Abstract
The latent nuclear antigen (LNA) of Kaposi's sarcoma-associated herpesvirus (KSHV) has an essential role in viral latent infection. LNA maintains the stability of KSHV episomes and modulates the expression of cellular genes. A novel cellular protein KLIP1 was identified to interact with LNA through yeast two-hybrid screening, and confirmed by a glutathione S-transferase pull down assay. Domain mapping showed that KLIP1 interacted with the N-terminal domain of LNA. Northern blot hybridization with a KLIP1 probe identified a major transcript of 1.8 kb and a minor transcript of 2.8 kb. cDNA library screening and 5′-RACE revealed that the major transcript encoded an open-reading-frame of 1,257 bp and had a 5′-untranslated region of 73 nucleotides. The major KLIP1 transcript was ubiquitously present in different cell types examined. A KLIP1 synthetic peptide antibody detected a doublet of 58-kDa and 63-kDa proteins in a Western blot assay. KLIP1 had two putative nuclear localization signals and showed punctate nuclear localization when expressed as a GFP-fusion protein. KLIP1 interacted with LNA in vivo, as demonstrated by coimmunoprecipitation using KSHV-infected cells and colocalization when they were expressed as GFP- and DsRed-fusion proteins, respectively. Consistent with its interaction with LNA, nuclear localization, and possession of two leucine zipper motifs, KLIP1 behaved like a transcriptional factor and repressed herpes simplex virus thymidine kinase (TK) promoter activity in a mammalian one-hybrid assay. In addition, cotransfection with LNA alleviated the transcriptional repression effect of KLIP1 on TK promoter activity. These results suggest that KLIP1 is a new member of cellular transcriptional repressors, and that LNA is involved in deregulating cellular transcription process.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also known as human herpesvirus 8, was discovered in a KS lesion from a patient with AIDS (6). Extensive epidemiologic studies with molecular and serologic assays have provided convincing evidence to support the essential role of KSHV in the development of KS (5). KSHV has also been associated with several other lymphoproliferative diseases including primary effusion lymphoma and a subset of multicentric Castleman's disease (5).
KSHV is closely related to Epstein-Barr virus (EBV), a lymphotropic herpesvirus associated with Burkitt's lymphoma, nasopharyngeal carcinoma, and posttransplant lymphoproliferative diseases, and leiomyosarcomas in immunosuppressed persons (16). EBV latent infection and the expression of viral latent antigens are essential for the development of EBV-related malignancies (16). Similar to EBV, KSHV establishes latent infection in the majority of tumor cells in KS lesions and expresses a limited number of viral latent genes, suggesting that KSHV latent infection has a crucial role in the development of KS lesions (32).
Latent nuclear antigen (LNA) encoded by KSHV open reading frame 73 (ORF73) is the immunodominant major latent antigen expressed in KS lesions (8, 12, 13, 22-24, 36). LNA is a doublet of 222- to 234-kDa proteins identified in KSHV-infected cell lines by Western blot assay using hyperimmune sera from KS patients (12, 13). In KSHV-infected cells, LNA has a punctate nuclear distribution pattern (12, 13, 23). LNA has been shown to be a multifunctional protein. It maintains the stability of KSHV genomes (3, 4, 7, 15, 26, 33, 39-41), and supports latent replication (18, 19). LNA is also a transcriptional regulator and has dual function in both activating and repressing transcription. LNA acts as a transcriptional activator of several promoters such as telomerase reverse transcriptase, interleukin-6, and E2F-regulated promoters (1, 25, 35). LNA's effects on EBV latent promoters are controversial, activating LMP-1 and Cp promoters in one occasion (17), while repressing EBNA-1 Cp and Qp promoters in another occasion (27). LNA also modulates the transcriptional activity of the long terminal repeat of HIV-1 (20, 37), and its own promoter (21, 37), although there is no evidence showing that LNA binds to its promoter (15). LNA interacts with tumor suppressor protein p53 and pRb, repressing p53-dependent transcription (10) as well as E2F-dependent transcription (35). LNA interacts with ATF4/CREB2 (30), CBP (29), and mSin3 corepressor complex (27) and modulates the transcriptional activities mediated by these proteins. LNA induces and relocates RING3 to nuclear heterochromatin regions (31, 34). In a stably transfected B cell line, LNA was shown to modulate the expression of six known cellular genes and nine-expressed sequence tags (37). In human endothelial cells, LNA was found to induce the expression of helix-loop-helix protein Id-1 (42). LNA also transforms rat primary fibroblasts in cooperation with oncogene Hras (35). Thus, similar to EBV latent proteins, LNA plays an important role in KSHV latent infection and cellular transformation.
The C terminus of LNA contains a sequence-specific DNA-binding domain that binds to a 20-bp imperfect palindrome within the terminal repeat region of KSHV genome (14, 39). LNA binding to the imperfect palindrome inhibits gene transcription in a reporter assay (15). Nevertheless, no specific binding site has been identified for LNA within cellular gene promoters.
Considering the complexity and multifunctional nature of LNA, we speculated that LNA might interact with more cellular proteins to modulate the expression of cellular genes and promote KSHV latent infection. Here, we report the identification of a novel cellular protein interacting with LNA by yeast two-hybrid screening, and subsequent characterization of this protein. The novel protein was named KLIP1, and found to be a nuclear protein involved in transcriptional repression.
MATERIALS AND METHODS
Plasmids.
Full-length LNA was PCR-amplified from the BC-1 cell line and cloned into the EcoRI/SalI sites of the pAS2-1 vector (Clontech, Palo Alto, Calif.) to produce the bait construct pAS2-1-LNA for yeast two-hybrid screening. The same LNA DNA fragment was also cloned into the EcoRI/BamHI sites of pBSKF vector (a gift from Phang-Lang Chen) to yield pBSKF-LNA, and into the XhoI/XbaI sites of pcDNA3.1His (Invitrogen, Carlsbad, Calif.) to yield pcDNA3.1His-LNA. The pDsRed-LNA vector expressing a LNA DsRed fusion protein was constructed by inserting the full-length LNA DNA into the BglII/SalI sites of pDsRed2-C1 (Clontech). For mapping of the KLIP1 LNA-interacting domain, the N-terminal, internal repeat and C-terminal domains of LNA were cloned into the EcoRI/SalI sites of pAS2-1 vector, respectively, resulting in three corresponding constructs pAS2-1-LNA-N (aa 1 to 317), pAS2-1-LNA-IR (aa 318 to 1011) and pAS2-1-LNA-C (aa 949 to 1162). To construct a green fluorescent protein (GFP)-KLIP1 plasmid expressing the KLIP1 GFP fusion protein, the full-length KLIP1 ORF was released by SpeI digestion from a cDNA clone pBSK-KLIP1 and inserted into the XbaI site of CHPL-GFP vector (a gift from Phang-Lang Chen). To generate a PM2-KLIP1 plasmid expressing the KLIP1 GAL4 DNA-binding domain (DBD) fusion protein, KLIP1 ORF released by SpeI digestion from pBSK-KLIP1 was inserted into the XbaI site of PM2 vector (44). The expression of GAL4 DBD in PM2 is directed by SV40 early promoter. The pGEX-3X-KLIP1C plasmid expressing truncated KLIP1 glutathione S-transferase (GST) fusion protein (GST-KLIP1C) was generated by cloning the KLIP1 C-terminal cDNA sequence identified in the yeast two-hybrid screening into the BamHI site of pGEX-3X vector (Amersham Biosciences, Piscataway, N.J.). All the constructs were verified by restriction enzyme digestion and DNA sequencing on an ABI 373-S sequencer, using a Big Dye Terminator Sequencing Kit (PE Bio-systems, Foster City, Calif.).
