Abstract
Members of the Argonaute (Ago) protein family associate with small RNAs and have important roles in RNA silencing. Here, we analysed Ago1- and Ago2-containing protein complexes in human cells. Separation of Ago-associated messenger ribonucleoproteins (mRNPs) showed that Ago1 and Ago2 reside in three complexes with distinct Dicer and RNA-induced silencing complex activities. A comprehensive proteomic analysis of Ago-containing mRNPs identified a large number of proteins involved in RNA metabolism. By using co-immunoprecipitation experiments followed by RNase treatment, we biochemically mapped interactions within Ago mRNPs. Using reporter assays and knockdown experiments, we showed that the putative RNA-binding protein RBM4 is required for microRNA-guided gene regulation.
Keywords: Argonaute proteins, gene silencing, microRNA, RNA interference
Introduction
RNA silencing is a conserved gene-silencing pathway that is triggered by double-stranded RNA (dsRNA). DsRNA molecules are processed to small interfering RNAs (siRNAs) or microRNAs (miRNAs), which are then assembled into various effector complexes, including the RNA-induced silencing complex (RISC). MiRNA precursors (pre-miRNAs) are excised from primary transcripts by a nuclear complex containing the RNase III enzyme Drosha and the dsRNA-binding domain protein DGCR8/Pasha. Pre-miRNAs are subsequently exported to the cytoplasm by the export receptor exportin 5, where dsRNAs and pre-miRNAs are further processed by the RNase III enzyme Dicer to duplexes of approximately 22 nucleotides. In a manner similar to Drosha, Dicer functions with the dsRNA-binding domain proteins TRBP (human immunodeficiency virus transactivating response RNA binding protein) and PACT (PKR activator; reviewed by Meister & Tuschl, 2004; Filipowicz et al, 2005; Zamore & Haley, 2005). Only one strand of the siRNA–miRNA duplex intermediate is loaded into the silencing effector complex to become the guide strand. SiRNAs and miRNAs either guide the sequence-specific degradation of complementary RNAs or inhibit the translation of partly complementary target messenger RNAs (Pillai et al, 2007). Recently, a third pathway of miRNA function has been identified in Drosophila, in which miRNAs guide the degradation of not perfectly complementary target mRNAs by recruiting de-adenylation and de-capping enzymes (Behm-Ansmant et al, 2006; Wu et al, 2006).
Members of the Argonaute (Ago) protein family are crucial components of RNA silencing effector complexes. Ago proteins contain PAZ and PIWI domains, and structural studies of archaeal Ago proteins show striking similarity between the PIWI domain and RNase H. Further functional analyses have shown that some Ago proteins contain endonucleolytic activity (Parker & Barford, 2006; Peters & Meister, 2007). Recently, another Ago domain, which binds to the m7G cap of mRNAs, was identified (Kiriakidou et al, 2007).
Biochemical purifications of Ago1 and Ago2 complexes from HeLa cell lysates identified the DExD box protein MOV10 (Moloney leukaemia virus 10 homlogue) and a protein termed TNRC6B (trinucleotide repeat containing 6B) as new components of the Ago complex (Eulalio et al, 2007a). Interestingly, TNRC6B is highly homologous to TNRC6A (GW182), which constitutes a marker protein for cytoplasmic processing bodies (P-bodies). P-bodies are cellular sites of RNA metabolism, and it has been shown that Ago proteins also localize to P-bodies (Eulalio et al, 2007a). MiRNAs and artificially bulged miRNA target mRNAs are also found in P-bodies, and it has been suggested that localization of mRNAs to P-bodies might prevent their translation (Liu et al, 2005; Pillai et al, 2005). Furthermore, a recent study showed that the P-body component RCK/p54 (DEAD box polypeptide 6) is required for miRNA-guided translational repression in human cells (Chu & Rana, 2006). Further studies showed that P-bodies are formed as a consequence of gene silencing, but their integrity is not required for gene silencing (Eulalio et al, 2007b).
Here, we report the biochemical identification and isolation of human Ago1 and Ago2 protein complexes. We identify three distinct Ago1 and Ago2 complexes, which we refer to as Ago complexes I–III. Using a comprehensive proteomic approach, we have identified the protein composition of the Ago complexes. Among these interactors, we found RBM4 (RNA binding motif protein 4) and show that it is required for small RNA-guided gene silencing.
