Abstract
In this study, we investigated the effect of pressure on protein structure and stability at high temperature. Thermoinactivation experiments at 5 and 500 atm were performed using the wild-type (WT) enzyme and two single mutants (D167T and T138E) of the glutamate dehydrogenase (GDH) from the hyperthermophile Thermococcus litoralis. All three GDHs were stabilized, although to different degrees, by the application of 500 atm. Interestingly, the degree of pressure stabilization correlated with GDH stability as well as the magnitude of electrostatic repulsion created by residues at positions 138 and 167. Thermoinactivation experiments also were performed in the presence of trehalose. Addition of the sugar stabilized all three GDHs; the degree of sugar-induced thermostabilization followed the same order as pressure stabilization. Previous studies suggested a mechanism whereby the enzyme adopts a more compact and rigid structure and volume fluctuations away from the native state are diminished under pressure. The present results on the three GDHs allowed us to further confirm and refine the proposed mechanism for pressure-induced thermostabilization. In particular, we propose that pressure stabilizes against thermoinactivation by shifting the equilibrium between conformational substates of the GDH hexamer, thus inhibiting irreversible aggregation.
Keywords: Pressure, pressure stabilization, Thermococcus litoralis, glutamate dehydrogenase, mutants, trehalose
Though not used as often as temperature or denaturants, pressure has nonetheless become an increasingly useful tool for the analysis of protein structure and function (Frauenfelder et al. 1990; Gross and Jaenicke 1994; Heremans and Smeller 1998). In particular, pressure has helped to elucidate protein-folding pathways. For example, pressure-jump studies of staphylococcal nuclease revealed that the volume of the protein-solvent system in the transition state of folding/unfolding is larger than in either the folded or unfolded states, consistent with a molten-globulelike intermediate (Vidugiris et al. 1995). In another recent study, high-pressure, stopped-flow measurements provided evidence for a dry molten-globulelike transition state; furthermore, the structure of the transition state varied with the concentration of denaturant, i.e., with the solution environment (Pappenberger et al. 2000). Besides its use as a research tool, pressure has both potential and proven practical applications. Pressures around 1–2 kilobars have been shown to dissociate aggregates and assist proteins to refold into their native structures (Gorovits and Horowitz 1998; St. John et al. 1999). Application of pressure in combination with low concentrations of guanidine hydrochloride was effective in reversing the effect of aggregation and promoting refolding of several proteins (St. John et al. 1999). Remarkably high recoveries (up to 100%) of properly folded proteins were obtained from inclusion bodies of human growth hormone, lysozyme, and β-lactamase (St. John et al. 1999). Other related applications of pressure include its use in the food industry and in vaccine development and virus inactivation (Hayashi and Balny 1996; Heremans 1997; Silva et al. 1996). In particular, high-pressure processes such as pascalization have been used to sterilize food stuffs because pressure inactivates microorganisms by destabilizing their biomembranes (Heremans and Smeller 1998).
Until recently, pressure generally has been viewed and employed as a destabilizer of protein structure; for example, in studies of pressure-induced unfolding and dissociation of monomeric as well as multimeric proteins (Jaenicke 1991; Panick et al. 1998; Weber and Drickamer 1983). However, moderately high pressures (<1000 atm) have been shown to stabilize proteins against both reversible unfolding and irreversible inactivation resulting from high temperatures (Hawley 1971; Millar et al. 1974; Mozhaev et al. 1996). In particular, there are many examples of pressure stabilizing thermophilic and hyperthermophilic proteins against thermal inactivation (Hei and Clark 1994; Michels et al. 1996; Sun et al. 1999). One example was with the glutamate dehydrogenase (GDH) from the hyperthermophile Pyrococcus furiosus (Pf), where Sun et al. (1999) showed that pressures in the range of 250–500 atm stabilized the GDH against thermoinactivation. The thermal half-life at 105°C as measured by remaining activity increased from 13 min at 5 atm to 360 min at 500 atm, the largest pressure-induced thermostabilization recorded for any protein. Based on the known effects of glycerol on protein structure and stability, a mechanism of pressure stabilization involving the compression of cavities and reduction of volume fluctuations in the native structure was proposed (Sun et al. 1999).
As part of our continuing effort to elucidate the effect of pressure on enzyme structure, flexibility, and stability at high temperatures, we have carried out studies with the highly homologous GDH from the hyperthermophile Thermococcus litoralis (Tl) (sharing 89% sequence identity with the Pf GDH). Tl is an archaeon isolated from marine thermal springs and grows optimally at 88°C (Neuner et al. 1990). The GDH from Tl is very thermostable, with a melting temperature of 109°C (Vetriani et al. 1998). Like Pf GDH, Tl GDH is a hexamer composed of six identical subunits.
In this paper, we describe results for the wild-type (WT) enzyme and two single mutants of Tl GDH: D167T and T138E. The single mutations originally were designed to alter electrostatic interactions at a particular intersubunit region of the GDH (Vetriani et al. 1998). Figure 1 ▶ shows half of a WT Tl GDH hexamer (i.e., a trimer) viewed along the trimer-trimer axis, exposing the trimer-trimer interface. The Thr138s and Asp167s are highlighted, illustrating their proximity to each other and to the subunit-subunit interfaces. As shown previously (unpubl.), ion-pair interactions as well as long-range electrostatic interactions involving residues at positions 138 and 167 are important to the thermostability of the GDH. In particular, long-range electrostatic repulsion among and between positions 138 and 167 was shown to correlate with the thermostability of the three GDHs: WT, D167T, and T138E.
Fig. 1.