Yeast two-hybrid screening.
To identify LNA-interacting proteins, yeast two-hybrid screening was carried out using pAS2-1-LNA as bait, as previously described with minor modifications (9). Briefly, the yeast Y190 trp+ transformant expressing the LNA GAL4 DBD fusion protein was transformed with a human B-cell cDNA library expressing the GAL4 activation domain (AD) fusion proteins (America Type Culture Collection, Rockville, Md.). The interacting clones were selected by measuring β-galactosidase activity in a colony lift assay and a liquid CPRG assay, using X-Gal and chlorophenyl-red-β-d-galactopyranoside as substrates, respectively (Roche, Indianapolis, Ind.). Clones with high β-galactosidase activity were selected for further examination.
GST pull down assay.
A GST pull down assay was performed as previously reported (28). 35S-labeled LNA was generated using the pBSKF-LNA construct via in vitro translation using a TNT T3 system kit according to the manufacturer's instructions (Promega, Madison, Wis.). The reaction was carried out in the presence of [35S]methionine with pBSKF-LNA DNA as template. KLIP1 GST fusion protein GST-KLIP1C was induced with IPTG in E. coli transformed with the pGEX-3X-KLIP1C plasmid. Glutathione-Sepharose beads (Amersham Biosciences, San Francisco, Calif.) were incubated with lysate from bacteria expressing GST-KLIP1C and control GST protein, and washed to eliminate unspecific binding proteins. The beads were then incubated with 35S-labeled LNA, washed, and analyzed in a SDS-PAGE. The images were captured with a GS-525 PhosphorImager and analyzed with a Multi-Analysis Program (Bio-Rad Laboratories, Richmond, Calif.).
In vivo coimmunoprecipitation assay.
GFP-KLIP1 DNA was transfected into BCBL-1 cells by electroporation, as described previously (33). At 48 h posttransfection, the cells were harvested and lysed in ice-cold lysis 250 buffer (50 mM Tris, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40) containing a cocktail of protease inhibitors including 100 μM phenylmethylsulfonyl fluoride, leupeptin (1 μg/ml), TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone) (10 μg/ml), and pepstatin A (1 μg/ml). After removal of the debris by centrifugation, the supernatant was incubated with a mouse anti-GFP monoclonal antibody B-2 (Santa Cruz Biotechnology, Santa Cruz, Calif.) or a rat anti-LNA monoclonal antibody (ABI Advanced Biotechnologies, Columbia, Md.), and followed by incubation with protein G Sepharose beads (Sigma, St. Louis, Mo.). The beads were washed 5 times with Lysis 250. The attached proteins were separated by SDS-PAGE, transferred to nitrocellulose membrane, and specific proteins were detected with anti-GFP and anti-LNA antibodies, respectively. BCBL-1 cells transfected with only the GFP vector were used as the negative control.
RNA isolation and Northern blot hybridization.
Total RNA was isolated from 107 cells with TRIzol reagent, following the manufacturer's instructions (Invitrogen). For Northern blot hybridization, 20 μg total RNA from each sample were separated in an 1% agarose gel containing 18% formaldehyde and transferred to a nylon membrane, which was hybridized with a radiolabeled riboprobe (43). Following hybridization, the image was captured and quantified with a GS-525 Molecular Imager (Bio-Rad).
cDNA library screening and DNA sequencing.
Screening of a cDNA library constructed from BC-3 cells was performed as previously reported (38). Briefly, plaques appearing on 150-mm-diameter agar plates at about 50,000 PFU/plate were transferred onto nitrocellulose membranes. The membranes were hybridized with a radiolabeled KLIP1 probe. Selected plaques were further subjected to secondary and tertiary screenings to confirm the specificity. KLIP1-specific plaques were then isolated, excised in vivo as pBluescript phagemids, and sequenced using both T3 and T7 primers. The DNA sequences were assembled and analyzed with Lasergene 99 program (DNASTAR Inc., Madison, Wis.).
RNA ligase-mediated 5′-rapid amplification of cDNA ends (RLM-RACE).
Sequences from the 5′ ends of full-length, capped mRNA were obtained by RLM-RACE using a commercial kit (Ambion, Inc., Austin, Tex.), as previously described (45). RNA isolated from BC-3 cells was used as template for RLM-RACE. The primers used in the first round PCR were 5′-RACE outer primer from the kit and KLIP1-R1 (5′AGAGGGCTGGGCACTAAG3′). The primers used for the second round PCR were 5′-RACE inner primer from the kit and KLIP1-R2 (5′GTCGAACACGTCAATAGG3′). For high-temperature reverse transcription, MasterAmp Tth DNA polymerase and MasterAmp PCR Enhancer containing betaine (trimethyl glycine) (Epicentre Technologies Corporation, Madison, Wis.) were used. PCR products were separated in an agarose gel, purified and sequenced. The DNA sequences were assembled and analyzed with the Lasergene 99 program.
Transient transfection and reporter assay.
For HeLa, COS-7, and 293 cells, transfection experiments were performed with Lipofectamine 2000 reagent according to the instructions of the manufacturer (Invitrogen). For primary human umbilical vein endothelial cells (HUVEC), transfection experiments were carried out with Lipofectin according to the instructions of the manufacturer (Invitrogen). For BJAB and BCBL-1 cells, transfection experiments were performed by electroporation, as described previously (33). A chloramphenicol acetyl transferase (CAT) assay was performed as previously reported (11). In all reporter assays, total amount of DNA was equalized by addition of salmon sperm carrier DNA. Transfection efficiencies were normalized by cotransfection with a reporter plasmid, pSV-β-galactosidase, and the β-galactosidase activity was determined following the instructions of the manufacturer (Promega). The conversion rate of the modified 14C-labeled chloramphenicol was calculated with the Multi-Analysis Program.
Confocal microscopy.
COS-7 cells transfected with GFP-KLIP1 alone or together with pDsRed-LNA constructs were fixed with 2% paraformaldehyde at 36 h posttransfection, and observed with a laser scan confocal microscope (Olympus FV-500). Cells with green and red florescence were visualized under 488 nm and 543 nm laser line, respectively. Digital photos were taken with the aid of the FluoView program (version 3.5), using a PlanApo 60 × 1.4 NA objective lens.
Generation of KLIP1 antibody.
A synthetic peptide from a highly antigenic region (aa 3 to 23) of KLIP1 protein was commercially synthesized and conjugated to KLH by a standard method (2). The peptide was used to prepare a mouse polyclonal antibody named anti-KLIP1-N according to standard procedures (2).
Western blot assay.
