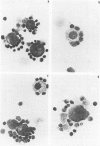
Abstract
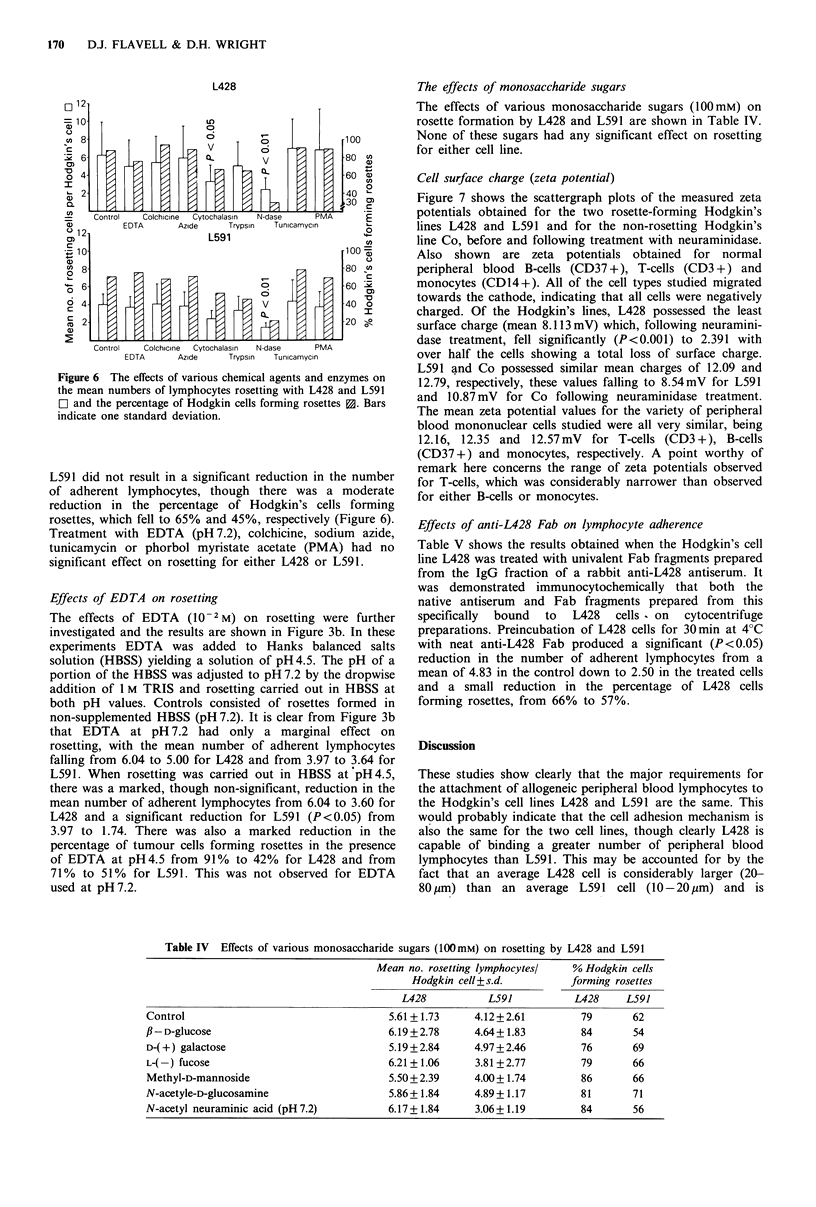
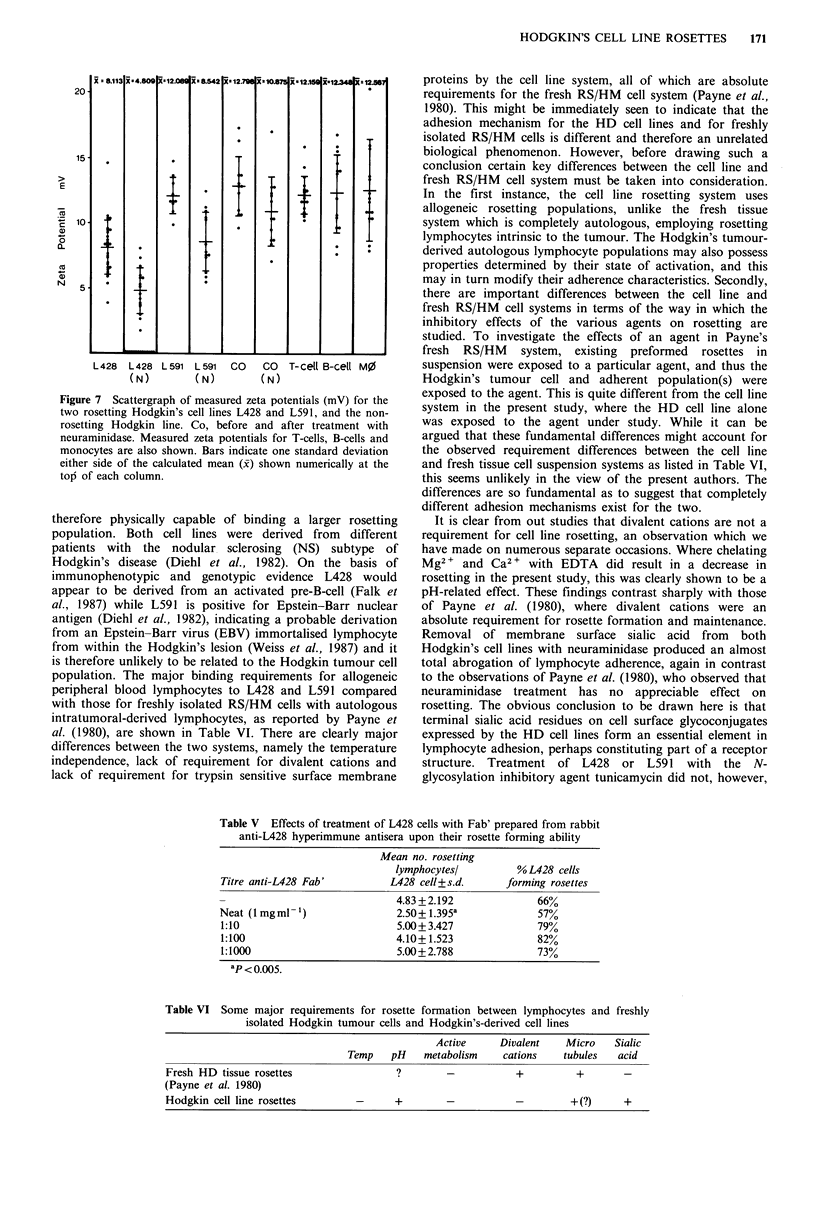
The properties of rosettes formed between the Hodgkin's cell lines, L428 and L591, and allogeneic peripheral blood mononuclear cell populations have been investigated. Immunocytochemical analysis showed that the majority of adherent cells were T-cells of both the CD4 and CD8 subsets. Only relatively few B-cells and monocytes were seen to adhere. However, when peripheral blood mononuclear cell populations were fractionated, it was found that monocytes were as good as T-cells at forming rosettes with both L428 and L591, though B-cells were shown to be poor at forming such associations. Treatment of both L428 and L591 with neuraminidase resulted in a significant reduction (P less than 0.01) in the mean number of adherent lymphocytes and in the numbers of Hodgkin's tumour cells which formed rosettes. Smaller, less significant effects were observed for Cytochalasin B and trypsin. EDTA (10(-2) M) at pH 7.2 had no significant effect on rosetting for L428 or L591. Adherence of allogeneic lymphocytes to L428 or L591 was pH dependent but did not appear to correlate with cell surface charge. Treatment of L428 cells with Fab fragments prepared from the IgG fraction of a hyperimmune rabbit anti-L428 antiserum, significantly (P less than 0.05) inhibited the adherence of allogeneic lymphocytes, but only when used at high concentration. The binding requirements of the Hodgkin's cell lines with allogeneic peripheral blood lymphocytes, as described in this study, appear to be quite different from those described for freshly isolated Hodgkin's tumour cells with autologous intratumoral lymphocytes. This suggests that the two phenomena may be unrelated. There would appear to be an absolute requirement for cell surface sialic acid for allogeneic lymphocyte attachment to the HD cell lines. This might suggest that the receptor-ligand system involved contains sialic acid as an integral part of the cell surface receptor structure involved in recognition of the appropriate ligand.