Results And Discussion
Human AGO1 and AGO2 associate with mRNPs
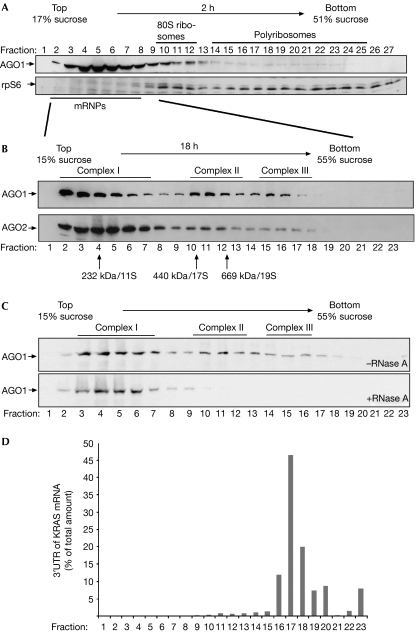
Previously, it has been shown that mammalian Ago proteins and miRNAs sediment with polyribosomes (Kim et al, 2004; Nelson et al, 2004; Maroney et al, 2006; Nottrott et al, 2006). In many studies, however, the majority of Ago proteins and miRNAs migrate together with messenger ribonucleoproteins (mRNPs; Kim et al, 2004; Nelson et al, 2004). For a detailed characterization of Ago protein complexes, we revisited Ago sedimentation in polyribosome fractionations (Fig 1A). Extracts from human embryonic kidney (HEK) 293 cells were separated on a sucrose gradient ranging from 17% to 51%. Fractions were analysed by western blotting against the ribosomal protein S6 (rpS6) to identify ribosome-containing fractions. RpS6 was detected in fractions 10–12, representing ribosomal subunits as well as monosomes, and in fractions 14–26, indicative of polyribosomes. Probing with antibodies against AGO1 showed that human AGO1 predominantly migrated in the fractions with low sucrose density. In addition, a small portion of AGO1 was found in higher molecular weight fractions that also contained polyribosomes (fractions 18–24).
Figure 1.
Human AGO1 and AGO2 associate with distinct protein–RNA complexes. (A) Individual fractions of polyribosome gradients were analysed by western blotting against endogenous AGO1 (upper panel) or rpS6 (lower panel). (B) Lysates from wild-type HEK 293 cells were separated by sucrose density centrifugation under conditions that allow the separation of mRNPs. Endogenous AGO1 and AGO2 were analysed using specific antibodies. (C) Lysates were analysed as in (B). Lysates shown in the lower panel were treated with 100 μg/ml RNase A before centrifugation. (D) A reporter construct containing the KRAS 3′-UTR was transfected into HEK 293 cells and lysates were separated as in (B). Total RNA was extracted from the individual fractions and analysed by qRT–PCR. The distribution of the KRAS 3′-UTR is shown as a percentage of the total amount of the KRAS 3′-UTR. AGO, Argonaute; HEK, human embryonic kidney; KRAS, Kirsten rat sarcoma viral oncogene homologue; mRNPs, messenger ribonucleoproteins; qRT–PCR, quantitative reverse transcription–PCR; rpS6, ribosomal protein S6; 3′-UTR, 3′-untranslated region.
To investigate AGO-containing mRNPs, we established gradient conditions that allowed further separation of the mRNP pool (Fig 1B). HEK 293 cell lysate was loaded onto a 15–55% sucrose gradient and fractionated by centrifugation for 18 h; AGO proteins were then analysed as above. Both AGO1 and AGO2 sedimented in three distinct complexes, which we refer to as AGO complexes I–III. A large portion of AGO1 or AGO2 was found in complex I, which has a molecular mass of about 250–350 kDa (lanes 2–7). Complex II constitutes a second prominent peak, which sediments similarly to a 19S particle and is about 600–700 kDa in size (lanes 10–13). Complex III peaks in fractions 15 and 16 are indicative of a molecular mass of more than 900 kDa or 25–30S (lanes 15,16).
The co-migration of AGO proteins with mRNPs in polyribosome gradients prompted us to investigate whether Ago complexes I–III contain mRNAs and form mRNPs (Fig 1C). HEK 293 cell lysates preincubated with or without RNase A were separated as described previously. AGO1 complexes II–III were clearly visible in the untreated lysates (upper panel), but not in the RNase-treated extracts (lower panel). Together, our data show that human AGO1 and AGO2 associate with three distinct RNA–protein complexes. Furthermore, AGO complexes II and III are sensitive to RNase treatment, suggesting that these complexes form mRNPs.
AGO complex III co-sediments with the KRAS mRNA
Next, we investigated whether AGO complexes II and III contain miRNA target mRNAs. We transfected a luciferase reporter construct carrying the 3′-untranslated region (3′-UTR) of KRAS (Kirsten rat sarcoma viral oncogene homologue), which has been shown to be translationally regulated by let-7a in human cells (Johnson et al, 2005). Cell lysates were separated on a 15–55% sucrose gradient. RNA was extracted from individual fractions and analysed by quantitative reverse transcription–PCR (qRT–PCR; Fig 1D). Strikingly, we detected high amounts of KRAS mRNA co-sedimenting with AGO complex III, suggesting that AGO complex III forms large mRNPs with miRNA target mRNAs.