Western blot assays were performed as described previously (12). Proteins from yeast or mammalian cells were resolved on an SDS-PAGE and transferred to a nitrocellulose membrane. To detect LNA expression, the membrane was reacted with an anti-LNA monoclonal antibody (ABI Advanced Biotechnologies) or a well-characterized LNA-positive hyper-immune serum from a KS patient (12). Specific bands were revealed with an anti-rat or anti-human IgG alkaline phosphatase conjugate using NBT/BCIP as substrate (Invitrogen). To detect KLIP1 protein expression, the membrane was reacted with anti-KLIP1-N antibody, and specific signals were revealed with an anti-mouse IgG alkaline phosphatase conjugate. Similarly, GFP was detected with a mouse monoclonal antibody B-2 to GFP and GST protein was detected with a mouse monoclonal antibody GST-2 (Sigma).
GenBank accession numbers.
The cDNA sequence of the new protein KLIP1 was deposited in GenBank (AF469667), including the 5′-untranslated sequence.
RESULTS
Identification of KLIP1 by yeast two-hybrid screening.
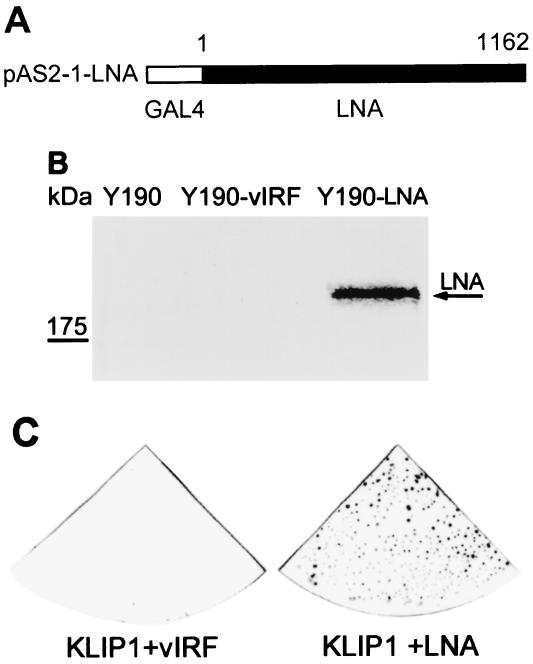
To identify cellular proteins interacting with LNA, a yeast two-hybrid screening was performed with the pAS2-1-LNA construct, in which the full-length LNA was fused to the GAL4 DBD, as a bait (Fig. 1A). LNA expression in yeast was confirmed by Western blot assay, using LNA specific antibodies (Fig. 1B). The LNA bait was used to screen a human B-cell library expressing proteins fused to the GAL4 AD. Repeated screenings identified a total of 60 primary positive clones that activated reporter gene expression in the presence of LNA fusion protein. Plasmid DNA from these clones was extracted and cotransformed with the bait plasmid into yeast, followed by a filter-lift assay. A total of 28 clones were confirmed to activate reporter gene expression in the presence of LNA fusion proteins, but not in the presence of the unrelated fusion protein vIRF (Fig. 1C). CPRG assay was performed with all of the 28 clones, and the resulting values ranged from 10 to 4,960 units, while the negative controls had 0 values. DNA sequencing revealed that 2 of the strongest clones with CPRG value of 4,960 and 4,860 shared the same DNA sequences with no sequence homology to any known genes but they partially matched an expression sequence tag in GenBank database at the time of the identification. We named this protein as KHSV LNA-interacting protein 1 (KLIP1).
FIG. 1.
Identification of LNA-interacting proteins by yeast two-hybrid screening. Full-length LNA (KSHV ORF73) was cloned into pAS2-1 vector (A). Detection of LNA GAL4 DBD fusion protein in Y190 yeast cells transformed with pAS2-1-LNA by Western blot assay (B). The arrowhead indicates the LNA band (lane 3). Y190 alone (lane1) and Y190 transformed with a recombinant pAS2-1 plasmid containing vIRF (KSHV ORF-K9) gene (lane 2) were used as negative controls. (C) The prey DNA was extracted from the positive clones after initial screening and transformed with either LNA bait construct or negative control vIRF construct to confirm the interaction. The novel clone was shown as a representative result.
Confirmation of LNA-KLIP1 interaction by GST pull down assay.
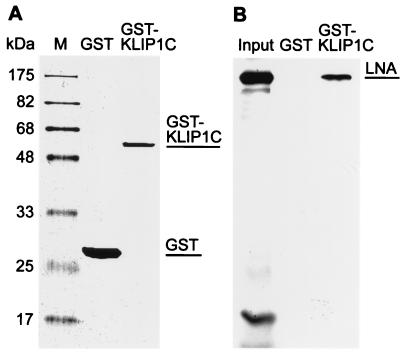
To further confirm the interaction between LNA and KLIP1 biochemically, a GST pull down assay was performed. The partial cDNA sequence of KLIP1 isolated in the yeast two-hybrid screening was expressed as GST fusion protein (GST-KLIP1C) (Fig. 2A). In the GST pull down assay, GST-KLIP1C bound to 35S-labeled LNA as expected (Fig. 2B). A specific band of about 220 kDa, corresponding to LNA, was visible in the pull down extract from reaction with initial input-containing LNA and GST-KLIP1C fusion protein. No specific signal was visible in a negative control pull down extract from reaction with initial input containing LNA and GST protein.
FIG. 2.
Confirmation of LNA and KLIP1 interaction by GST pull down assay. (A) Purified GST (lane 1), GST-KLIP1C (lane 2) fusion proteins visualized by SDS-PAGE and Coomassie blue staining. (B) [35S]methionine-labeled LNA protein was pulled-down by the GST-KLIP1C fusion protein (lane 3) but not by GST (lane 2). Lane 1 showed the amount of input LNA.
KLIP1 binds to the N-terminal domain of LNA.
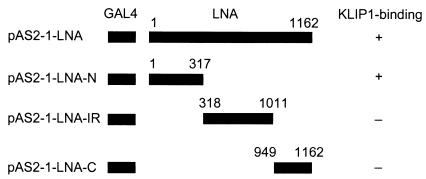
To map the LNA domain interacting with KLIP1, the N-terminal, C-terminal, or internal repeat domains of LNA were fused with GAL4 DBD (Fig. 3), and cotransformed with pACT-KLIP1 into yeast, respectively, to test their abilities to activate the reporter gene expression. The N-terminal domain, but not the C-terminal and internal repeat domains of LNA, activated reporter gene expression (Fig. 3). These results indicated that the LNA domain interacting with KLIP1 is located in the N-terminal domain of the protein.
FIG. 3.
Mapping of LNA domain interacting with KLIP1 protein by yeast-two hybrid assay. Schematic diagram of the full-length LNA and different truncated LNA fragments fused to GAL4 DBD, and their abilities to activate reporter after cotransfection with pACT-KLIP1 plasmid isolated through the initial yeast two-hybrid screening.
Identification of KLIP1 transcripts and examination of KLIP1 expression in different cell types.