Full text
PDF

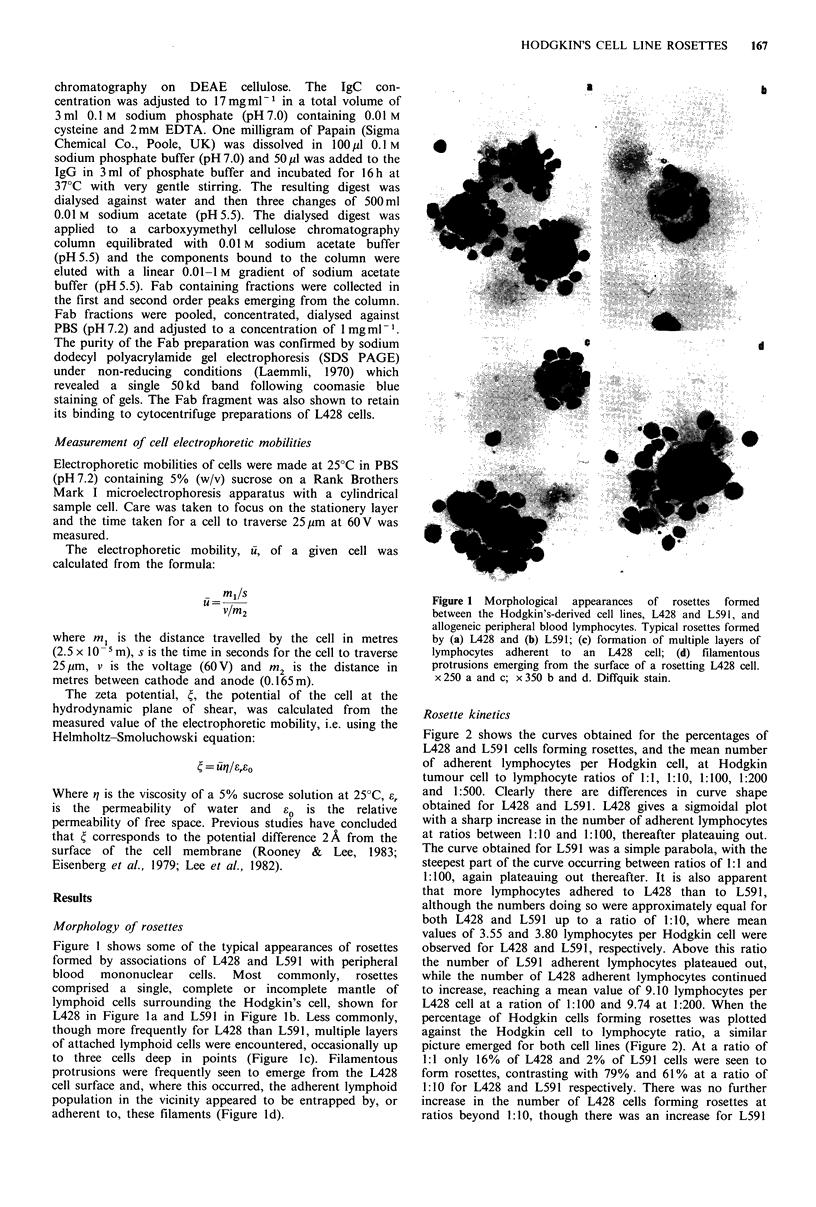


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulaziz Z., Mason D. Y., Stein H., Gatter K. C., Nash J. R. An immunohistological study of the cellular constituents of Hodgkin's disease using a monoclonal antibody panel. Histopathology. 1984 Jan;8(1):1–25. doi: 10.1111/j.1365-2559.1984.tb02318.x. [DOI] [PubMed] [Google Scholar]
- Archibald R. B., Frenster J. H. Quantitative ultrastructural analysis of in vivo lymphocyte-Reed-Sternberg cell interactions in Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:239–245. [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Diehl V., Kirchner H. H., Burrichter H., Stein H., Fonatsch C., Gerdes J., Schaadt M., Heit W., Uchanska-Ziegler B., Ziegler A. Characteristics of Hodgkin's disease-derived cell lines. Cancer Treat Rep. 1982 Apr;66(4):615–632. [PubMed] [Google Scholar]
- Dorreen M. S., Habeshaw J. A., Wrigley P. F., Lister T. A. Distribution of T-lymphocyte subsets in Hodgkin's disease characterized by monoclonal antibodies. Br J Cancer. 1982 Apr;45(4):491–499. doi: 10.1038/bjc.1982.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M. H., Tesch H., Stein H., Diehl V., Jones D. B., Fonatsch C., Bornkamm G. W. Phenotype versus immunoglobulin and T-cell receptor genotype of Hodgkin-derived cell lines: activation of immature lymphoid cells in Hodgkin's disease. Int J Cancer. 1987 Aug 15;40(2):262–269. doi: 10.1002/ijc.2910400223. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Bubbers J. E. Antigen-T lymphocyte interactions: inhibition by cytochalasin B. J Immunol. 1973 Jul;111(1):85–90. [PubMed] [Google Scholar]
- Jondal M., Klein E., Yefenof E. Surface markers on human B and T lymphocytes. VII. Rosette formation between peripheral T lymphocytes and lymphoblastoid B-cell lines. Scand J Immunol. 1975;4(3):259–266. doi: 10.1111/j.1365-3083.1975.tb02625.x. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Scott C. S., Wright D. H., Stein H., Beverley P. C., Payne S. V., Crawford D. H. Phenotypic analysis of an established cell line derived from a patient with Hodgkin's disease (HD). Hematol Oncol. 1985 Apr-Jun;3(2):133–145. doi: 10.1002/hon.2900030205. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Newcom S. R., Gold S. B., Stites D. P. Letter: Origin of Hodgkin's cell. Lancet. 1974 Jul 20;2(7873):167–168. doi: 10.1016/s0140-6736(74)91602-x. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Hodgkin's disease: a war between T-lymphocytes and transformed macrophages? Recent Results Cancer Res. 1976;(56):111–121. doi: 10.1007/978-3-642-81049-7_15. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee A. G., East J. M., Jones O. T., McWhirter J., Rooney E. K., Simmonds A. C. Interaction of fatty acids with the calcium-magnesium ion dependent adenosinetriphosphatase from sarcoplasmic reticulum. Biochemistry. 