AGO complexes I–III associate with miRNAs
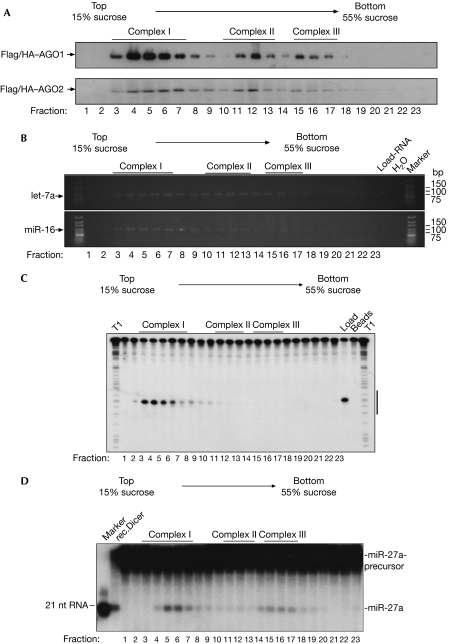
A detailed molecular characterization of the AGO complexes I–III requires immunoprecipitations and functional analyses of the precipitates. As the antibodies used for western blotting proved to be too inefficient for immunoprecipitation, we recapitulated AGO protein complex associations using Flag/haemagglutinin (HA)-tagged AGO proteins (Flag/HA–AGO; Fig 2A). Flag/HA–AGO1 and Flag/HA–AGO2 were expressed in HEK 293 cells and the lysates were separated by centrifugation as described above. Individual fractions were analysed using HA antibodies. Consistently, the same distinct AGO1 and AGO2 complexes as in wild-type HEK 293 lysates were observed, indicating that Flag/HA–AGO1 and Flag/HA–AGO2 associated with native protein complexes and could therefore be used for further analyses.
Figure 2.
Argonaute complexes associate with distinct Dicer and RISC activities. (A) HEK 293 cell extracts containing Flag/HA–AGO1 (upper panel) or Flag/HA–AGO2 (lower panel) were separated by gradient centrifugation. The presence of Flag/HA–AGO1 and Flag/HA–AGO2 was analysed by western blotting using HA antibodies. (B) Lysates from HEK 293 cells expressing Flag/HA–AGO1 or Flag/HA–AGO2 were separated as in (A). Fractions were immunoprecipitated using Flag antibodies. RNA was extracted and the presence of endogenous let-7a (upper panel) and miR-16 (lower panel) was determined using RT–PCR. (C) Lysates from Flag/HA–AGO2-transfected HEK 293 cells were separated and immunoprecipitated as described in (A). Immunoprecipitates were incubated with a 32P-cap-labelled RNA, which contained a perfect complementary sequence to the endogenous miR-19b. Lanes indicated with T1 show RNase T1 digestions of the RNA substrates. The RNA sequence complementary to miR-19b is indicated by a black bar to the right. (D) Flag/HA–AGO1-containing HEK 293 lysate was separated and immunoprecipitated as described in (A). The immunoprecipitates or recombinant Dicer were incubated with an internally labelled pre-miR-27a substrate. A 21-nucleotide marker is shown to the left. AGO, Argonaute; HA, haemagglutinin; HEK, human embryonic kidney; qRT–PCR, quantitative reverse transcription–PCR; RISC, RNA-induced silencing complex.
As AGO proteins are the binding partners of mature miRNAs, we analysed the miRNA content of various AGO complexes. HEK 293 lysates containing Flag/HA–AGO1 or Flag/HA–AGO2 were separated as described above. Proteins were immunoprecipitated from each fraction using Flag antibodies, and the associated RNA was extracted and analysed by semiquantitative RT–PCR for miR-16 or let-7a (Fig 2B). Notably, both miR-16 and let-7a were found in all AGO-containing fractions, whereas only weak signals were found in other fractions (Fig 2B). Together, the three distinct human AGO1 and AGO2 complexes associate with mature miRNAs.
Analysis of AGO-associated RISC and Dicer activity
AGO2 is the endonucleolytic component of human RISC (Liu et al, 2004; Meister et al, 2004); therefore, we investigated which of the observed AGO2 complexes associates with RISC activity. Lysate from HEK 293 cells transfected with Flag/HA–AGO2 was fractionated and immunoprecipitated as described above. Individual immunoprecipitates were incubated with a 32P-cap-labelled RNA complementary to endogenous miR-19b (Fig 2C). Fractions 3–6, as well as the total lysate, showed strong cleavage activity, whereas no cleavage activity was observed in higher molecular weight fractions, indicating that AGO2 complex I represents active human RISC.