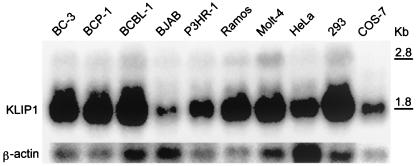
To determine the expression of KLIP1 gene in cells, we examined the expression of its transcript(s) in several cell lines by Northern blot hybridization. A major transcript of approximately 1.8 kb was detected in all the cell lines examined (Fig. 4). Another weak band containing a transcript of approximately 2.8 kb was also detected in some cell lines, which might represent a minor KLIP1 transcript. The 1.8-kb major transcript was present in all the human cell lines tested, including B cells (BC-3, BCP-1, BCBL-1, BJAB, P3HR-1, and Ramos), T cells (Molt-4), and epithelial cells (HeLa and 293), and an African green monkey fibroblast cell line COS-7. The expression of a KLIP1 major transcript in all human cell lines examined indicates that KLIP1 is likely a ubiquitous cellular protein. The detection of KLIP1 major transcript in COS-7 cells also indicates the presence of a similar gene in African green monkey cells, and the sequence conservation of this gene in these two species.
FIG. 4.
Expression of KLIP1 transcripts in different cell lines detected by Northern blot hybridization. Total RNA from cells was separated in a denaturing agarose gel, transferred to a membrane, and probed with a KLIP1 riboprobe. β-actin probe was used to hybridize the same membrane after striping of the membrane to calibrate the RNA loading.
Identification of KLIP1 coding sequences by cDNA library screening and DNA sequencing.
The original KLIP1 cDNA clone identified through yeast two-hybrid screening is shorter than the transcript size detected by Northern blot hybridization and has an open 5′ end, indicating that it is a partial cDNA. To identify the full-length KLIP1 coding sequence, we screened a cDNA library constructed from BC-3 cells using the isolated KLIP1 cDNA as a probe. Twenty-eight positive clones were selected after three-round of screening. Most clones had an insert of 2.1 kb, while the other clones had inserts of either 1.6 or 1.7 kb. DNA from these clones were subjected to restriction enzyme digestion with KpnI and EcoRI, and clones with representative restriction patterns were selected and sequenced.
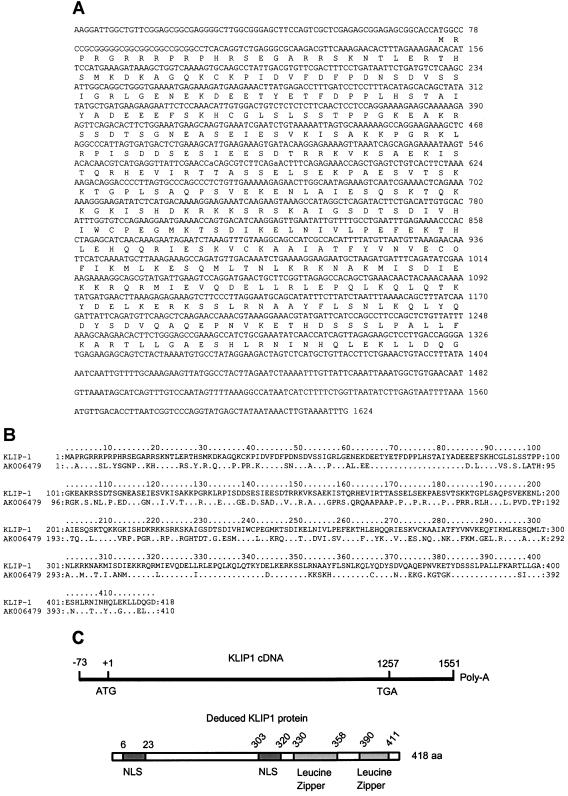
Analysis of the KLIP1 cDNA sequences identified the largest potential ORF of KLIP1, which was 1,257 bp in size (Fig. 5A). All of the clones except the clones with 2.1-kb insert contained the full-length sequence of the ORF. A GenBank database search with the KLIP1 cDNA sequence failed to identify any homologous genes with known functions, except several expressed sequence tags, a human cDNA sequence with unknown function (FJ23468) that was 195 nucleotides (nt) shorter at its 5′ end than that of KLIP1 cDNA, and a mouse gene with unknown function (AK006479) that had 56% identity to KLIP1 at the amino acid level (Fig. 5B).
FIG. 5.
cDNA sequence of the major KLIP1 transcript, deduced amino acid sequence of KLIP1 protein, and its structural features. (A) cDNA sequence of the major KLIP1 transcript and deduced amino acid sequence. There are 73 nt at the 5′ end and 294 nt at the 3′ end of the untranslated regions of KLIP1 transcript, respectively. (B) Sequence alignment of the KLIP1 deduced amino acid sequence with a mouse homologous sequence (AK006479). Identical amino acids in the homologous mouse sequence are indicated. (C) The deduced 418 aa protein contains two putative nuclear localization signals (NLS) and two Leucine zipper motifs.
Full-length transcript of KLIP1 gene.
To map the full-length KLIP1 mRNA, we performed 5′-RLM-RACE. A specific single band was obtained from the KLIP1 transcript with intact 5′ end. The PCR products were cloned into pBluescript SK vector and confirmed by restriction enzyme digestion. DNA sequencing showed that there were 73 nt upstream of the ATG initiation codon with a G+C content of 67% (Fig. 5A).
Alignment of the 3′ end of KLIP1 cDNA with the cDNA sequence (FJ23468) in GenBank showed that the poly(A) sequence of KLIP1 cDNA started at 294 nt downstream of the stop codon. Thus, the full-length KLIP1 transcript is 1,624 nt excluding the poly(A) sequence, which is consistent with the 1.8-kb KLIP1 major transcript identified by Northern blot hybridization (Fig. 4). Two of the KLIP1 cDNA clones had 143 and 915 nt longer sequences downstream of the poly(A) signal sequence, respectively. These clones might correspond to the 2.8-kb KLIP1 transcript, or were merely due to artifacts generated during the construction of the cDNA library.
The entire KLIP1 cDNA sequences could be aligned with sequences from chromosome 4, indicating that the authentic KLIP1 gene is located on this chromosome. The 5′ end (700 bp) of KLIP1 cDNA also had 99 to 100% sequence identity with chromosome 3 while most of KLIP1 cDNA sequences had 84 to 85% sequence identity with sequences from chromosomes 11 and 12, suggesting the presence of KLIP1 pseudogenes or other members from the same gene family on these chromosomes. Sequence alignment with the incomplete chromosome 4 sequences indicated that KLIP1 had at least 13 exons. Several cancer-related genes such as interferon regulatory factor 2, FAT tumor suppressor (Drosophila) homolog, and apoptosis-related cystine protease were found in the proximity of KLIP1 gene.
KLIP1 ORF has 40.33% G+C content, and encodes a 418-aa polypeptide with a theoretical molecular mass of 47.5 kDa and an isoelectric point of 9.14. There are two putative nuclear localization signals (NLS) located at aa 6 to 23 and 303 to 320, respectively (Fig. 5C). Protein analysis of the deduced amino acid sequences showed that the protein is highly hydrophilic and antigenic, which is consistent with the characteristics of nuclear proteins. At the C-terminal domain, there are two leucine zipper motifs at aa 330 to 358 and 390 to 411, respectively (Fig. 5C). Using the gene family identification system, the predicted secondary structure of KLIP1 protein was found to be similar to the kinesin motor domain pattern and the myosin heavy chain superfamily.
Antibody to KLIP1 detects a doublet of two bands by Western blot assay.