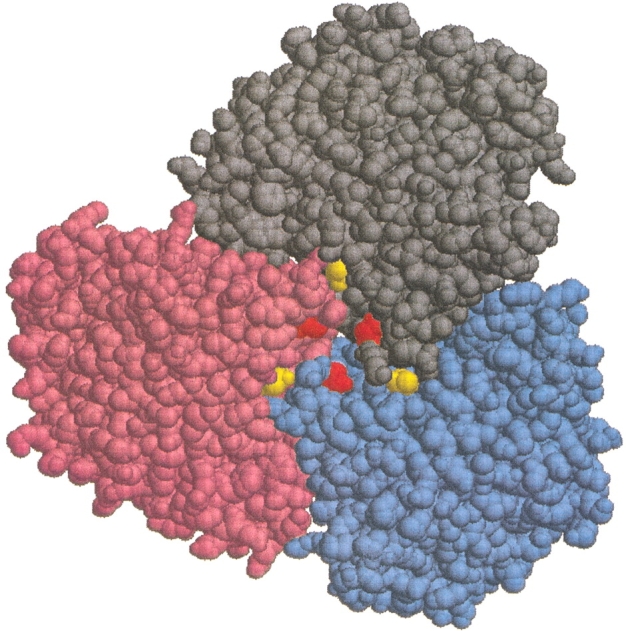
Thermococcus litoralis wild-type glutamate dehydrogenase viewed along the threefold (trimer-trimer) axis with the top trimer removed, thus exposing the trimer-trimer interface. Subunits A, B, and C are colored gray, blue, and pink, respectively, and the Thr138s and Asp167s are colored yellow and red, respectively. The distance between any two Asp167s is ∼10 Å, while that between any two Thr138s is ∼20 Å. Figure created using the program RasMol.
The goal of this study was to further examine the thermostabilization affected by moderately high pressures and to extend our understanding of the pressure effects on protein structure and stability at high temperatures. To this end, we investigated the effect of pressure on the kinetic thermostability of the WT GDH and the two single mutants D167T and T138E. All three enzymes were stabilized by pressure against thermoinactivation. Interestingly, the degree of pressure stabilization differed significantly between the three enzymes, suggesting changes in the structure and/or dynamics of GDH caused by the mutations. Thermoinactivation experiments performed in the presence of trehalose indicated that stabilization by a stabilizing sugar also differed for the three GDHs. Our results, along with the known mechanism of protein stabilization by sugars and recent studies of protein structure and dynamics under pressure, provide further insights into the mechanism of pressure stabilization of GDH.
Results
Most hyperthermophilic proteins studied to date (Hiller et al. 1997), including Tl GDH, undergo irreversible thermoinactivation. Therefore, the thermoinactivation experiments performed in this work were analyzed in terms of the kinetics and not the thermodynamics of GDH inactivation. The time course of GDH thermoinactivation was followed by activity assays of samples withdrawn at fixed time intervals from a bioreactor as described previously (Sun et al. 1999). The percentage of GDH activity remaining in solution then was plotted as a function of incubation time. The remaining activity was confirmed to be proportional to the amount of nondenatured GDH remaining in solution (unpubl.).
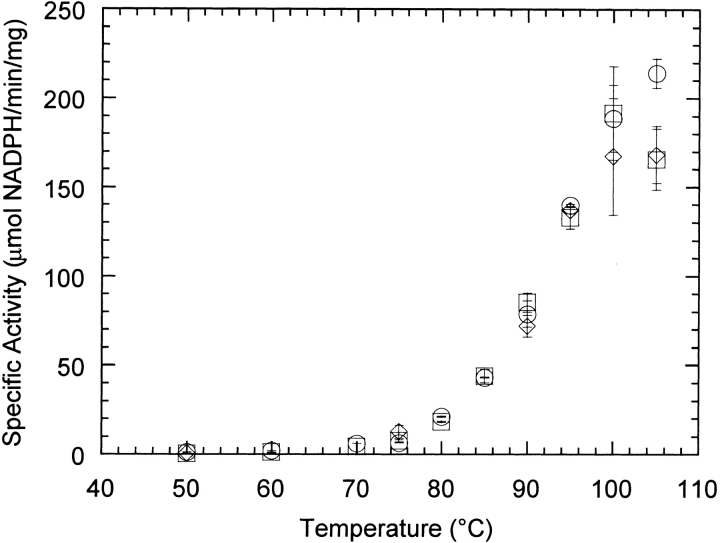
We showed previously that the single mutations D167T and T138E altered the kinetic stability of Tl GDH (unpubl.). Specifically, the D167T and T138E single mutants are, respectively, slightly more and much less thermostable than the WT GDH. However, all three GDHs assembled into hexameric forms that were indistinguishable by native polyacrylamide gel electrophoresis (data not shown). Furthermore, the activity-temperature profiles of both the D167T and T138E mutants are very similar to that of the WT GDH (Fig. 2 ▶). Therefore, neither mutation appears to grossly alter the conformation of GDH.
Fig. 2.
Specific activity of wild-type (○), D167T (⋄), and T138E (□) glutamate dehydrogenases as a function of temperature. Error bars represent average deviations of two or more duplicate assays.
