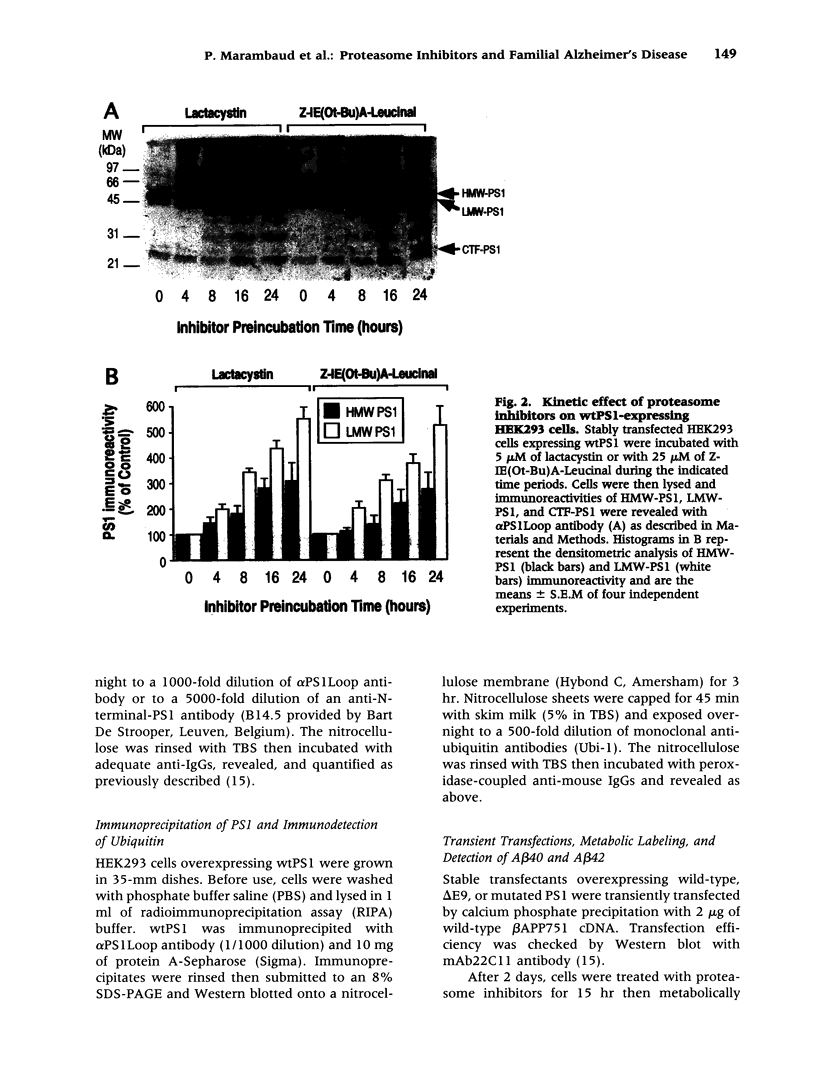
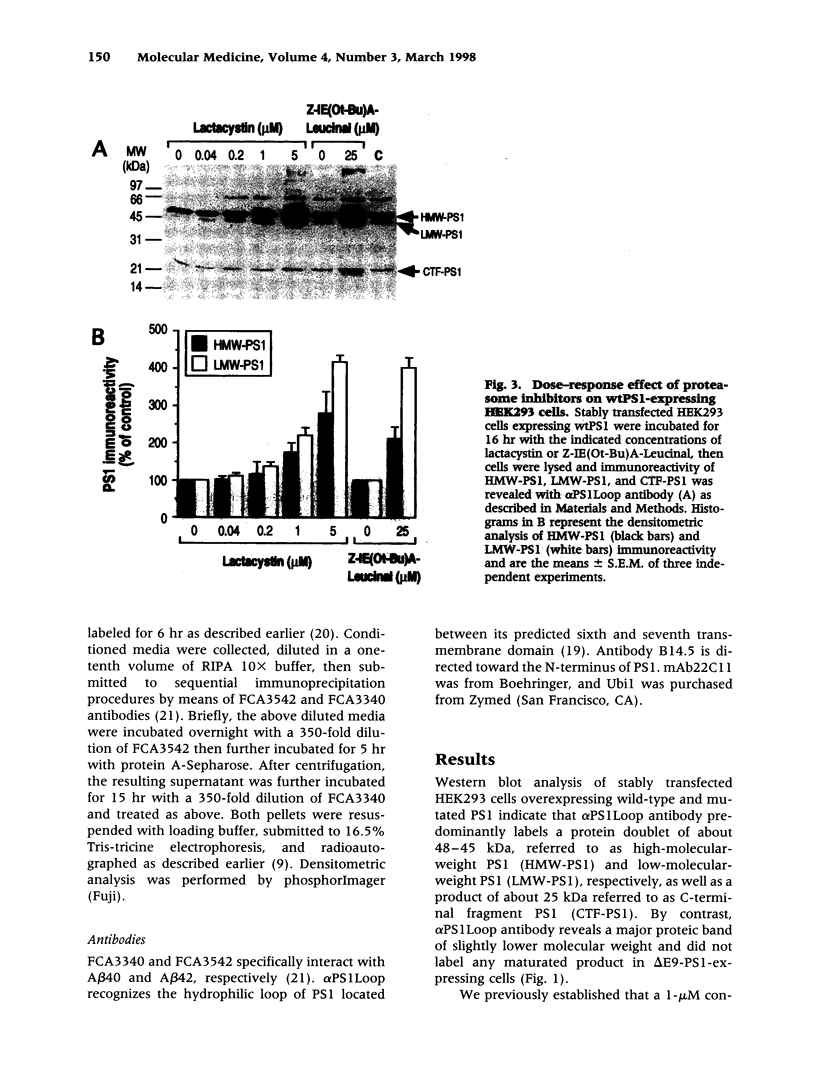
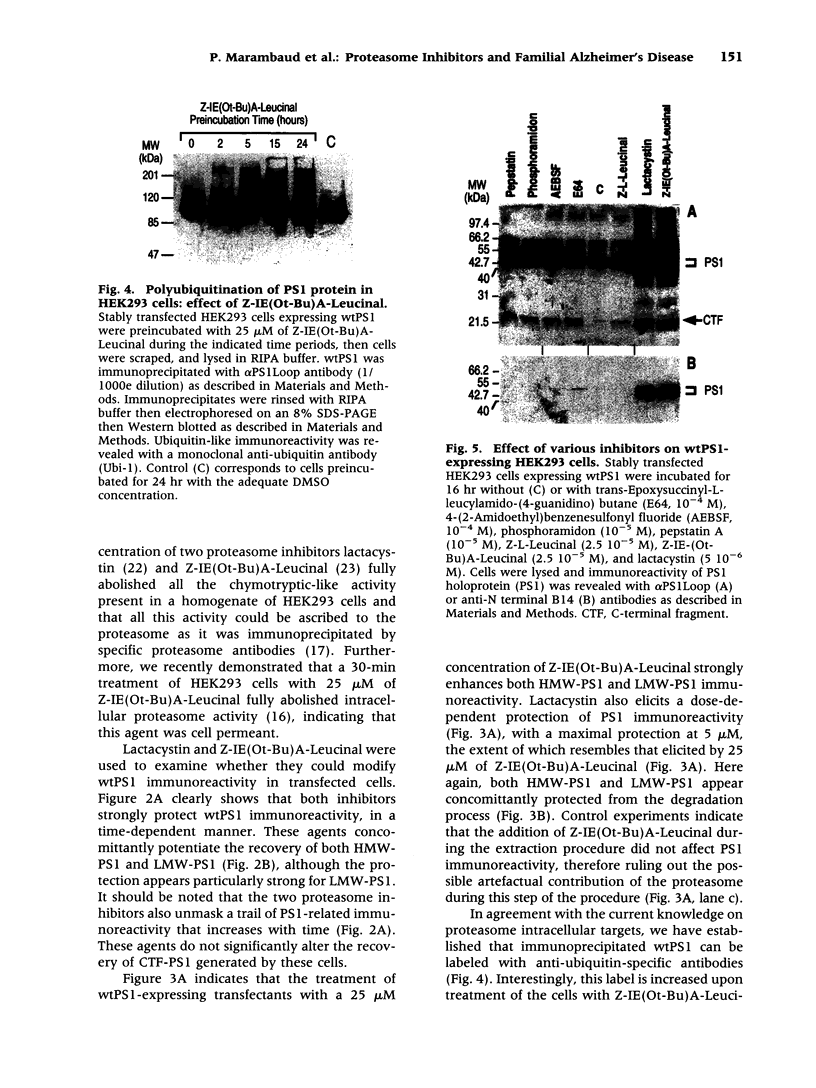
Abstract
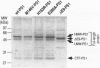
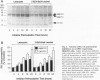
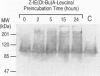
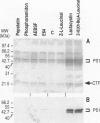
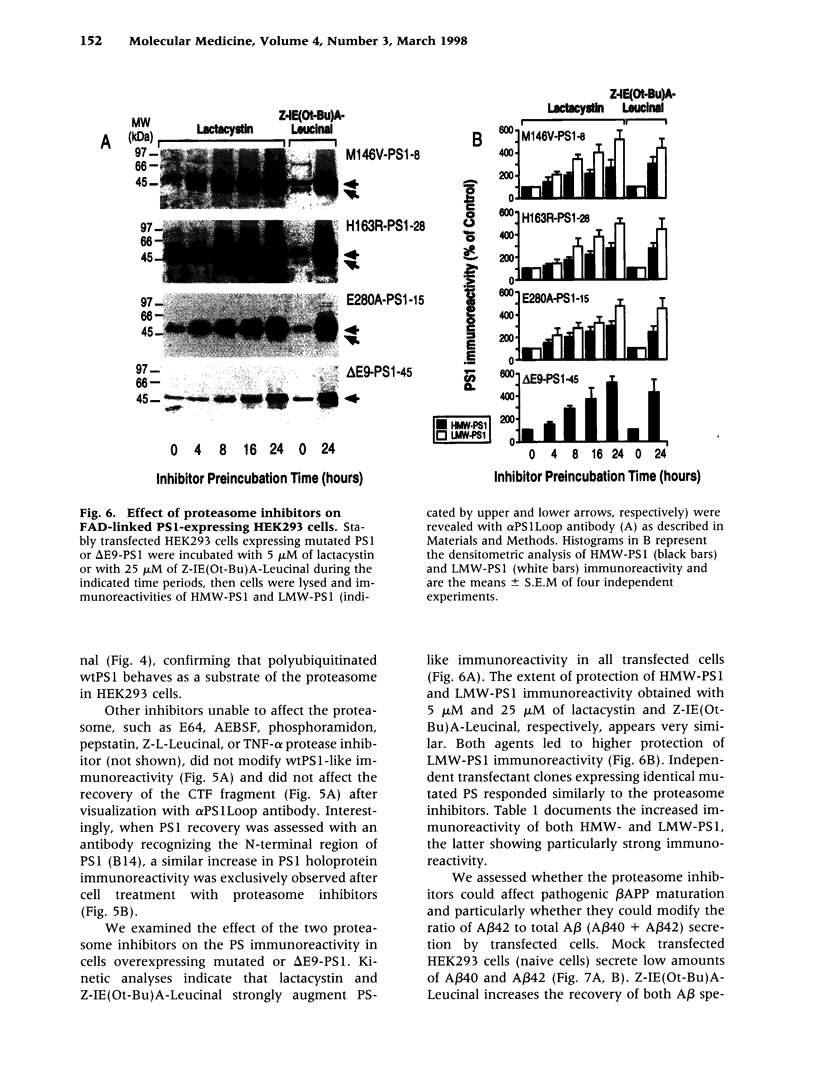
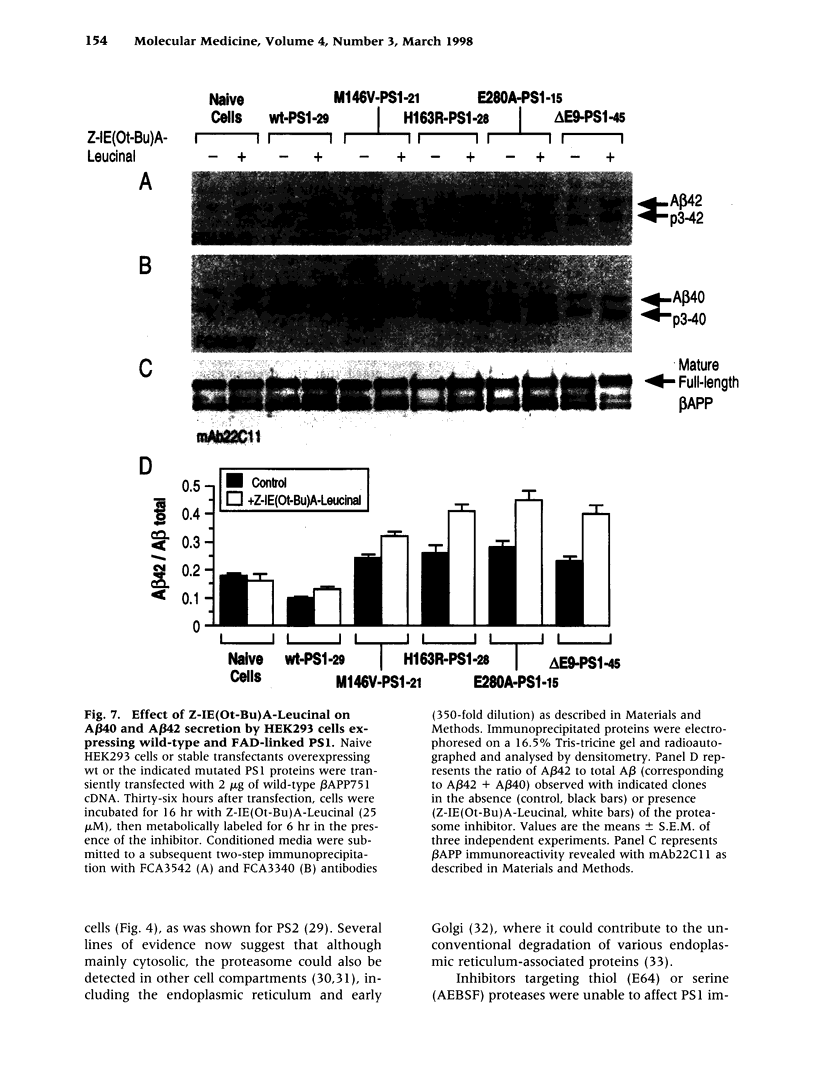
BACKGROUND: Several lines of evidence suggest that most of the early-onset forms of familial Alzheimer's disease (FAD) are due to inherited mutations borne by a chromosome 14-encoded protein, presenilin 1 (PS1). This is likely related to an increased production of amyloid beta-peptide (A beta) 42, one of the main components of the extracellular deposits called senile plaques that invade human cortical areas during the disease. MATERIALS AND METHODS: We set up stably transfected HEK293 cells overexpressing wild-type (wt) and various FAD-linked mutated PS1. By Western blot analysis, we examined the influence of specific proteasome inhibitors on PS1-like immunoreactivities. Furthermore, by means of metabolic labeling and immunoprecipitation with A beta 40 and A beta 42-directed specific antibodies, we assessed the effect of the inhibitors on the production of A beta s by wt and mutated PS1-expressing cells transiently transfected with beta APP751. RESULTS: We show that two distinct proteasome inhibitors, Z-IE (Ot-Bu)A-Leucinal and lactacystin, increase in a time- and dose-dependent manner the immunoreactivities of both wt and mutated PS1. Furthermore, we demonstrate that PS1 is polyubiquitinated in these cells. Other inhibitors, ineffective on the proteasome, fail to protect wt and mutated PS1-like immunoreactivities. We also establish that the FAD-linked mutations of PS1 trigger a selective increased