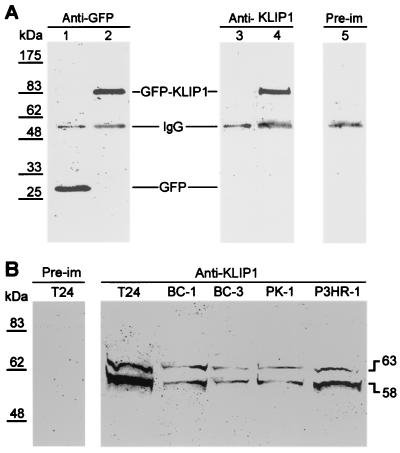
To identify the cellular protein(s) encoded by the KLIP1 gene, a polyclonal antibody, named anti-KLIP1-N, was generated using a synthetic polypeptide corresponding to a highly antigenic region (aa 3 to 23) in KLIP1 protein. To determine the specificity of the antibody, we overexpressed KLIP1 as a GFP fusion protein by transient transfection in 293 cells, and used the fusion protein as a positive control in immunoprecipitation and Western blot assay (Fig. 6A). Antibody to GFP detected both GFP and GFP-KLIP1 proteins in cells transfected with control GFP and GFP-KLIP1, respectively. Anti-KLIP1-N detected the GFP-KLIP1 fusion protein, but not GFP alone, while preimmune serum did not detect GFP-KLIP1, suggesting that the anti-KLIP1-N peptide antibody specifically recognized KLIP1 protein (Fig. 6A). The anti-KLIP1-N peptide antibody was then used to examine the expression of KLIP1 in different cell lines. As shown in Fig. 6B, preimmune serum again failed to detect any protein band in T24 cells, while anti-KLIP1-N detected a doublet of two bands of 58 and 63 kDa in all cell lines tested, including T24, BC-1, BC-3, PK-1, and P3HR-1 (Fig. 6B).
FIG. 6.
Detection of KLIP1 protein in different cell lines by immunoprecipitation and Western blot assay. (A) GFP vector (lane 1 and 3) or GFP-KLIP1 (lanes 2, 4, and 5) were transfected into 293 cells. The cell lysate was immunoprecipitated with an anti-GFP monoclonal antibody and further immunoblotted with the anti-GFP monoclonal antibody (lanes 1 and 2), anti-KLIP1-N peptide antibody (lanes 3 and 4), or preimmune serum (lane 5). Anti-KLIP1 peptide antibody detected the fusion protein GFP-KLIP1 but not GFP alone, while preimmune serum failed to detect GFP-KLIP1 protein band. (B) In Western blot assay, the anti-KLIP1-N synthetic peptide antibody, but not the preimmune serum detected a doublet of 58 and 63 kDa in all cell lines examined.
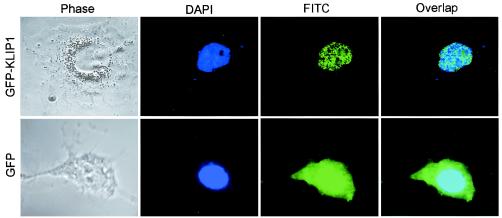
KLIP1 is a nuclear protein.
The fact that KLIP1 interacts with nuclear protein LNA and has two potential NLS (Fig. 5C) suggests that it is a nuclear protein. To study the subcellular localization of KLIP1 protein, GFP-KLIP1 construct was transiently transfected into COS-7 cells. Figure 7 showed that cells transfected with GFP-KLIP1 DNA had a distinct nuclear speckle fluorescence pattern, while cells transfected with GFP vector had both cytoplasmic and nuclear fluorescence distribution.
FIG. 7.
KLIP1 is a nuclear protein. Speckle fluorescence distribution was observed in the nucleus of COS-7 cells transiently transfected with GFP-KLIP1 plasmid DNA. COS-7 cells transfected with GFP vector control had whole cell fluorescence distribution.
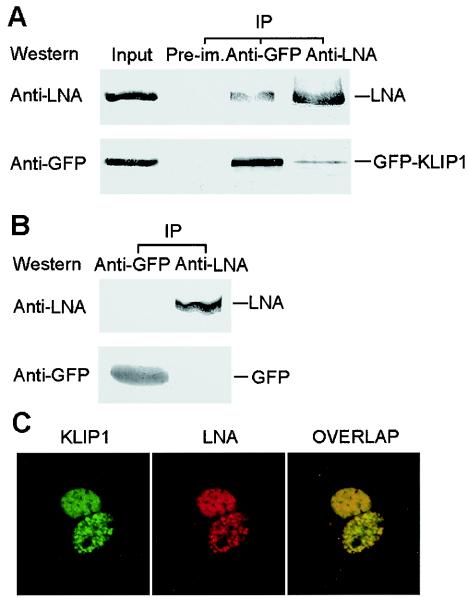
KLIP1 interacts with LNA in vivo.
As stated in the above, we have demonstrated that KLIP1 interacts with LNA in vitro. To determine the biological relevance of a KLIP1-LNA interaction, their in vivo interaction was further examined. The GFP expression vector or the GFP-KLIP1 construct was transfected into BCBL-1 cells and a reciprocal coimmunoprecipitation experiment was performed with anti-GFP and anti-LNA antibodies. As shown in Fig. 8A, anti-LNA antibody coimmunoprecipitated LNA and GFP-KLIP1, while anti-GFP antibody coprecipitated GFP-KLIP1 and LNA in BCBL-1 cells transfected with the GFP-KLIP1 construct. In GFP vector-transfected cells, both anti-GFP and anti-LNA antibodies failed to coimmunoprecipitate each other, indicating that the interaction between KLIP1 and LNA is specific (Fig. 8B).
FIG. 8.
KLIP1 interacts with LNA in vivo. (A) Lysate from GFP-KLIP1-transfected BCBL-1 cells was immunoprecipitated with either anti-GFP (lane 3) or anti-LNA (lane 4) antibodies and then subjected to Western blotting with these two antibodies, respectively. The cell lysate was also precipitated with preimmune serum (lane 2, denoted Pre-im.) as a negative control. Lysates that were equal to 35% of those used in immunoprecipitation were run in PAGE and detected by Western blotting with anti-GFP and anti-LNA antibodies (lane 1). (B) Reciprocal coimmunoprecipitation was performed with GFP-transfected BCBL-1 cells to verify the specificity of the interaction between LNA and KLIP1. (C) Speckle green and red fluorescence distribution was observed in the nucleus of COS-7 cells transiently transfected with GFP-KLIP1 and pDsRed-LNA plasmid DNA. The distribution of both KLIP1 and LNA fusion proteins was identical and overlapped with each other, indicating colocalization of both proteins.
To observe whether KLIP1 and LNA colocalize in the cells, GFP-KLIP1 and pDsRed-LNA constructs were cotransfected into COS-7 cells. The cells were examined with a confocal microscope. Figure 8C showed that both KLIP1 and LNA exhibited the same distribution pattern of nuclear speckles, and overlapped with each other. These results demonstrated a KLIP1-LNA interaction in vivo.
KLIP1 has transcriptional repression activity.