Effect of pressure on stability
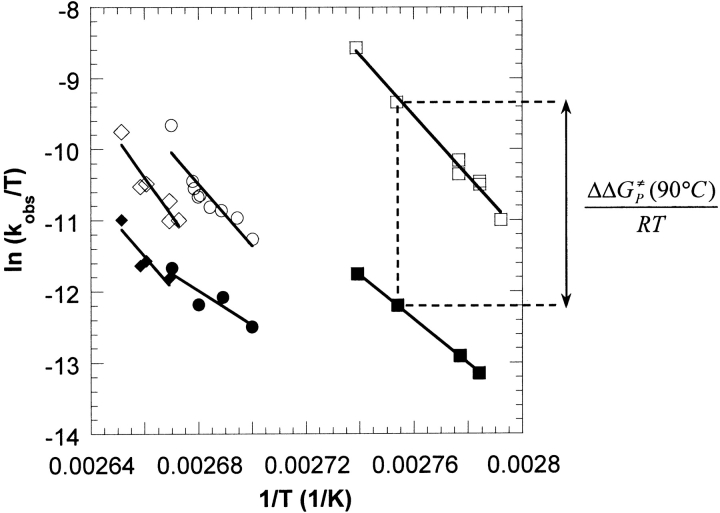
Thermoinactivation experiments at low and high pressures were performed using the WT, D167T, and T138E GDHs at different temperatures. Thermoinactivation trajectories were fitted as described in Materials and Methods, from which apparent first-order rate constants, kobs, were determined. Figure 3 ▶ shows Eyring plots of the first-order inactivation rate constants for the thermoinactivation of the GDHs at 5 (open symbols) and 500 atm (filled symbols). Application of 500 atm stabilized all three GDHs against thermoinactivation as evidenced by the downward shifts in the respective Eyring plots (reflecting a decrease in the rate constants of inactivation). In addition, the downward shifts are different in magnitude, indicating different degrees of pressure stabilization. Because of the large difference in stability between the T138E mutant and the other two GDHs, thermoinactivation of all three enzymes at a common temperature was not possible. Instead, thermoinactivation experiments for each GDH were performed over as wide a range of temperature as was experimentally feasible, resulting in half-lives between 10 and 1000 min. The lower limit on the half-life (10 min) was constrained by the time required to pressurize the sample and reach thermal equilibrium, whereas the upper limit was a matter of practicality. Nevertheless, over this range of half-lives, the degree of pressure stabilization follows the order: D167T < WT < T138E, with the largest stabilization corresponding to the T138E mutant.
Fig. 3.
Eyring plots showing the effect of pressure on the thermostability of Tl T138E (□,▪), wild type (○,•), and D167T (⋄,♦) glutamate dehydrogenases (GDHs). The open and filled symbols represent thermoinactivation experiments at 5 and 500 atm, respectively. As shown for the T138E GDH at 90°C, the difference in the ΔG≠ of inactivation at low and high pressure (i.e., ΔΔG≠P) is proportional to the vertical distance on an Eyring plot.
The inactivation rate constants were further analyzed using transition-state theory and presented as the difference in the free energy of activation (for the inactivation process), ΔΔG≠P, between thermoinactivation at 5 and 500 atm (Table 1). Positive values of ΔΔG≠P indicate stabilization by pressure. Table 1 confirms the order in the degree of pressure stabilization shown graphically in Figure 1 ▶. In addition, the values of ΔΔG≠P indicate that the degree of pressure stabilization for each individual GDH tends to increase with the experimental temperature; for example, the ΔΔG≠P values for the WT are 3.8, 4.9, and 6.3 kJ/mol at 98.8, 100, and 101.4°C, respectively. This trend suggests that the stabilizing effect of pressure increases as the stability decreases. The same trend was observed for the pressure stabilization of the highly homologous native Pf GDH at various temperatures (Sun et al. 1999).
Table 1.
Quantifying the effect of pressure on the thermostability of D167T, WT, and T138E GDHs
| Enzyme | Temp. (°C) | t½ [5 atm] (min)a | ΔΔG≠p (kJ/mol)a,b |
| D167T | 101.5 | 80 | 3.4 |
| D167T | 102.9 | 67 | 3.4 |
| D167T | 104 | 32 | 3.9 |
| WT | 98.8 | 97 | 3.8 |
| WT | 100 | 79 | 4.9 |
| WT | 101.4 | 29 | 6.3 |
| T138E | 86 | 68 | 8.0 |
| T138E | 87 | 60 | 7.6 |
| T138E | 90 | 22 | 8.6 |
| T138E | 92 | 10 | 9.6 |
a Half-lives and ΔΔG≠p were determined based on kobs (for further details, see Materials and Methods).
b ΔΔG≠p = ΔG≠500 atm − ΔG≠5 atm .
GDH, glutamate dehydrogenase; WT, wild type.
Effect of trehalose on stability
The effect of a stabilizing sugar, trehalose, on the thermostability of the GDHs also was investigated. Table 2 gives the half-lives of all three GDHs in the absence and presence of 0.5 M trehalose. Because of differences in stability between the enzymes, thermoinactivation temperatures were chosen to give approximately the same half-life in the absence of trehalose. The results indicate that trehalose stabilized all three GDHs against thermoinactivation. Furthermore, the degree of stabilization conferred by trehalose was dependent on the enzyme. Trehalose had the largest stabilizing effect on the T138E mutant, with a ΔΔG≠tre of 6.0 kJ/mol. The effect on the WT and D167T enzymes was more modest, with ΔΔG≠tre values of 2.6 and 1.5 kJ/mol, respectively.
Table 2.
Effect of trehalose on the thermoinactivation of GDHs
| Half-life (min)a,b | ||||
| TCH Enzyme | Temp. (°C) | No trehalose | 0.5 M trehalose | ΔΔG≠tre (kJ/mol)a,c |
| D167T | 101.5 | 80 ± 5 | 129 ± 9 | 1.5 ± 0.3 |
| WT | 100.0 | 80 ± 1 | 185 ± 20 | 2.6 ± 0.3 |
| T138E | 86.0 | 69 ± 2 | 504 ± 39 | 6.0 ± 0.2 |
a Half-lives and ΔΔG≠tre were determined based on kobs (for further details, see Materials and Methods).
b Errors are average deviations of two experiments.
c ΔΔG≠tre = ΔG≠tre. Errors were propagated following Equation 9. GDH, glutamate dehydrogenase; WT, wild type.
