.1016/S0006-3495(01)76010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E.A., Kaczmarek L.K. Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol. 1996;6:318–323. doi: 10.1016/s0959-4388(96)80114-0. [DOI] [PubMed] [Google Scholar]
- Jones S.W. On the resting potential of isolated frog sympathetic neurons. Neuron. 1989;3:153–161. doi: 10.1016/0896-6273(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Ketchum K.A., Joiner W.J., Sellers A.J., Kaczmarek L.K., Goldstein S.A.N. A new family of outwardly-rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kim D., Fujita A., Horio Y., Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1) Circ. Res. 1998;82:513–518. doi: 10.1161/01.res.82.4.513. [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang H., Kim D. TASK-3, a new member of the tandem pore K+ channel family Biophys. J 78 2000. 207A(Abstr.) [DOI] [PubMed] [Google Scholar]
- Koh D.S., Jonas P., Brau M.E., Vogel W. A TEA-insensitive flickering potassium channel active around the resting potential in myelinated nerve. J. Memb. Biol. 1992;130:149–162. doi: 10.1007/BF00231893. [DOI] [PubMed] [Google Scholar]
- Koyano K., Tanaka K., Kuba K. A patch-clamp study on the muscarine-sensitive potassium channel in bullfrog sympathetic ganglion cells. J. Physiol. 1992;454:231–246. doi: 10.1113/jphysiol.1992.sp019262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D., Gray A.T., Winegar B.D., Kindler C.H., Harada M., Taylor D.M., Chavez R.A., Forsayeth J.R., Yost C.S. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J. Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Levitan I.B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu. Rev. Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Lopes C.M.B., Gallagher P.G., Wong C., Buck M., Goldstein S.A.N. OATsopen, acid-sensitive, two P domain K+ channels from mouse heart J. Biophys 74 1998. A44(Abstr.) [Google Scholar]
- Lopes C.M.B., Gallagher P.G., Buck M.E., Butler M.H., Goldstein S.A.N. Proton block and voltage-gating are potassium-dependent in the cardiac leak channel Kcnk3. J. Biol. Chem. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- Maingret F., Fosset M., Lesage F., Lazdunski M., Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Manjunath N.A., Bray-Ward P., Goldstein S.A.N., Gallagher P.G. Assignment of the 2 P domain, acid-sensitive potassium channel gene OAT1 (KCNK3) to human chromosome 2p23.3-p24.1 and murine chromosome band 5B by in situ hybridization. Cytogen. Cell Gen. 1999;86:242–243. doi: 10.1159/000015349. [DOI] [PubMed] [Google Scholar]
- Margiotta J.F., Berg D.K., Dionne V.E. Cyclic AMP regulates the proportion of functional acetylcholine receptors on chicken ciliary ganglion neurons. Proc. Natl. Acad. Sci. USA. 1987;84:8155–8159. doi: 10.1073/pnas.84.22.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J. Gen. Physiol. 1988;92:549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honore E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honore E., Lesage F., Fink M., Romey G., Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Simoni A., Pellegrino M. Two types of K+ channels in excised patches of somatic membrane of the leech AP neuron. Brain Res. 1989;483:294–300. doi: 10.1016/0006-8993(89)90173-x. [DOI] [PubMed] [Google Scholar]
- Pountney D.J., Gulkarov I., de Miera E.V., Holmes D., Saganich M., Rudy B., Artman M., Coetzee W.A. Identification and cloning of TWIK-originated similarity sequence (TOSS)a novel human 2-pore K+ channel principal subunit. FEBS Lett. 1999;450:191–196. doi: 10.1016/s0014-5793(99)00495-0. [DOI] [PubMed] [Google Scholar]
- Premkumar L.S., Chung S.H., Gage P.W. GABA-induced potassium channels in cultured neurons Proc. R. Soc. Lond. B. Biol. Sci. 241 1990. 153 158a [DOI] [PubMed] [Google Scholar]
- Premkumar L.S., Gage P.W., Chung S.H. Coupled potassium channels induced by arachidonic acid in cultured neurons Proc. R. Soc. Lond. B. Biol. Sci. 242 1990. 17 22b [DOI] [PubMed] [Google Scholar]