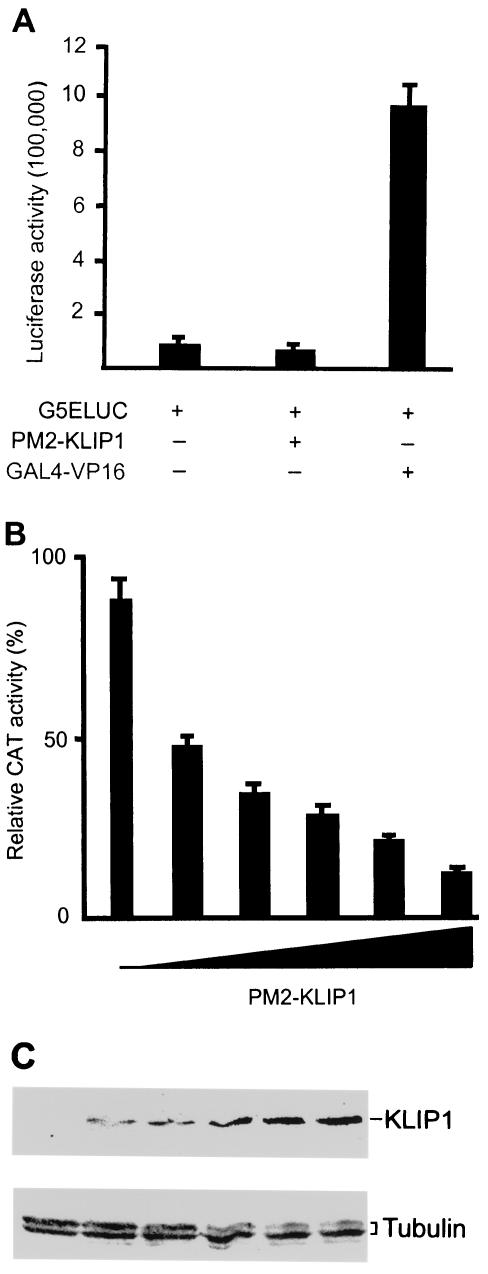
In previous studies, it was demonstrated that LNA regulates the expression of cellular genes through interaction with cellular transcriptional factors such as p53, pRb, ATF4, and proteins of the mSin3 complex, etc. Since KLIP1 is a nuclear protein, has two leucine zipper motifs, and interacts with LNA, we speculated that KLIP1 might be a transcriptional factor. To test this hypothesis, we performed a mammalian yeast one-hybrid assay. The full-length KLIP1 ORF was fused in-frame with GAL4 DBD into PM2 vector. The resulting PM2-KLIP1 construct was cotransfected with the G5ELUC reporter vector, in which the sequence recognized by GAL4-DBD was placed upstream of the luciferase reporter gene (44), into 293 cells to test KLIP1's transactivation activity. As shown in Fig. 9A, no transactivation activity was observed. The same results were also obtained in HeLa, COS-7, and NIH 3T3 cells (data not shown).
FIG. 9.
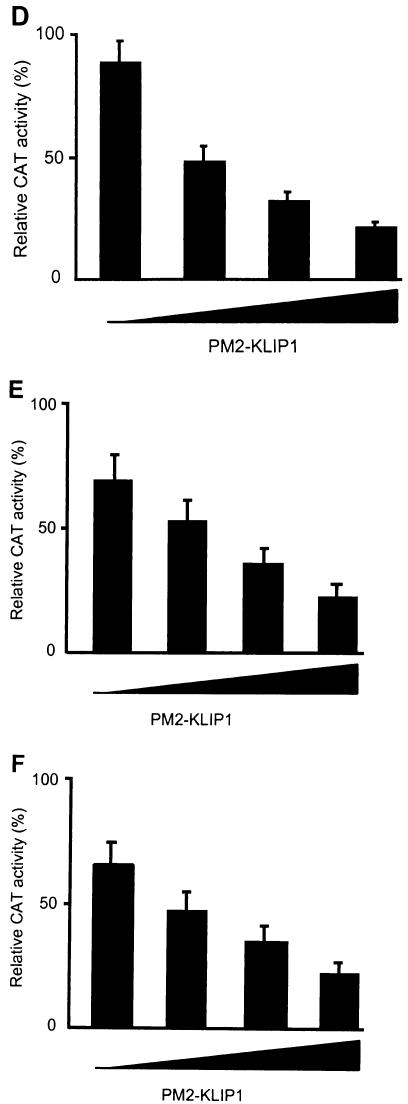
KLIP1 repressed TK promoter activity in a dose-dependent fashion in a mammalian one-hybrid assay. (A) Luciferase activity in 293 cells either transfected with 1 μg G5Eluc vector alone or cotransfected with 1 μg G5Eluc and 1 μg PM2-KLIP1. GAL4-VP16 was cotransfected with G5Eluc as a positive control. (B) Relative CAT activity in 293 cells cotransfected with 1 μg of GAL4-TK-CAT reporter plasmid and 0, 0.25, 0.5, 075, 1, or 2 μg of PM2-KLIP1. At 2 μg, KLIP1 repressed up to 87% of TK promoter activity. (C) Western blotting assay was carried out with anti-GAL4 DBD antibodies to examine the expression of PM2-KLIP1 fusion protein in 293 cells transfected with different doses of the plasmid DNA cited in (B). α-tubulin expression was used as loading control. (D to F) Relative CAT activity in HeLa (D), BJAB (E) and HUVEC (F) cells cotransfected with 1 μg of GAL4-TK-CAT reporter and 0, 0.5, 1, or 2 μg of PM2-KLIP1 plasmid DNA. Percentage denotes the ratio between the acetylated substrate and the whole amount of substrate used in the assay. Results are the averages and standard deviations from three independent experiments except in panels A and F, which are the averages and the high and low values from two independent experiments.
To determine whether KLIP1 has transcriptional repression activity, we cotransfected the PM2-KLIP1 construct and a GAL4-thymidine kinase (TK)-CAT reporter into HeLa cells. GAL4-TK-CAT reporter construct has a UAS upstream of the TK promoter that can recruit the GAL4 DBD-KLIP1 fusion protein to the TK promoter. As shown in Fig. 9B, GAL4-TK-CAT had strong CAT activity when transfected alone into 293 cells. Expression of KLIP1 repressed 87% of the CAT reporter activities in a dose-dependent fashion (Fig. 9B and C). Similar results were also obtained with HeLa, BJAB and HUVEC cells. In HeLa cells, KLIP1 repressed 75% of the CAT reporter activities (Fig. 9D), while in BJAB and HUVEC cells, the CAT reporter activities were repressed 67% and 66%, respectively (Fig. 9E and F). In parallel experiments, GFP-KLIP1 did not repress GAL4-TK-CAT reporter activity while PM2-KLIP1 failed to repress the expression of CAT reporter in a PBLCAT2 vector, in which there was no UAS sequence upstream of TK promoter (data not shown), thus confirming the specificity of the KLIP1's repression activity.
LNA abolishes the transcriptional repression activity of KLIP1.