Discussion
Previous experiments demonstrated that the single mutations D167T and T138E affected the stability of Tl GDH (unpubl.). Because both positions 138 and 167 are located on the surface of the GDH monomer and occupy loop regions between secondary structural elements, it is unlikely that either mutation induces major changes in the secondary or tertiary structure of the monomer. Figure 2 ▶ shows that the activity-temperature profiles of the three GDHs are very similar up to 95°C. The similarity in specific-activity profiles is further evidence that all three GDHs are structurally alike at least at temperatures below the onset of thermal denaturation.
Like the GDH from Pf, Figure 3 ▶ shows that pressure has a substantial stabilizing effect on all three GDHs from Tl. The vertical distance between the 5 and 500 atm data points is proportional to the difference in the ΔG≠s for the denaturation process between 5 and 500 atm (Fig. 3 ▶). It is clear that the degree of pressure stabilization is different between the three GDHs, with the degree of pressure stabilization following the order: D167T < WT < T138E, where the largest stabilization corresponds to the T138E mutant.
Table 1 quantifies some of the data points in Figure 3 ▶ by listing low-pressure half-lives and ΔΔG≠Ps at several temperatures. For each GDH, pressure has a somewhat larger stabilizing effect as the temperature is increased. However, the degree of pressure stabilization is not solely a function of the thermoinactivation temperature, as the largest pressure stabilization is observed for T138E at temperatures more than 10°C lower than those used for D167T or WT. Any molecular model explaining the effect of pressure must be consistent with the following observations: (1) pressure stabilizes all three GDHs against thermoinactivation, (2) the degree of pressure stabilization follows the order D167T < WT < T138E, and (3) pressure stabilization increases with temperature for each GDH.
Studies of the effects of temperature and pressure on the structure and dynamics of proteins pointed to the existence of substates within a given state of the protein (Frauenfelder et al. 1988, 1990, 1991). These substates, termed conformational substates (CS), are similar, but not identical in structure and energy, and perform the same function (Frauenfelder et al. 1990). The arrangement of CS is hierarchical, resulting in different levels or tiers of CS. For example, myoglobin contains three CS of tier 0 (i.e., CS0); each of these CS0s is, in turn, more finely divided into further CS of tier 1 (i.e., CS1) (Frauenfelder et al. 1990). The existence of CS2 also has been shown, and it is likely that CS3 and CS4 also are present; however, differences in properties (e.g., structure, energy, volume) are greatest between members of CS0 tier (Frauenfelder et al. 1990). Above a characteristic temperature (200 K in the case of myoglobin) all CS within all tiers are in equilibrium (Frauenfelder et al. 1990, 1991). Most significant for our work, it was found that pressure shifted the equilibrium between two members of the CS0 tier of myoglobin, indicating a difference in volume. The difference in volume was a function of temperature with a value of about 15 cm3/mol at room temperature (Frauenfelder et al. 1990). By means of an equilibrium between CS, Frauenfelder and coworkers (1990) have demonstrated a mechanism that explains the observed effect of pressure on the structure, dynamics, and function (e.g., binding of carbon monoxide to myoglobin) of proteins.
Our own previous study on the effect of pressure on GDH from Pf suggested a possible model of pressure stabilization whereby the enzyme adopts a more compact and rigid structure and volume fluctuations away from the native state are diminished (Sun et al. 1999). The results obtained in this study with the WT and mutant GDHs, along with recent findings on the mechanism of protein aggregation (Kendrick et al. 1998), have led to further confirmation and refinement of the molecular mechanism for pressure stabilization of GDH previously put forth by us (Sun et al., 1999). Specifically, we now propose that pressure stabilizes by modulating the equilibrium between CS of the GDH hexamer, thus reducing unfolding and eventual aggregation. This refined model combines the idea and existence of CS (Frauenfelder et al., 1988, 1990, 1991) with the recent mechanism proposed for the aggregation of human interferon-γ (Kendrick et al. 1998). Under this model, the apparent first-order inactivation of GDH is modified to:
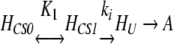 |
1 |
where HCS0 and HCS1 are CS that comprise the native-state ensemble of the GDH hexamer, HU is a partially unfolded hexamer that then is able to aggregate to form the aggregated species A, K1 (= HCS1/HCS0) is the equilibrium constant for the conversion between HCS0 and HCS1, and ki is the first-order inactivation constant for the formation of HU. For simplicity, we have assumed the existence of only two CS, with HCS0 being the lower energy and volume CS. Equation 1 assumes that only HCS1 can irreversibly unfold into an aggregate-competent (term used by Kendrick et al. 1998) species HU. The mechanism shown by Equation 1 is consistent with the observation that thermoinactivation of all Tl GDHs follows apparent first-order kinetics and that the rate-limiting step precedes aggregation (unpubl.). Kendrick and coworkers also found that aggregation of recombinant human interferon-γ follows apparent first-order kinetics, leading the authors to the same conclusion that a poly-molecular process such as aggregation was not rate-limiting (Kendrick et al. 1998).
Assuming that both HCS0 and HCS1 are active at the nondenaturing assay temperature of 85°C, Equation 1 can be solved for the remaining GDH activity as a function of time:
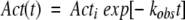 |
2 |
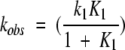 |
3 |
where Acti and Act(t) are, respectively, the GDH activity measured initially and at time t. The rate constant kobs is the first-order inactivation constant determined from fits to thermoinactivation experiments (see Materials and Methods). According to Equations 1–3, pressure can stabilize GDH by shifting the equilibrium between CS towards that of HCS0. The relation between the equilibrium constant K and pressure is characterized by a volume difference:
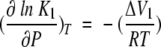 |
4 |
where P is pressure, ΔV1 is the difference in volume between HCS1 and HCS0 (i.e., V[HCS1] – V[HCS0]), R is the universal gas constant, and T is the temperature. We propose that GDH inactivation follows Equation 1 and that the volume of HCS1 is greater than that of HCS0 (i.e., ΔV1 >0). Therefore, according to Equation 4, an increase in pressure will result in a decrease of K1; this, in turn, will stabilize GDH against thermoinactivation by decreasing kobs (Equation 3). This is consistent with Le Châtelier's principle, which predicts that pressure will shift the equilibrium towards the state of lowest volume (i.e., HCS0), thus inhibiting formation of HU and aggregation.