It has been shown that human AGO proteins associate stably with Dicer and that this complex is able to generate small RNAs from dsRNA precursors (Gregory et al, 2005; Meister et al, 2005). Therefore, we tested individual AGO complexes for Dicer activity. HEK 293 lysates containing Flag/HA–AGO1 were fractionated and immunoprecipitated using Flag antibodies. The immunoprecipitates were incubated with an internally 32P-labelled miR-27a precursor and the cleavage products were analysed by 15% denaturing RNA polyacrylamide gel electrophoresis (Fig 2D). AGO1 complex I (fractions 3–7) and AGO1 complex III (fractions 15–17) were associated with Dicer activity, whereas only very weak Dicer activity was observed in AGO1 complex II (fractions 10–13). Together, we have shown AGO2 complex I is a low-molecular-weight RISC, whereas AGO complexes I and III are associated with Dicer. Interestingly, AGO complex II does not contain RISC and shows little detectable Dicer activity.
Proteomic analysis of AGO complexes I–III
We analysed the protein composition of AGO complexes I–III to identify cofactors that function together with AGO1 or AGO2. Flag/HA–AGO1 or Flag/HA–AGO2 was transiently expressed in HEK 293 cells, and the lysates were separated by gradient centrifugation. Fractions 3–8, 10–13 and 15–18, representing AGO complexes I, II and III, respectively, were combined and AGO complexes were immunoprecipitated using Flag antibodies. The co-immunoprecipitated proteins were analysed using mass spectrometry (supplementary Fig 1 online). Antibodies that were not specific to the Flag tag were used for control purifications (supplementary Fig 2 online). After investigating only a few visible bands in our previous study (Meister et al, 2005), our aim was now to analyse all proteins that were present in the AGO immunoprecipitates. Table 1 shows a list of proteins that were specifically found in AGO precipitations, but not in control purifications (see also the supplementary tables online).
Table 1.
Proteins associated with human AGO1 and AGO2
| Name | Domains/motif | AGO1 complex | AGO2 complex | Accession no. |
|---|---|---|---|---|
| Proteins involved in gene silencing | ||||
| Dicer | DEAD box, RNase III, PAZ, dsRBD, DUF | I, III | I, III* | gi∣21665773/gi∣5019620 |
| TNRC6B | RRM | — | I | gi∣14133235 |
| MOV10 | DExH box | III | III | gi∣14424568 |
| TRBP | dsRBD | I* | I* | gi∣107904 |
| Gemin3 | DEAD box | II* | gi∣14209614 | |
| Gemin4 | Leucin zipper | II*, III | II, III | gi∣7657122 |
| DEAD/DEAH box-containing proteins | ||||
| RNA helicase A (RHA)/DHX9 | DEAH box, helicase domain, dsRBD, DUF1605 | II, III | II, III | gi∣1806048/gi∣1082769 |
| DHX30 | DEAH box, helicase domain, dsRBD, DUF1605 | II, III | II, III | gi∣20336294 |
| RENT1/Upf1 | DEAD box, exoV | III | — | gi∣1575536 |
| DHX36 | DEAH box, helicase domain, DUF1605 | II*, III* | II* | gi∣7959237/gi∣23243423 |
| DDX21/RNA helicase GuA | DEAD box, helicase domain, GUCT | II, III | II | gi∣2135315 |
| DDX50/RNA helicase GuB | DEAD box, helicase domain, GUCT, RESIII | III | — | gi∣55664207 |
| DDX46 | DEAH box, helicase domain, DUF1605 | II* | II* | gi∣2696613 |
| DDX48 | DEAD box, helicase domain | II*, III | — | gi∣496902 |
| DDX18 | DEAD box, helicase domain | III | — | gi∣1498229 |
| DDX5/p68 | DEAD box, helicase domain | — | II* | |
| DDX39/BAT1 | DEAD box, helicase domain | III* | II* | gi∣1905998 |
| DDX47 | DEAD box, helicase domain, apolipoprotein L | III | — | gi∣20149629 |
| Heterogeneous nuclear ribonucleoprotein particles | ||||
| hnRNP-U | SAP, SPRY, SCOP | II, III | II, III | gi∣32358 |
| hnRNP-U-like | SAP, SPRY, SCOP | I* | — | gi∣3319956 |