formation of A beta 42 as reflected by higher A beta 42 over total A beta ratios when compared with wtPS1-expressing cells. Interestingly, this augmentation was further amplified by proteasome inhibitors in cells expressing mutated but not wtPS1. CONCLUSION: Altogether, our data indicate that PS1 undergoes polyubiquitination in HEK293 cells and that the proteasome contributes to the degradation of wt and FAD-linked PS1, thereby directly influencing the A beta production in human cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ancolio K., Marambaud P., Dauch P., Checler F. Alpha-secretase-derived product of beta-amyloid precursor protein is decreased by presenilin 1 mutations linked to familial Alzheimer's disease. J Neurochem. 1997 Dec;69(6):2494–2499. doi: 10.1046/j.1471-4159.1997.69062494.x. [DOI] [PubMed] [Google Scholar]
- Barelli H., Lebeau A., Vizzavona J., Delaere P., Chevallier N., Drouot C., Marambaud P., Ancolio K., Buxbaum J. D., Khorkova O. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med. 1997 Oct;3(10):695–707. [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996 Nov;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995 Oct;65(4):1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Chevallier N., Jiracek J., Vincent B., Baur C. P., Spillantini M. G., Goedert M., Dive V., Checler F. Examination of the role of endopeptidase 3.4.24.15 in A beta secretion by human transfected cells. Br J Pharmacol. 1997 Jun;121(3):556–562. doi: 10.1038/sj.bjp.0701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. G., Sung J. C., Golde T. E., Felsenstein K. M., Wojczyk B. S., Tanzi R. E., Trojanowski J. Q., Lee V. M., Doms R. W. Expression and analysis of presenilin 1 in a human neuronal system: localization in cell bodies and dendrites. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., Cordell B., Moechars D., Bollen M., Fraser P., George-Hyslop P. S. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer's disease-associated presenilins. J Biol Chem. 1997 Feb 7;272(6):3590–3598. doi: 10.1074/jbc.272.6.3590. [DOI] [PubMed] [Google Scholar]
- Dewji N. N., Singer S. J. Specific transcellular binding between membrane proteins crucial to Alzheimer disease. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12575–12580. doi: 10.1073/pnas.93.22.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., Hutton M., Buee L., Harigaya Y., Yager D. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996 Oct 24;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Fenteany G., Standaert R. F., Lane W. S., Choi S., Corey E. J., Schreiber S. L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995 May 5;268(5211):726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Fuller S. J., Storey E., Li Q. X., Smith A. I., Beyreuther K., Masters C. L. Intracellular production of beta A4 amyloid of Alzheimer's disease: modulation by phosphoramidon and lack of coupling to the secretion of the amyloid precursor protein. Biochemistry. 1995 Jun 27;34(25):8091–8098. doi: 10.1021/bi00025a015. [DOI] [PubMed] [Google Scholar]
- Gearing M., Tigges J., Mori H., Mirra S. S. A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging. 1996 Nov-Dec;17(6):903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Haass C. Presenile because of presenilin: the presenilin genes and early onset Alzheimer's disease. Curr Opin Neurol. 1996 Aug;9(4):254–259. [PubMed] [Google Scholar]
- Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991 Oct;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Kim T. W., Pettingell W. H., Hallmark O. G., Moir R. D., Wasco W., Tanzi R. E. Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J Biol Chem. 1997 Apr 25;272(17):11006–11010. doi: 10.1074/jbc.272.17.11006. [DOI] [PubMed] [Google Scholar]
- Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., Merriam D. E., Hollister R. D., Hallmark O. G., Mancini R., Felsenstein K. M. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996 Feb;2(2):224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Lemere C. A., Lopera F., Kosik K. S., Lendon C. L., Ossa J., Saido T. C., Yamaguchi H., Ruiz A., Martinez A., Madrigal L. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med. 1996 Oct;2(10):1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Iwatsubo T., Cairns N. J., Lantos P. L., Nochlin D., Sumi S. M., Bird T. D., Poorkaj P., Hardy J., Hutton M. Amyloid beta protein (Abeta) deposition in chromosome 14-linked Alzheimer's disease: predominance of Abeta42(43). Ann Neurol. 1996 Aug;40(2):149–156. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Iwatsubo T., Nochlin D., Sumi S. M., Levy-Lahad E., Bird T. D. Amyloid (Abeta) deposition in chromosome 1-linked Alzheimer's disease: the Volga German families. Ann Neurol. 1997 Jan;41(1):52–57. doi: 10.1002/ana.410410110. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Chevallier N., Barelli H., Wilk S., Checler F. Proteasome contributes to the alpha-secretase pathway of amyloid precursor protein in human cells. J Neurochem. 1997 Feb;68(2):698–703. doi: 10.1046/j.1471-4159.1997.68020698.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Lopez-Perez E., Wilk S., Checler F. Constitutive and protein kinase C-regulated secretory cleavage of Alzheimer's beta-amyloid precursor protein: different control of early and late events by the proteasome. J Neurochem. 1997 Dec;69(6):2500–2505. doi: 10.1046/j.1471-4159.1997.69062500.