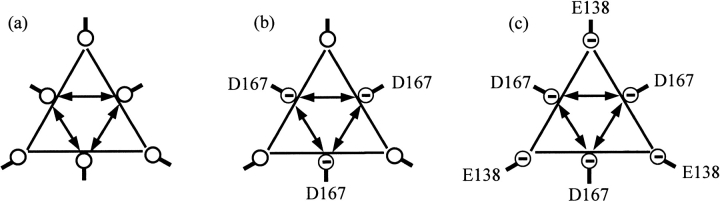
The proposed model of pressure stabilization also should be able to explain the varying degree of pressure stabilization between the three GDHs (Fig. 3 ▶). Figure 4 ▶ is a schematic of positions 138 and 167 as viewed along the trimer-trimer axis (same line-of-sight as Fig. 1 ▶) for the D167T, WT, and T138E GDHs. The number of negatively charged residues occupying these positions increases from D167T (none) to WT (Asp167s) to T138E (Asp167s + Glu138s). Thermostability experiments with KCl in concert with theoretical calculations have shown that the electrostatic repulsion among these positions weakens intersubunit interactions near the core of the GDH hexamer (unpubl.). In particular, with no added salts in the thermoinactivation solution, stability was inversely correlated with the magnitude of electrostatic repulsion and follows the order: T138E < WT < D167T.
Fig. 4.
Schematic representation of positions 138 and 167 within a glutamate dehydrogenase trimer looking along the trimer-trimer axis (same line-of-sight as Fig. 1 ▶). (a) D167T: all six residues are occupied by uncharged Thr. (b) Wild type: Asp at positions 167 are negatively charged. (c) T138E: Asp at positions 167 and Glu at positions 138 are negatively charged. The distance between any two closest neighbors is about 10 Å.
We propose that the destabilizing electrostatic repulsion originating around positions 138 and 167 also creates larger conformational fluctuations of the entire hexamer. In terms of Equation 1, one way that larger fluctuations can be manifested is by an increase in the volume of the higher-energy CS HCS1. Assuming this, stronger electrostatic repulsion will result in larger values of ΔV1s for the equilibrium step between CS. The conformational fluctuations should be smallest in the case of the D167T mutant because positions 138 and 167 are both occupied by uncharged threonines (Fig. 4 ▶). On the other extreme, both positions are negatively charged in the T138E mutant, resulting in the largest conformational fluctuations (Fig. 4 ▶). Fluctuations of the WT GDH should be intermediate because only positions 167 are occupied by negatively charged residues (Fig. 4 ▶). Thus, the magnitude of ΔV1s is predicted to follow the same order as the electrostatic repulsion — D167T < WT < T138E — with the largest volume difference corresponding to the T138E enzyme. And because the effect of pressure is governed by ΔV1, the observed order in the magnitude of pressure stabilization between the GDHs can be rationalized by differences in the electrostatic repulsion around positions 138 and 167.
In support of our model of pressure stabilization, we took advantage of the well-known effects of sugars on protein stability, structure, and dynamics to probe our system. Numerous studies have found that sugars (e.g., glucose, sucrose, and trehalose) stabilized proteins against denaturation by high temperature, low pH, and denaturants (Lee and Timasheff 1981; Arakawa and Timasheff 1982; Timasheff 1993 Timasheff 1998; Kita et al. 1994; Hall et al. 1995; Lin and Timasheff 1996; Kendrick et al. 1997, 1998; Xie and Timasheff 1997). The origin of this effect lies predominantly in the higher surface tension of aqueous sugar solutions (Lee and Timasheff 1981; Lin and Timasheff 1996), leading to the inhibition of protein processes (e.g., unfolding) that are accompanied by an increase in surface area (Sinanoglu and Abdulnur 1964,Sinanoglu and Abdulnur 1965).
Because of the increase in surface tension, we can expect sugars to shift the equilibrium between CS. As mentioned above, CS differ slightly in structure, and in particular, volume (Frauenfelder et al. 1990). According to Richards (1979), the only way to generate volume fluctuations is through the expansion and contraction of interior cavities. It therefore is reasonable that CS with larger volumes also expose larger surface areas; that is, protein expansion as a result of an increase in the number and/or volume of cavities also results in greater surface areas. In this manner, sugars should shift the equilibrium towards the GDH CS with smaller volume and surface area (i.e., HCS0) and thus stabilize against thermoinactivation.
Following the line of reasoning presented above, we examined the effect of trehalose on the thermostability of the WT, D167T, and T138E GDHs. Table 2 gives the half-lives of all three enzymes with and without 0.5 M trehalose. Because of the difference in stability between the GDHs, the experimental temperatures were selected to obtain similar half-lives for all GDHs in the absence of trehalose. Consistent with our model of inactivation involving shifts between CS that differ in volume as well as surface area (Equation 1), trehalose stabilized all three GDHs. More importantly, the degree of stabilization was different between enzymes and followed the order D167T < WT < T138E — the same order as in the case of pressure-induced stabilization. The correspondence between the order of sucrose- and pressure-induced stabilization is consistent with the proposal that the volume and surface area differences between the CS HCS1 and HCS0 increase with the magnitude of electrostatic repulsion around positions 138 and 167. Our proposed model of pressure and trehalose stabilization of GDHs also is consistent with the observation by Almagor and coworkers (1998) that various cosolvents (ranging from sucrose to dextran) reduced the specific volume and adiabatic compressibility of myoglobin.