- Reyes R., Duprat F., Lesage F., Fink M., Salinas M., Farman N., Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Salinas M., Reyes R., Lesage F., Fosset M., Heurteaux C., Romey G., Lazdunski M. Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem. 1999;274:11751–11760. doi: 10.1074/jbc.274.17.11751. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Stampfli R. The effect of tetraethylammonium chloride on single Ranvier's nodes. Pflügers Arch. Gesamte Physiol. Menschen Tiere. 1966;287:311–325. [PubMed] [Google Scholar]
- Schopperle W.M., Holmqvist M.H., Zhou Y., Wang J., Wang Z., Griffith L.C., Keselman I., Kusinitz F., Dagan D., Levitan I.B. Slob, a novel protein that interacts with the Slowpoke calcium-dependent potassium channel. Neuron. 1998;20:565–573. doi: 10.1016/s0896-6273(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Shen K.Z., North R.A., Surprenant A. Potassium channels opened by noradrenaline and other transmitters in excised membrane patches of guinea-pig submucosal neurones. J. Physiol. 1992;445:581–599. doi: 10.1113/jphysiol.1992.sp018941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S.A., Camardo J.S., Kandel E.R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982;299:413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F.J., Sine S.M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderman E.R., Zagotta W.N. Mechanism of allosteric modulation of rod cyclic nucleotide-gated channels. J. Gen. Physiol. 1999;113:601–620. doi: 10.1085/jgp.113.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope S.L., Huganir R.L. Binding of the nicotinic acetylcholine receptor to SH2 domains of Fyn and Fyk protein tyrosine kinases. J. Biol. Chem. 1994;269:29817–29824. [PubMed] [Google Scholar]
- Talley E.M., Lei Q.B., Sirois J.E., Bayliss D.A. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Theander S., Fahraeus C., Grampp W. Analysis of leak current properties in the lobster stretch receptor neurone. Acta. Physiol. Scand. 1996;157:493–509. doi: 10.1046/j.1365-201X.1996.510271000.x. [DOI] [PubMed] [Google Scholar]
- Van Wagoner D.R., Pond A.L., McCarthy P.M., Trimmer J.S., Nerbonne J.M. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Wagner P.G., Dekin M.S. cAMP modulates an S-type K+ channel coupled to GABAB receptors in mammalian respiratory neurons. Neuroreport. 1997;8:1667–1670. doi: 10.1097/00001756-199705060-00021. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhou Y., Wen H., Levitan I.B. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J. Neurosci. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-10-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- Wei A., Jegla T., Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Wilson G.F., Kaczmarek L.K. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993;366:433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]
- Wilson G.F., Magoski N.S., Kaczmarek L.K. Modulation of a calcium-sensitive nonspecific cation channel by closely associated protein kinase and phosphatase activities. Proc. Natl. Acad. Sci. USA. 1998;95:10938–10943. doi: 10.1073/pnas.95.18.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.V., Rubinstein C.T., Shrager P. Single channel characterization of multiple types of potassium channels in demyelinated Xenopus axons. J. Neurosci. 1993;13:5153–5163. doi: 10.1523/JNEUROSCI.13-12-05153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D.T., Marban E. A novel cardiac potassium channel that is active and conductive at depolarized potentials. Pflügers Arch. 1988;413:127–133. doi: 10.1007/BF00582522. [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Aldrich R.W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Schopperle W.M., Murrey H., Jaramillo A., Dagan D., Griffith L.C., Levitan I.B. A dynamically regulated 14-3-3. Slob, and slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wulf R.A., Schwarz M., Isbrandt D., Pongs O. Characterization of human Kv4.2 mediating a rapidly-inactivating transient voltage-sensitive K+ current. Recept. Chan. 1999;6:387–400. [PubMed] [Google Scholar]