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Wilk S., Checler F. Protein kinase A phosphorylation of the proteasome: a contribution to the alpha-secretase pathway in human cells. J Neurochem. 1996 Dec;67(6):2616–2619. doi: 10.1046/j.1471-4159.1996.67062616.x. [DOI] [PubMed] [Google Scholar]
- Mengual E., Arizti P., Rodrigo J., Giménez-Amaya J. M., Castaño J. G. Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS. J Neurosci. 1996 Oct 15;16(20):6331–6341. doi: 10.1523/JNEUROSCI.16-20-06331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Ikehara Y., Omura S. Lactacystin, an inhibitor of the proteasome, blocks the degradation of a mutant precursor of glycosylphosphatidylinositol-linked protein in a pre-Golgi compartment. Biochem Biophys Res Commun. 1996 Feb 27;219(3):800–805. doi: 10.1006/bbrc.1996.0314. [DOI] [PubMed] [Google Scholar]
- Palmer A., Rivett A. J., Thomson S., Hendil K. B., Butcher G. W., Fuertes G., Knecht E. Subpopulations of proteasomes in rat liver nuclei, microsomes and cytosol. Biochem J. 1996 Jun 1;316(Pt 2):401–407. doi: 10.1042/bj3160401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Hoffman L., Dubiel W. The multicatalytic and 26 S proteases. J Biol Chem. 1993 Mar 25;268(9):6065–6068. [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 1989 Jul 25;264(21):12215–12219. [PubMed] [Google Scholar]
- Seeger M., Nordstedt C., Petanceska S., Kovacs D. M., Gouras G. K., Hahne S., Fraser P., Levesque L., Czernik A. J., George-Hyslop P. S. Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5090–5094. doi: 10.1073/pnas.94.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996 Jul;17(1):181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Tomita T., Maruyama K., Saido T. C., Kume H., Shinozaki K., Tokuhiro S., Capell A., Walter J., Grünberg J., Haass C. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Capell A., Grünberg J., Pesold B., Schindzielorz A., Prior R., Podlisny M. B., Fraser P., Hyslop P. S., Selkoe D. J. The Alzheimer's disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum. Mol Med. 1996 Nov;2(6):673–691. [PMC free article] [PubMed] [Google Scholar]
- Walter J., Grünberg J., Capell A., Pesold B., Schindzielorz A., Citron M., Mendla K., George-Hyslop P. S., Multhaup G., Selkoe D. J. Proteolytic processing of the Alzheimer disease-associated presenilin-1 generates an in vivo substrate for protein kinase C. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5349–5354. doi: 10.1073/pnas.94.10.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. V., Davis J. B., Gray C. W., Barton A. J., Bresciani L. G., Caivano M., Murphy V. F., Duff K., Hutton M., Hardy J. Presenilin-1 is processed into two major cleavage products in neuronal cell lines. Neurodegeneration. 1996 Dec;5(4):293–298. doi: 10.1006/neur.1996.0040. [DOI] [PubMed] [Google Scholar]
- Weidemann A., Paliga K., Dürrwang U., Czech C., Evin G., Masters C. L., Beyreuther K. Formation of stable complexes between two Alzheimer's disease gene products: presenilin-2 and beta-amyloid precursor protein. Nat Med. 1997 Mar;3(3):328–332. doi: 10.1038/nm0397-328. [DOI] [PubMed] [Google Scholar]
- Werner E. D., Brodsky J. L., McCracken A. A. Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13797–13801. doi: 10.1073/pnas.93.24.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Zhang J., Kholodenko D., Citron M., Podlisny M. B., Teplow D. B., Haass C., Seubert P., Koo E. H., Selkoe D. J. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997 Mar 21;272(12):7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]