Kendrick et al. (1998) also took advantage of the known effects of sugars to elucidate the mechanism leading to the aggregation of human interferon-γ. As mentioned above, the proposed mechanisms for the aggregation of both human interferon-γ and GDH (Equation 1) are essentially the same; they both invoke the existence of an equilibrium between native conformational species preceding an irreversible step and leading to eventual aggregation. In agreement with our results, sucrose stabilized against aggregation, consistent with a shift of the equilibrium between CS caused by the increase in surface tension upon addition of sucrose (Kendrick et al. 1998).
Finally, the observation that for each GDH, pressure stabilization increases with temperature (Table 1) should be addressed in light of our model of pressure stabilization. Previous studies with the Pf GDH also have revealed an increase in the degree of pressure stabilization with temperature (Sun et al. 1999). That observation was rationalized by noting that the compressibility, volume, and volume fluctuations of a protein all increase with temperature (Cooper 1976; Sun et al. 1999), and that the increase is most likely a result of the expansion of cavities and free spaces between atoms (Richards 1979; Frauenfelder et al. 1987; Young et al. 1994). Therefore, it seemed reasonable that pressure-induced stabilization involving the compression of structure and reduction of volume fluctuations should be amplified at higher temperatures where compressibility and volume fluctuations are greatest (Sun et al. 1999). This explanation can be further elaborated with our present mechanism involving CS. In this case, volume fluctuations are taken into account explicitly by higher-energy CS with larger volumes (i.e., HCS1). As temperature increases, we hypothesize the appearance of new CS of even higher energy and volume beyond that of HCS1. For example, GDH thermoinactivation may proceed through a slightly different mechanism such as
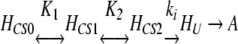 |
5 |
where HCS2 is a new, higher-energy and volume CS that is populated to a significant extent only at higher temperatures. For simplicity, we have assumed that the formation of the aggregation-competent species, HU, now occurs only through HCS2. This equation can be solved, yielding a slightly more complicated expression for the observed rate of GDH thermoinactivation (compare with Equation 3)
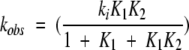 |
6 |
where K2 (= HCS2/HCS1) is the equilibrium constant for the conversion between HCS1 and HCS2. The dependence of K2 on pressure is governed by the volume difference between HCS2 and HCS1, ΔV2, and follows an equation analogous to Equation 4. The effect of pressure on the two expressions for kobs (Equation 3 and 6) were analyzed by substituting in some realistic values for ΔVs and Ks. As an example, for values of ΔVs from 100–300 Å3 (i.e., <0.1% of the volume of a GDH hexamer) and K values between 0.01–1, the stabilizing effect of pressure was found to be ∼2–3 times greater for the mechanism of thermoinactivation hypothesized to occur at higher temperatures. This simple analysis demonstrates that the observed increase in pressure stabilization with temperature (Table 1) can be accounted by a model where pressure shifts the equilibrium between CS.
Materials and methods
GDH Purification
GDH was purified as described previously (Vetriani et al. 1998). Final purified GDHs exhibited single bands when analyzed by SDS-PAGE and stained with Coomasie blue. The concentration of GDH was determined by ultraviolet (UV) absorption at 280 nm of GDH dissolved in 7.84 M guanadine hydrochloride using an extinction coefficient of 82,740 M−1 cm−1 for the unfolded GDH monomer (Edelhoch 1967; Gill and von Hippel 1989).
Activity assays
GDH activity was measured by monitoring the glutamate-dependent reduction of NADP+ as described previously (Sun et al. 1999). Assays were performed in an AVIV 14NT-UV-VIS spectrophotometer equipped with a Peltier heater and a magnetic stirrer for accurate temperature control (AVIV Instruments, Inc.). The cell holder was inside a pressure chamber capable of hyperbaric pressurization to 5 atm, allowing assays above 100°C.
Thermoinactivation experiments
Thermoinactivation experiments were performed as described previously in a custom bioreactor kept in a constant-temperature oven (Sun et al. 1999). All thermoinactivation experiments were performed at either 5 or 500 atm of hyperbaric pressure using helium as the pressurizing gas. At 100°C and 500 atm of helium, the liquid mole fraction of helium in water is 0.003094 (Clever 1979); thus, the influence of dissolved helium should be negligible. Temperatures of the thermoinactivation experiments were accurate to within ± 0.1°C. Unless otherwise indicated, GDH (24–28 μg/mL final concentration) was incubated in 100 mM EPPS (Sigma), at a pH of 7.1–7.2 at the thermoinactivation temperature. For correction of pH as a function of temperature, a ΔpH/ΔT value of −0.0114 was experimentally determined for 100 mM EPPS, with or without 0.5 M trehalose (Sigma). Samples from the bioreactor were withdrawn periodically and immediately assayed for activity at 85°C and 1 atm (Sun et al. 1999).
Analysis of irreversible thermoinactivation
The thermoinactivation trajectories of all three GDHs deviated from a single-exponential decay. As discussed previously, the available evidence suggests that the apparent non–single-exponential thermoinactivation of the GDHs is because of the existence of misfolded and less-stable form(s) of GDH (unpubl.). Specifically, thermoinactivation appears to involve the simultaneous denaturation of less-stable form(s) of GDH and the native, more stable, form of GDH, which inactivates via an irreversible, rate-limiting transition between the native hexamer and an inactivated species. Following this model, the thermoinactivation trajectories were fitted to a double-exponential model with four adjustable parameters:
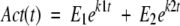 |
7 |
where Act is the remaining activity at time t (i.e., defined as the percentage of the initial activity), E1 and E2 are the percentages of the total activity contributed by the less-stable and native forms of GDH, respectively, and k1 and k2 are the respective apparent first-order inactivation rate constants. The double-exponential model fitted all thermoinactivation trajectories well. As expected, the fitted parameters E1 and E2 summed to nearly 100% in all of the fits. In all samples, the more stable form comprised at least 60% of the total GDH.