1982 Dec 7;21(25):6441–6446. doi: 10.1021/bi00268a019. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. I. Characteristics of the antigen-independent-binding of guinea pig thymocytes and lymphocytes to syngeneic macrophages. J Exp Med. 1973 Oct 1;138(4):900–924. doi: 10.1084/jem.138.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Cooper S. A., Jones D. B. Use of the monoclonal antibody WR17, identifying the CD37 gp40-45 Kd antigen complex, in the diagnosis of B-lymphoid malignancy. J Pathol. 1987 May;152(1):13–21. doi: 10.1002/path.1711520103. [DOI] [PubMed] [Google Scholar]
- Morris C. S., Stuart A. E. Reed-Sternberg/lymphocyte rosette: lymphocyte subpopulations as defined by monoclonal antibodies. J Clin Pathol. 1984 Jul;37(7):767–771. doi: 10.1136/jcp.37.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. V., Jones D. B., Wright D. H. Reed-Sternberg-cell/lymphocyte interaction. Lancet. 1977 Oct 8;2(8041):768–769. doi: 10.1016/s0140-6736(77)90281-1. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Rooney E. K., Lee A. G. Binding of hydrophobic drugs to lipid bilayers and to the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1983 Jul 27;732(2):428–440. doi: 10.1016/0005-2736(83)90060-3. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sussman E. H., Luce G. E., Cossman J., Shaw S. Molecular pathways of adhesion in spontaneous rosetting of T-lymphocytes to the Hodgkin's cell line L428. Cancer Res. 1988 Jan 1;48(1):37–40. [PubMed] [Google Scholar]
- Schaadt M., Diehl V., Stein H., Fonatsch C., Kirchner H. H. Two neoplastic cell lines with unique features derived from Hodgkin's disease. Int J Cancer. 1980 Dec 15;26(6):723–731. doi: 10.1002/ijc.2910260605. [DOI] [PubMed] [Google Scholar]
- Stuart A. E., Williams A. R., Habeshaw J. A. Rosetting and other reactions of the Reed-Sternberg cell. J Pathol. 1977 Jun;122(2):81–90. doi: 10.1002/path.1711220205. [DOI] [PubMed] [Google Scholar]
- Suomalainen H. A., Gahmberg C. G., Patarroyo M., Beatty P. G., Schröder J. Genetic assignment of GP90, leukocyte adhesion glycoprotein to human chromosome 21. Somat Cell Mol Genet. 1986 May;12(3):297–302. doi: 10.1007/BF01570789. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Warnke R. A., Purtilo D. T., Sklar J. Epstein-Barr viral DNA in tissues of Hodgkin's disease. Am J Pathol. 1987 Oct;129(1):86–91. [PMC free article] [PubMed] [Google Scholar]
- van Parys G., Van den Oord J., de Wolf-Peeters C., de Vos R., Desmet V. J. Reed-Sternberg cells, lymphocytes, and interdigitating reticulum cell rosettes in Hodgkin's disease. J Clin Pathol. 1985 Nov;38(11):1316–1316. doi: 10.1136/jcp.38.11.1316-a. [DOI] [PMC free article] [PubMed] [Google Scholar]