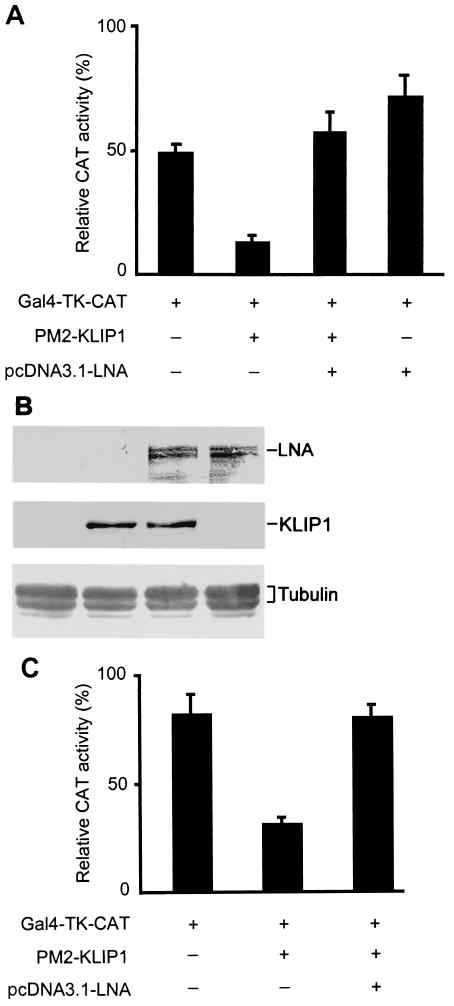
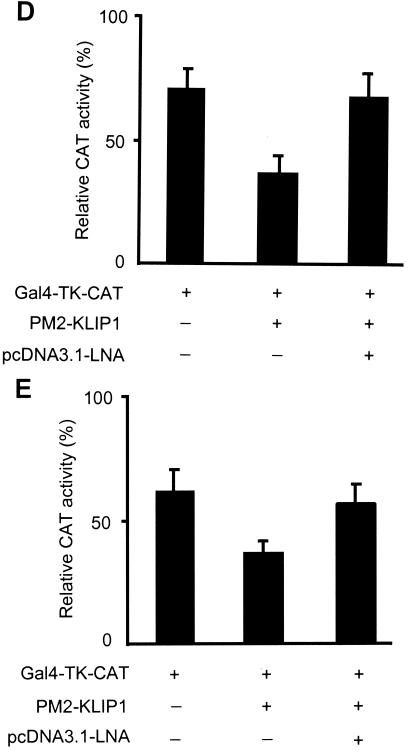
To examine whether LNA-KLIP1 interaction interferes with KLIP1's transcriptional repression function, we cotransfected LNA mammalian expression plasmid pcDNA3.1His-LNA into the above reporter system, in which the TK promoter activity was repressed by PM2-KLIP1. Figure 10A showed that LNA relieved TK promoter activity repressed by KLIP1, resulting in an increase in the reporter activity in 293 cells. The expression of KLIP1 and LNA protein was detected in the transfected cells (Fig. 10B). Similar results were also obtained in HeLa cells (Fig. 10C), BJAB cells (Fig. 10D), and HUVEC (Fig. 10E).
FIG. 10.
LNA alleviates KLIP1's transcriptional repression effect in a mammalian one-hybrid assay. (A) Relative CAT activity in 293 cells transfected with GAL4-TK-CAT reporter (1 μg) either alone, or cotransfected together with PM2-KLIP1 (1 μg) or pcDNA3.1-LNA (1 μg), respectively, or with both PM2-KLIP1 (1 μg) and pcDNA3.1-LNA (1 μg). (B) The expression of LNA and PM2-KLIP1 in 293 cells transfected with pcDNA3.1-LNA was detected by Western blot assay using anti-LNA antibodies and anti-GAL4 DBD antibodies, respectively. α-tubulin expression was used as loading control. (C through E) Relative CAT activity in HeLa (C), BJAB (D) and HUVEC (E) cells, transfected with GAL4-TK-CAT reporter (1 μg) either alone, or cotransfected together with PM2-KLIP1 (1 μg) or both PM2-KLIP1 (1 μg) and pcDNA3.1-LNA (1 μg). Percentage indicates the ratio between the acetylated substrate and the whole amount of substrate used in the assay. Results are the averages and standard deviations from three independent experiments.
DISCUSSION
A novel cellular protein interacting with KSHV LNA was identified through yeast two-hybrid screening from a human cDNA library and named KLIP1. The interaction between KLIP1 and LNA was confirmed by a GST pull down assay in vitro and by coimmunoprecipitation as well as colocalization examination in vivo. Northern blot hybridization detected two KLIP1 transcripts: a major transcript of 1.8 kb and a minor transcript of 2.8 kb. Mapping of the major transcript by cDNA library screening and 5′-RACE revealed an ORF of 1,257 bp encoding KLIP1. No homologous gene with known function in GenBank database has been identified for KLIP1, indicating that KLIP1 is a novel gene. Only a shorter cDNA sequence with an unknown protein product is identical to a portion of the KLIP1 sequences in the GenBank database. A homologous mouse gene with an unknown function is present in the database, indicating the presence of a similar gene in the mouse genome. The major KLIP1 transcript is also present in an African green monkey cell line, suggesting that KLIP1 is likely a conserved mammalian gene.
RLM-RACE analysis of the 5′ end of KLIP1 full-length mRNA identified a 73 nt untranslated region upstream of the KLIP1 ATG initiation codon. The 5′-end untranslated region of the KLIP1 transcript has a high G+C content, resulting in the formation of a mRNA stem loop secondary structure that prevents efficient reverse transcription in vitro (45). Thus, cDNA clones isolated through screening of a cDNA library had a shorter 5′-end sequence than the natural transcripts. High temperature reaction in combination with the use of Tth DNA polymerase was applied to extend the reaction through the high G+C region in reverse transcription. Sequence alignment identified 294 nt at the 3′-end untranslated region downstream of the stop codon.
The major KLIP1 transcript is present in all human cell lines examined, including B cells, T cells, epithelial cells, and fibroblast cells, indicating that KLIP1 is a housekeeping gene. Antiserum to a synthetic peptide representing a unique region of KLIP1 detected a doublet comprised of proteins of 58 and 63 kDa in a Western blot assay. Both proteins were larger than the theoretical molecular mass of 48 kDa predicted for KLIP1. The doublet could result from posttranslation modifications such as phosphorylation, alternative splicing or other undetermined reasons. Indeed, several potential phosphorylation sites are present in KLIP1. Future investigations will determine whether these sites are functional.
Consistent with its possession of two leucine zipper motifs, two nuclear localization signals and its interaction with LNA, KLIP1 has transcriptional repression activity, suggesting that KLIP1 is involved in controlling cellular gene transcription. We have demonstrated that KLIP1 represses transcription in a reporter assay after being recruited to the TK promoter through a GAL4 DBD fusion. It is possible that, in the cellular context, KLIP1 could function as a specific transcriptional repressor that represses the transcriptional activity of certain cellular gene promoters; KLIP1 could also function as a transcription corepressor by interacting with other transcription factors through its leucine zipper domain(s), a domain that is known to mediate protein-protein interaction. Whether KLIP1 itself has DNA-binding activity requires further investigation.