| hnRNP-H2/H' | RRM, RNPHF zinc finger | II* | — | gi∣6065880 |
| hnRNP-F | RRM, RNPHF zinc finger | II* | I* | gi∣16876910 |
| hnRNP-C | RRM | II, III | II, III | gi∣13937888/gi∣14250048 |
| hnRNP-E2 | KH1, KH2 | III* | — | |
| NSAP1 | Phox-like, PX-associated motif, RRM | II, III | — | gi∣5031512 |
| hnRNP-L | Enoyl-CoA hydratase/isomerase, RRM | III* | — | gi∣11527777 |
| Messenger RNA-binding proteins | ||||
| Poly-A-binding proteins | RRM | II, III | II, III | gi∣46367787/gi∣693937 |
| Nuclear cap-binding protein 80 kDa | MIF4G | III | — | gi∣3153873 |
| YB-1 | Cold-shock domain | II | II, III | gi∣181486/gi∣55451 |
| FMRp | Agenet, KH1 | III* | — | gi∣182673 |
| FXR1 | Agenet, KH1 | — | III | gi∣1730139 |
| FXR2 | Agenet, KH1 | III | — | gi∣4758410 |
| ZBP-1 | RRM, KH1 | II, III | III | gi∣7141072/gi∣56237027 |
| ZBP-3 | RRM, KH1 | III | — | gi∣30795212 |
| HuR | RRM | III* | — | gi∣1022961 |
| RBM4 | RRM, zinc finger | — | III* | gi∣4506445 |
| Proteins involved in RNA metabolism | ||||
| NF90/ILF3/NFAR-1 | dsRBD, DZF | II, III | II | gi∣1082856/gi∣5006602 |
| NF45/ILF2 | DZF | II, III | II, III | gi∣532313 |
| SART3 | Lsm interaction motif, RRM | I, II, III | — | gi∣7661952 |
| RBM10 | D111/G-patch, RRM, zinc finger, Ran binding | — | I*, II* | gi∣12644371 |
| Fibrillarin | Fibrillarin motif | — | II*, III* | gi∣182592 |
| NOP56 | Pre-mRNA processing RNP, NOP5NT, NOSIC | III | — | gi∣2230878 |
| Nucleolin | RRM | III | — | gi∣128841 |
| eIF2bδ | Initiation factor 2B | I* | — | gi∣6563202 |
| eIF4b | RRM | — | I* | gi∣288100 |
| FLJ20758 | Pentatricopeptide repeat | II | II | gi∣38683855 |
| Other proteins | ||||
| Myb-binding protein 1a | DNA polymerase V | III | III* | gi∣7657351 |
| Matrin 3 | RRM, zinc finger | III* | III* | gi∣6563246 |
| Motor protein | — | II, III | gi∣516764 | |
| ZNF326 | AKAP95 | II, III | — | gi∣31807861/gi∣47125447 |
| Ku70 | Ku70/80 motif, DNA-binding SAP | — | II* | gi∣57165052 |
| DDB1 | CPSF A subunit | I | I* | gi∣418316 |
| RuvB-like II | AAA ATPase, Tip49b | I | I, II | gi∣5730023/gi∣12653319 |
| Coatomer protein | WD-40, COPB2 | III | II | gi∣1002369 |
| *Identified by a single peptide. | ||||
As expected from the Dicer activity assays, we found Dicer only in AGO1/2 complexes I and III, whereas TRBP was identified only in AGO complex I. TNRC6B, MOV10, RHA (DEAH box polypeptide 9), Gemin3 and Gemin4, which have been found in AGO complexes previously (Mourelatos et al, 2002; Meister et al, 2005; Robb & Rana, 2007), were also among the identified proteins. Proteins that have not yet been implicated in RNA silencing in mammals were grouped according to their domains and function (Table 1). Among the DEAD/DEAH box helicases, we found DDX5, an orthologue of Drosophila p68, which has been shown to associate with Drosophila Ago2 (Meister & Tuschl, 2004), and DDX18, a putative helicase that has been implicated in Drosha function (Gregory et al, 2004). Consistent with the hypothesis that AGO complexes II and III are mRNPs, we found various isoforms of poly-A-binding proteins, indicating that mRNAs were present in the purifications. Strikingly, we found many mRNA-binding proteins that are involved in translational regulation, including FMRp and its homologues FXR1 and FXR2. It was reported previously that FMRp associates with Ago proteins as well as miRNAs in both human and Drosophila cells (Meister & Tuschl, 2004). Further identified proteins with regulatory functions in translation are NSAP1/SYNCRIP, YB-1, HuR, RBM4, ZBP1 and ZBP3. We also found various ribosomal proteins in the Ago complexes (supplementary tables online), suggesting that ribosomal proteins might have other functions as components of mRNPs.