The rate constant k2 corresponding to that of the native GDH, and henceforth identified as kobs, was further analyzed in terms of the activation barrier for thermoinactivation using transition state theory (Eyring 1935):
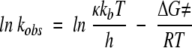 |
8 |
where ϰ; is the transmission factor, kb the Boltzmann constant, T the absolute temperature in Kelvin, h the Planck constant, ΔG≠ the free energy of activation, and R the gas constant. The difference in the free energy of activation between 5 and 500 atm (ΔΔG≠P) were calculated following the analysis of Pappenberger et al. (1997):
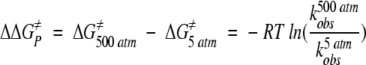 |
9 |
The difference in free energy between conditions of 0 and 0.5 M trehalose, ΔΔG≠tre, was calculated using the analogous equation and rate constants determined with and without trehalose.
Acknowledgments
This work was supported by the National Science Foundation (BES 9410687), the Schlumberger Fellowship of DSC, and by Kyowa Hakko Kogyo Co. Ltd.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.4001.
References
- Almagor, A., Priev, A., Barshtein, G., Gavish, B., and Yedgar, S. 1998. Biochim. Biophys. Acta. 1382 151–156. [DOI] [PubMed] [Google Scholar]
- Arakawa, T. and Timasheff, S.N. 1982. Stabilization of protein structure by sugars. Biochemistry 21 6536–6544. [DOI] [PubMed] [Google Scholar]
- Clever, H.L. 1979. Solubility data series, vol. 1. Pergamon Press, Oxford, UK.
- Cooper, A. 1976. Thermodynamic fluctuations in protein molecules. Proc. Natl. Acad. Sci. 73 2740–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch, H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6 1948–1954. [DOI] [PubMed] [Google Scholar]
- Eyring, H. 1935. The activated complex in chemical reactions. J. Chem. Phys. 3 107–115. [Google Scholar]
- Frauenfelder, H., Alberding, N.A., Ansari, A., Braunstein, D., Cowen, B.R., Hong, M.K., Iben, I.E.T., Johnson, J.B., Luck, S., Marden, M.C., et al. 1990. Proteins and pressure. J. Phys. Chem. 94 1024–1037. [Google Scholar]
- Frauenfelder, H., Hartmann, H., Karplus, M., Kuntz, I.D., Jr., Kuriyan, J., Parak, F., Petsko, G.A., Ringe, D., Tilton, R.F., Jr., Connolly, M.L., et al. 1987. Thermal expansion of a protein. Biochemistry 26 254–261. [DOI] [PubMed] [Google Scholar]
- Frauenfelder, H., Parak, F., and Young, R.D. 1988. Conformational substates in proteins. Ann. Rev. Biophys. Biophys. Chem. 17 451–479. [DOI] [PubMed] [Google Scholar]
- Frauenfelder, H., Sligar, S.G., and Wolynes, P.G. 1991. The energy landscapes and motions of proteins. Science 254 1598–1603. [DOI] [PubMed] [Google Scholar]
- Gill, S.C. and von Hippel, P.H. 1989. Calculation of protein extinction coefficients from amino acid sequence data [published erratum appears in Anal. Biochem. 1990. 189: 283]. Anal. Biochem. 182: 319–326. [DOI] [PubMed]
- Gorovits, B.M. and Horowitz, P.M. 1998. High hydrostatic pressure can reverse aggregation of protein folding intermediates and facilitate acquisition of native structure. Biochemistry 37 6132–6135. [DOI] [PubMed] [Google Scholar]
- Gross, M. and Jaenicke, R. 1994. Proteins under pressure. Eur. J. Biochem. 221 617–630. [DOI] [PubMed] [Google Scholar]
- Hall, D.R., Jacobsen, M.P., and Winzor, D.J. 1995. Stabilizing effect of sucrose against irreversible denaturation of rabbit muscle lactate dehydrogenase. Biophys. Chem. 57 47–54. [DOI] [PubMed] [Google Scholar]
- Hawley, S.A. 1971. Reversible pressure-temperature denaturation of chymotrypsinogen. Biochemistry 10 2436–2442. [DOI] [PubMed] [Google Scholar]
- Hayashi, R. and Balny, C., eds. 1996. High pressure bioscience and biotechnology. Elsevier Science B.V., Amsterdam.
- Hei, D.J. and Clark, D.S. 1994. Pressure stabilization of proteins from extreme thermophiles. Appl. Env. Microb. 60 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans, K. 1997. High pressure research in bioscience and biotechnology. Leuven University Press, Leuven, Belgium.