LNA is a major viral latent protein expressed in KS, primary effusion lymphoma, multicentric Castleman's disease, and KSHV-infected cell lines, and has essential function in sustaining KSHV latent infection (8, 12, 13, 23). Besides its role in ensuring KSHV episomal stability and segregation into daughter cells during cell mitosis (3, 7, 41), and targeting p53 and pRb tumor suppressor pathways to evade host antiviral and/or antitumor defenses (10, 35), LNA is also involved in transcription modulation via regulating the expression of cellular genes. LNA transactivates an artificial promoter carrying the cell cycle transcription factor E2F DNA-binding sequences, cyclin E (CCNE1) promoter, and the telomerase reverse transcriptase promoter (25, 35). Using microarray, LNA was shown to modulate the expression of 6 known cellular genes and 9 expressed sequence tags (37). LNA also auto-transactivates its own promoter, and functions as a transcriptional repressor and represses activated expression from the EBV Qp and Cp latency promoters (27). These data suggest that LNA regulates the transcription of cellular genes and EBV genes in KSHV-infected or KSHV-EBV dually infected cells to favor KSHV latent replication.
The specific DNA-binding activity of LNA on cellular promoters has not been established. However, several studies have shown that LNA modulates gene transcription through direct interaction with transcriptional activators and repressors, or modulation of chromatin structure. LNA binds to CBP to interfere with its interaction with c-fos and histone acetyltransferase activity (29), with ATF4/CREB2 to inhibit its transcriptional activation activity (30), and with proteins of mSin3 corepressor complex including SAP30, mSin3A, and CIR to mediate transcription repression (27). In addition, LNA binds, induces and relocates RING3, a human homologue of the fsh (female sterile homeotic) gene product of Drosophila, to nuclear heterochromatin regions (31, 34). We have shown that KLIP1 interacts with the LNA N-terminal domain, with which proteins of mSin3 corepresser complex also interact (27). It is conceivable that KLIP1 is a previous unidentified component of the mSin3 corepressor complex or other transcriptional complexes.
Our results have shown that LNA is capable of alleviating the transcriptional repression effect of KLIP1 in a reporter assay. This is likely due to the specific interaction between these two proteins, since LNA itself has little effect on reporter expression in the absence of KLIP1 (Fig. 10). The mechanism could be summarized in a model, in which, when KLIP1 is recruited to the promoter region by GAL4, it interacts with chromatin remodeling proteins or basal transcription machinery, resulting in the repression of reporter expression. When LNA is applied, it disrupts the interaction between KLIP1 and other factors, releasing KLIP1's repression effect. Previous studies had demonstrated that LNA has transcriptional repression activity in a similar one-hybrid assay (39), although some other studies showed that it is capable of transactivating certain promoters or binding sites (37). We failed to observe synergistic effect or at least repression when LNA was applied in the reporter assay. These data suggested that LNA might have certain specificity in deregulating cellular transcription processes.
Together with previous reports, these data further confirm that LNA interacts with cellular transcriptional factors to deregulate cellular transcriptional machinery. This strategy is commonly used by other tumor viruses to evade host antiviral defenses and is likely essential for KSHV latent infection. Further studies are warranted to elucidate the biologic functions of KLIP1, and how LNA interacts with KLIP1 to deregulate its function.
Acknowledgments
This work was supported in part by funds from the Elsa U. Pardee Foundation and the Association for International Cancer Research and by NIH grants HL60604-01 and CA096512 to S.-J. Gao.
We thank Charles Gauntt in the Department of Microbiology and Immunology, University of Texas Health Science Center at San Antonio, for his helpful comments; Victoria Frohlich for her technical assistance in confocal microscopy; and Phang-Lang Chen for providing pBSKF and CHPL-GFP plasmids.
REFERENCES
- 1.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi's sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Current protocols in molecular biology, vol. 1-2. John Wiley and Sons, New York, N.Y.
- 3.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 4.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff, C., and R. A. Weiss. 2001. Epidemiology and pathogenesis of Kaposi's sarcoma-associated herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:517-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Cotter, M. A., and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 8.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durfee, T., K. Becherer, P. L. Chen, S. H. Yeh, Y. Yang, A. E. Kilburn, W. H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. [DOI] [PubMed] [Google Scholar]
- 10.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 11.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 12.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 13.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 14.Garber, A., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 15.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin, B. E. 2000. Epstein-Barr virus (EBV) and human disease: facts, opinions and problems. Mutat. Res. 462:395-405. [DOI] [PubMed] [Google Scholar]
- 17.Groves, A. K., M. A. Cotter, C. Subramanian, and E. S. Robertson. 2001. The latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters. J. Virol. 75:9446-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, J., A. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated virus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun, T. S., C. Subramanian, M. A. Cotter II, R. A. Thomas, and E. S. Robertson. 2001. Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 75:8761-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 24.Kellam, P., C. Boshoff, D. Whitby, S. Matthews, R. A. Weiss, and S. J. Talbot. 1997. Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1:19-29. [PubMed] [Google Scholar]
- 25.Knight, J., M. Cotter, I. I., and E. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 275:22971-22978. [DOI] [PubMed] [Google Scholar]
- 26.Krithivas, A., M. Fujimuro, M. Weiner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, S., C. Y. Ku, A. A. Farmer, Y. S. Cong, C. F. Chen, and W. H. Lee. 1998. Identification of a novel cytoplasmic protein that specifically binds to nuclear localization signal motifs. J. Biol. Chem. 273:6183-6189. [DOI] [PubMed] [Google Scholar]
- 29.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of CREB-binding protein is modulated by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpes virus. J. Biol. Chem. 25:31016-31022. [DOI] [PubMed] [Google Scholar]
- 30.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 31.Mattsson, K., C. Kiss, G. M. Platt, G. R. Simpson, E. Kashuba, G. Klein, T. F. Schulz, and L. Szekely. 2002. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J. Gen. Virol. 83:179-188. [DOI] [PubMed] [Google Scholar]
- 32.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt, G. M., G. R. Simpson, S. Mittnacht, and T. F. Schulz. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 36.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, vol. 2. Cold Spring Harbor Laboratories, Cold Spring Harbor, N.Y.
- 39.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinohara, H., M. Fukushi, M. Higuchi, M. Oie, O. Hoshi, T. Ushiki, J.-I. Hayashi, and M. Fujii. 2002. Chromosome binding site of latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced histone H1. J. Virol. 76:12917-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szekely, L., C. Kiss, K. Mattsson, E. Kashuba, K. Pokrovskaja, A. Juhasz, P. Holmvall, and G. Klein. 1999. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J. Gen. Virol. 80:2889-2900. [DOI] [PubMed] [Google Scholar]
- 42.Tang, J., G. M. Gordon, M. G. Muller, M. Dahiya, and K. E. Foreman. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77:5975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, X. P., Y. J. Zhang, J. H. Deng, H. Y. Pan, F. C. Zhou, E. A. Montalvo, and S. J. Gao. 2001. Characterization of the promoter region of the viral interferon regulatory factor encoded by Kaposi's sarcoma-associated herpesvirus. Oncogene 20:523-530. [DOI] [PubMed] [Google Scholar]
- 44.Wu, L. C., Z. W. Wang, J. T. Tsan, M. A. Spillman, A. Phung, X. L. Xu, M. C. Yang, L. Y. Hwang, A. M. Bowcock, and R. Baer. 1996. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat. Genet. 14:430-440. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Y.-J., H.-Y. Pan, and S.-J. Gao. 2001. Reverse transcription slippage over the mRNA secondary structure of the LIP1 gene. BioTechniques 31:1286-1294. [DOI] [PubMed] [Google Scholar]