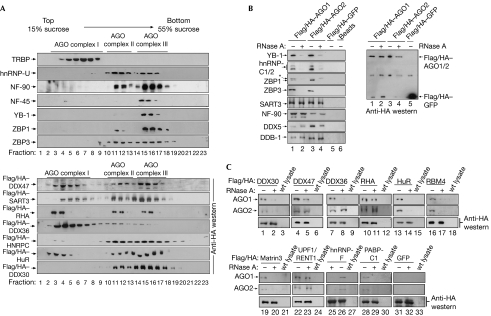
Next, using western blotting, we examined whether the identified factors specifically co-sediment with AGO-containing fractions in sucrose gradients (Fig 3A). Consistent with the proteomic data, hnRNP-U, NF-90, ZBP1 and ZBP3 co-migrated with both AGO complexes II and III, whereas TRBP was found in low-molecular-weight fractions co-migrating with AGO complex I; however, NF-45 and YB-1 were detected in fractions containing AGO complex III. For a more comprehensive analysis, we expressed Flag/HA-tagged DDX47, DDX36, DDX30, RHA (DHX9), hnRNPC, HuR as well as SART3 and investigated co-sedimentation with AGO proteins. All tagged proteins migrated in fractions also containing AGO complexes II and III. Notably, we found a larger portion of the tagged proteins migrating at the top of the gradient, presumably owing to overexpression.
Figure 3.
Proteins identified by mass spectrometry interact with Argonaute complexes. (A) HEK 293 cell extracts were separated by gradient centrifugation and fractions were analysed by western blotting against the proteins indicated to the left (upper panels). HEK 293 cells were transiently transfected with Flag/HA-tagged expression constructs as indicated to the left and analysed by western blotting using HA antibodies. (B) HEK 293 cells were transfected as indicated. AGO complexes were immunoprecipitated using Flag antibodies and probed using specific antibodies with (lanes 2 and 4) or without (lanes 1 and 3) RNase A treatment (left panel). The asterisk denotes unspecific interactions of the ZBP1 antibody. A western blot using HA antibodies is shown to the right. (C) HEK 293 cells expressing Flag/HA-tagged proteins were treated as indicated. Immunoprecipitations and RNase treatment were carried out as in (A). Wild-type HEK 293 lysate was used as a control. Interactions were analysed by western blotting against AGO1 (upper panels), AGO2 (middle panels) or HA (control; lower panels). AGO, Argonaute; GFP, green fluorescent protein; HA, haemagglutinin; HEK, human embryonic kidney; hnRNP, heterogeneous nuclear ribonucleoprotein particles; TRBP, human immunodeficiency virus transactivating response RNA binding protein.
To validate a specific association with AGO complexes, we carried out co-immunoprecipitations (Fig 3B) and Flag/HA–AGO1 or Flag/HA–AGO2 was immunoprecipitated from HEK 293 lysates using Flag antibodies. RNase A-treated and untreated samples (supplementary Fig 3 online) were analysed using western blotting. HnRNP-C1/C2, ZBP1, ZBP3 and YB-1 disappeared from the Flag/HA–AGO1/2 immunoprecipitates when RNase A was added, indicating that the tested proteins were not associated with AGO proteins through protein–protein interactions, but bound to the same RNAs. NF-90, SART3, DDX5 and DDB-1 immunoprecipitated with Flag/HA–AGO1/2 in the presence of RNase A, which is indicative of protein–protein interactions. To analyse a specific AGO association of mRNP components for which no specific antibodies were available, we expressed Flag/HA-tagged fusions (Fig 3C). The tagged proteins were immunoprecipitated using Flag antibodies and the precipitates were analysed by western blotting using HA (lanes 1–33, lower panel), AGO1 (upper panel) or AGO2 (middle panel) antibodies. Endogenous AGO1 and AGO2 clearly co-precipitated with all Flag/HA-tagged proteins (lanes 1–33). The binding of DDX30, HuR, RBM4, hnRNP-F, PABP-C1 and Matrin3 to AGO1 and AGO2 complexes was sensitive to RNase A treatment, whereas the binding of DDX36, DDX47, RHA and UPF1/RENT1 was not, suggesting protein–protein interactions.