- Heremans, K. and Smeller, L. 1998. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta. 1386 353–370. [DOI] [PubMed] [Google Scholar]
- Hiller, R., Zhou, Z.H., Adams, M.W.W., and Englander, S.W. 1997. Stability and dynamics in a hyperthermophilic protein with melting temperature close to 200 degree C. Proc. Natl. Acad. Sci. 94 11329–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke, R. 1991. Protein stability and molecular adaptation to extreme conditions. Eur. J. Biochem. 202 715–728. [DOI] [PubMed] [Google Scholar]
- Kendrick, B.S., Carpenter, J.F., Cleland, J.L., and Randolph, T.W. 1998. A transient expansion of the native state precedes aggregation of recombinant human interferon-gamma. Proc. Natl. Acad. Sci. 95 14142–14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick, B.S., Chiang, B.S., Arakawa, T., Peterson, B., Randolph, T.W., Manning, M.C., and Carpenter, J.C. 1997. Preferential exclusion of sucrose from recombinant interleukin-1 receptor antagonist: Role in restricted conformational mobility and compaction of native state. Proc. Natl. Acad. Sci. 94 11917–11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita, Y., Arakawa, T., Lin, T.-Y., and Timasheff, S.N. 1994. Contribution of the surface free energy perturbation of protein-solvent interactions. Biochemistry 33 15178–15189. [DOI] [PubMed] [Google Scholar]
- Lee, J.C. and Timasheff, S.N. 1981. The stabilization of proteins by sucrose. J. Biol. Chem. 256 7193–7201. [PubMed] [Google Scholar]
- Lin, T.-Y. and Timasheff, S.N. 1996. On the role of surface tension in the stabilization of globular proteins. Protein Sci. 5 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels, P.C., Hei, D., and Clark, D.S. 1996. Pressure effects on enzyme activity and stability at high temperatures. Adv. Protein Chem. 48 341–376. [DOI] [PubMed] [Google Scholar]
- Millar, D.B., Grafius, M.A., Wild, J.R., and Palmer, D.A. 1974. High pressure stabilization of acetylcholinesterase sizeozymes against thermal denaturation. Biophys. Chem. 2 189–192. [DOI] [PubMed] [Google Scholar]
- Mozhaev, V.V., Lange, R., Kudryashova, E.V., and Balny, C. 1996. Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol. Bioeng. 52 320–331. [DOI] [PubMed] [Google Scholar]
- Neuner, A., Jannasch, H.W., Belkin, S., and Stetter, K.O. 1990. Thermococcus litoralis: A new species of extremely thermophilic marine archaebacteria. Arch. Microbiol. 153 205–207. [Google Scholar]
- Panick, G., Malessa, R., Winter, R., Rapp, G., Frye, K.J., and Royer, C.A. 1998. Structural characterization of the pressure-denatured state and unfolding/refolding kinetics of staphylococcal nuclease by synchrotron small-angle x-ray scattering and Fourier-transform infrared spectroscopy. J. Mol. Biol. 275 389–402. [DOI] [PubMed] [Google Scholar]
- Pappenberger, G., Saudan, C., Becker, M., Merbach, A.E., and Kiefhaber, T. 2000. Denaturant-induced movement of the transition state of protein folding revealed by high-pressure stopped-flow measurements. Proc. Natl. Acad. Sci. 97 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenberger, G., Schurig, H., and Jaenicke, R. 1997. Disruption of an ionic network leads to accelerated thermal denaturation of D-glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima. J. Mol. Biol. 274 676–683. [DOI] [PubMed] [Google Scholar]
- Richards, F.M. 1979. Packing defects, cavities, volume fluctuations and access to the interior of proteins. Carlsberg Res. Commun. 44 47–63. [Google Scholar]
- Silva, J.L., Foguel, D., Da Poian, A.T., and Prevelige, P.E. 1996. The use of hydrostatic pressure as a tool to study viruses and other macromolecular assemblages. Curr. Opin. Struct. Biol. 6 166–175. [DOI] [PubMed] [Google Scholar]
- Sinanoglu, O. and Abdulnur, S. 1964. Hydrophobic stacking of bases and the solvent denaturation of DNA. Photochem. Photobiol. 3 333–342. [Google Scholar]
- ———. 1965. Effect of water and other solvents on the structure of biopolymers. Federation Proc. 24: Suppl. 15, 12–23. [PubMed] [Google Scholar]
- St. John, R.J., Carpenter, J.F., and Randolph, T.W. 1999. High pressure fosters protein refolding from aggregates at high concentrations. Proc. Natl. Acad. Sci. 96 13029–13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M.M.C., Tolliday, N., Vetriani, C., Robb, F.T., and Clark, D.S. 1999. Pressure-induced thermostabilization of glutamate dehydrogenase from the hyperthermophile Pyrococcus furiosus. Protein Sci. 8 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff, S.N. 1993. The control of protein stability and association by weak interactions with water: How do solvents affects these processes? Ann. Rev. Biophys. Biomol. Struct. 22 67–97. [DOI] [PubMed] [Google Scholar]
- ———. 1998. Control of protein stability and reactions by weakly interacting cosolvents: The simplicity of the complicated. Adv. Prot. Chem. 51 355–432. [DOI] [PubMed] [Google Scholar]
- Vetriani, C., Maeder, D.L., Tolliday, N., Yip, K.-S., Stillman, T.J., Britton, K.L., Rice, D., Klump, H.H., and Robb, F.T. 1998. Protein thermostability above 100°C: A key role for ionic interactions. Proc. Natl. Acad. Sci. 95 12300–12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidugiris, G.J.A., Markley, J.L., and Royer, C.A. 1995. Evidence for a molten globule-like transition state in protein folding from determination of activation volumes. Biochemistry 34 4909–4912. [DOI] [PubMed] [Google Scholar]
- Weber, G. and Drickamer, H.G. 1983. The effect of high pressure upon proteins and other biomolecules. Q. Rev. Biophys. 16 89–112. [DOI] [PubMed] [Google Scholar]
- Xie, G. and Timasheff, S.N. 1997. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 64 25–43. [DOI] [PubMed] [Google Scholar]
- Young, A.C.M., Tilton, R.F., and Dewan, J.C. 1994. Thermal expansion of hen egg-white lysozyme: Comparison of the 1.9 Å resolution structures of the tetragonal form of the enzyme at 100 K and 298 K. J. Mol. Biol. 235 302–317. [DOI] [PubMed] [Google Scholar]