RBM4 is required for miRNA-guided gene silencing
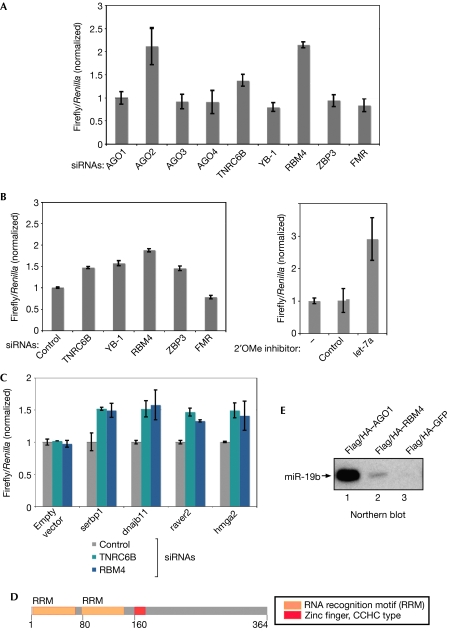
To investigate the relevance of identified AGO mRNP components for miRNA function, we generated a luciferase construct containing a perfectly complementary miR-21 target site in the 3′-UTR (Fig 4A). As expected, knockdown of AGO1, AGO3 and AGO4 had no effect, whereas siRNAs against AGO2 or TNRC6B led to a significant increase of luciferase expression. No effect was observed with a mutated miR-21-binding site (supplementary Fig 4 online). Strikingly, knockdown of RBM4 resulted in a strong increase of luciferase activity, indicating that RBM4 modulates miR-21-guided RNA cleavage. Notably, the interaction of RBM4 and AGO2 is reduced when RNase A is added (Fig 3C). It is reasonable to assume that miRNA degradation by RNase A affects AGO2 interactions. Alternatively, RBM4 could also enhance the binding of AGO proteins to mRNAs by increasing the accessibility of miRNA target sites.
Figure 4.
RNA binding motif protein 4 is required for microRNA-guided gene silencing. (A) SiRNAs against the indicated proteins were pre-transfected into HeLa cells. After 2 days, a luciferase reporter containing a complementary binding site for miR-21 was transfected. (B) Experiments were carried out as described in (A). A luciferase reporter construct containing the 3′-UTR of KRAS was used (left panel). 2′OMe inhibitors of the indicated miRNAs were pre-transfected into HeLa cells (right panel). (C) Experiments were carried out as in (A). Luciferase reporter constructs carrying the 3′-UTRs of the indicated mRNAs were transfected and normalized to control siRNA. (D) Schematic illustration of RBM4. (E) Lysates from HEK 293 cells transfected with the indicated constructs were immunoprecipitated using Flag antibodies, and northern blotting against miR-19b was performed. GFP, green fluorescent protein; HEK, human embryonic kidney; KRAS, Kirsten rat sarcoma viral oncogene homologue; miRNA, microRNA; RBM4, RNA binding motif protein 4; siRNAs, small interfering RNAs; TNRC6B, trinucleotide repeat containing 6B; UTR, untranslated region.
Next, we analysed whether RBM4 is also required for the regulation of natural miRNA targets (Fig 4B). A luciferase construct containing the KRAS 3′-UTR was transfected into HEK 293 cells in which let-7a was inhibited or TNRC6B, YB-1, RBM4, ZBP3 or FMRp was depleted by RNA interference. Knockdown of TNRC6B, RBM4, YB-1 and ZBP3 resulted in stronger luciferase activity. Overexpression of RBM4 but not ZBP3 led to decreased luciferase activity (supplementary Fig 6 online), suggesting that RBM4 functions on the 3′-UTR of KRAS.
Recently, hmga2, serbp, dnajb11 and raver2 have been identified and validated as miRNA targets (Beitzinger et al, 2007; Mayr et al, 2007). Therefore, we transfected luciferase reporter constructs containing the respective 3′-UTRs and measured luciferase activity in an RBM4- or TNRC6B-knockdown background (Fig 4C). Indeed, luciferase activity was significantly increased in the RBM4- or TNRC6B-knockdown cells, indicating that RBM4 functions on different miRNA targets. As RBM4 contains two RNA recognition motifs (Fig 4D), we investigated whether RBM4 co-immunoprecipitates with miRNAs (Fig 4E). Flag/HA–RBM4 was immunoprecipitated using Flag antibodies and the associated RNA was extracted. Strikingly, miR-19b was detected in the Flag/HA–RBM4 precipitate, whereas no miR-19b was precipitated in a control reaction.
Our data indicate that RBM4 co-immunoprecipitates with miRNAs and functions in AGO-mediated gene silencing; therefore, we have identified a new component of human gene silencing.
Methods
Cell extracts and gradient centrifugation. Polyribosome fractionation from HEK 293 cells was carried out according to Pillai et al (2005). For complex purification and co-immunoprecipitations, HEK 293 cells were lysed in buffer containing 25 mM Tris–HCl (pH 7.4), 150 mM KCl, 0.5% NP-40, 2 mM EDTA, 1 mM NaF, 0.5 mM dithiothreitol and protease inhibitors (Roche, Penzberg, Germany) and centrifuged at 10,000g for 10 min at 4°C. For fractionations, gradients from 15% (w/v) to 55% (w/v) sucrose in 150 mM KCl, 25 mM Tris (pH 7.4) and 2 mM EDTA were used. Lysates were separated by centrifugation at 30,000 r.p.m. for 18 h in an SW41 rotor at 4°C. To determine indicated S values, catalase (11S), apoferritin (17S) and thyroglobin (19S) were used. For the analysis of RNA-dependent interactions, extracts were preincubated with 100 μg/ml RNase A (Qiagen, Hilden, Germany) at 4°C for 1 h.
Immunoprecipitation of Flag/HA-tagged AGO complexes. AGO-containing gradient fractions were pooled and incubated with 100 μl Flag M2 agarose beads (Sigma, Taufkirchen, Germany) for 2 h at 4°C. Beads were subsequently washed with immunoprecipitation buffer (300 mM NaCl, 5 mM MgCl2, 0.1% NP-40 and 50 mM Tris–HCl, pH 7.5) and phosphate-buffered saline (PBS).
Co-immunoprecipitation experiments. Lysates were incubated with 30 μl Flag M2 agarose at 4°C for 1.5 h, and washed with IP buffer and PBS. Beads were distributed equally into two tubes and 1.5 ml PBS or PBS containing 100 μg/ml RNase A was added. After incubation at 4°C for 1.5 h, beads were washed with PBS and denatured by adding 25 μl of protein sample buffer.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
We thank S. Behrens for NF-45/NF-90-specific antibodies, S. Hüttelmaier for ZBP1/3-specific antibodies and S. Jentsch for support. Our research was supported by the Max-Planck-Society, Deutsche Forschungsgemeinschaft (DFG) grant ME 2064/2-1 and European Union grant LSHG-CT-2005-037900. L.W. receives a fellowship of the Boehringer Ingelheim Funds.
References
- Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 20: 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitzinger M, Peters L, Zhu JY, Kremmer E, Meister G (2007) Identification of human microRNA targets from isolated Argonaute protein complexes. RNA Biol 4: e1–e9 [DOI] [PubMed] [Google Scholar]
- Chu CY, Rana TM (2006) Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol 4: e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Izaurralde E (2007a) P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol 8: 9–22 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E (2007b) P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol 27: 3970–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS (2005) Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol 15: 331–341 [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R (2004) The Microprocessor complex mediates the genesis of microRNAs. Nature 432: 235–240 [DOI] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R (2005) Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123: 631–640 [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ (2005) RAS is regulated by the let-7 microRNA family. Cell 120: 635–647 [DOI] [PubMed] [Google Scholar]
- Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, Ruvkun G (2004) Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci USA 101: 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z (2007) An mRNA m(7)G cap binding-like motif within human ago2 represses translation. Cell 129: 1141–1151 [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R (2005) MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney PA, Yu Y, Fisher J, Nilsen TW (2006) Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol 13: 1102–1107 [DOI] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP (2007) Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315: 1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Tuschl T (2004) Mechanisms of gene silencing by double-stranded RNA. Nature 431: 343–349 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T (2004) Human argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15: 185–197 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, Tuschl T (2005) Identification of novel argonaute-associated proteins. Curr Biol 15: 2149–2155 [DOI] [PubMed] [Google Scholar]
- Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G (2002) miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 16: 720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Hatzigeorgiou AG, Mourelatos Z (2004) miRNP:mRNA association in polyribosomes in a human neuronal cell line. RNA 10: 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottrott S, Simard MJ, Richter JD (2006) Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat Struct Mol Biol 13: 1108–1114 [DOI] [PubMed] [Google Scholar]
- Parker JS, Barford D (2006) Argonaute: a scaffold for the function of short regulatory RNAs. Trends Biochem Sci 31: 622–630 [DOI] [PubMed] [Google Scholar]
- Peters L, Meister G (2007) Argonaute proteins: mediators of RNA silencing. Mol Cell 26: 611–623 [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W (2005) Inhibition of translational initiation by let-7 MicroRNA in human cells. Science 309: 1573–1576 [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W (2007) Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17: 118–126 [DOI] [PubMed] [Google Scholar]
- Robb GB, Rana TM (2007) RNA helicase a interacts with RISC in human cells and functions in RISC loading. Mol Cell 26: 523–537 [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco JG (2006) MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA 103: 4034–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Haley B (2005) Ribo-gnome: the big world of small RNAs. Science 309: 1519–1524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information