Abstract
The Methoprene-tolerant (Met) gene of Drosophila melanogaster is involved in both juvenile hormone (JH) action and resistance to JH insecticides, such as methoprene. Although the consequences of Met mutations on development and methoprene resistance are known, no studies have examined Met+ overexpression. Met+ was overexpressed in transgenic lines with various promoters that drive overexpression to different levels. Flies expressing either genomic or cDNA Met+ transgenes showed higher susceptibility to both the morphogenetic and toxic effects of methoprene, consistent with the hormone-binding property of MET. Both the sensitive period and lethal period were the same as seen for non-overexpressing Met+ flies. However, continual exposure of high-overexpressing Met+ larvae to borderline-toxic or higher methoprene doses advanced the sensitive period from prepupae to first instar and the lethal period from pharate adults to larvae and early pupae. When expression of transgenic UAS-Met+ was driven to high levels by either an actin-GAL4 or tubulin-GAL4 promoter, larvae showed high mortality in the absence of methoprene, indicating that high MET titer is lethal, perhaps resulting from expression in an inappropriate tissue. Adults overexpressing Met+ did not show enhanced oogenesis, ruling out MET as a limiting factor for this hormone-driven physiology.
Keywords: Methoprene, Met gene, insecticide resistance, hormone action
1. Introduction
Insect development and reproduction are hormonally regulated by the steroid hormone 20-hydroxyecdysone and the sesquiterpenoid juvenile hormone (JH). JH is essential for a diverse array of functions, including maintenance of larval development (Riddiford, 1994; Riddiford, 1996), adult oogenesis, and male accessory gland function (Wyatt and Davey, 1996). Roles for JH, specifically JH III (which will be referred to in this paper as “JH”), in Drosophila melanogaster are best understood in adults, especially during reproduction (Bownes, 1989; Riddiford, 1993; Soller et al., 1999; Dubrovsky et al., 2002; Flatt et al., 2005). JH has been shown to be necessary for the endocytotic uptake of vitellogenin by oocytes (Postlethwait and Weiser, 1973; Kambysellis and Heed, 1974; Wilson, 1982; Soller et al., 1999) and is involved in female receptivity (Kerr et al., 1997). In male D. melanogaster, JH promotes the synthesis of accessory gland protein (Yamamoto et al., 1988; Shemshedini et al., 1990). Examples of other roles for JH in adults include an acceleration of the autolysis of the larval fat body during the first 2 days of adult life (Postlethwait and Jones, 1978; Wilson, 1982) and an intriguing involvement in life-span (Tatar et al., 2001). The hormone acts to regulate gene expression at the transcriptional level in various insects, including D. melanogaster, but only a few genes that are directly regulated have been identified (Dubrovsky et al., 2000; Li et al., 2007). Over the past four decades, insecticidal JH analogs (JHAs), such as methoprene, have appeared (Staal, 1975; Dhadialla et al., 1998) and shown to be effective against certain insects, including D. melanogaster. It is clear from numerous studies that methoprene and perhaps all JHAs act as JH agonists (Wilson, 2004). When applied to D. melanogaster at the onset of metamorphosis, these insecticides act to disrupt metamorphosis of both external and internal tissues (Ashburner, 1970; Madhavan, 1973; Postlethwait, 1974; Wilson and Fabian, 1986; Riddiford and Ashburner, 1991; Restifo and Wilson, 1998). Metamorphic disruption may result from misexpression of secondary-response genes, resulting in lethality or morphogenetic defects of sternal bristle patterns and malrotation of the male genitalia during pupal development (Restifo and Wilson, 1998; Zhou and Riddiford, 2002).
The Methoprene-tolerant (Met) gene was identified from a screen of mutagenized D. melanogaster as conferring resistance to both the toxic and morphogenetic effects of methoprene (Wilson and Fabian, 1986). Met was shown to confer resistance to application of the endogenous hormone, JH III (Wilson and Fabian, 1986), and to a more powerful JHA, pyriproxyfen (Riddiford and Ashburner, 1991), but not to other classes of insecticides (Wilson and Fabian, 1986). Judging from the ability of MET to bind JH (Shemshedini and Wilson, 1990; Miura et al., 2005; Konopova and Jindra, 2007) and the involvement of Met in JH-regulated physiology, such as male accessory gland development or oogenesis (Shemshedini et al., 1990; Ashok et al., 1998; Wilson and Ashok, 1998; Miura et al., 2005), we believe that Met participates in the action of endogenous and exogenous JH III, possibly as a component of a JH receptor. Recently, Tribolium castaneum was shown to display precocious metamorphosis following RNAi suppression of expression of the Met ortholog of this beetle (Konopova and Jindra, 2007), further demonstrating the involvement of this gene in JH action during molting.
Cloning and sequence analysis of Met identifed the gene as a member of the bHLH-PAS transcription factor family. bHLH-PAS proteins in both vertebrates and invertebrates play important roles during development and as transcriptional regulators in response to environmental signals such as light, sundry environmental chemicals, and hypoxia (Gu et al., 2000). For example, the dioxin receptor partners AHR and ARNT act as a ligand-activated heterodimeric transcription factor to regulate gene expression in response to xenobiotic chemicals (Hankinson, 1995). Likewise, MET has been hypothesized (Ashok et al., 1998) and shown (Miura et al., 2005) to be capable of transcriptional regulation. Thus, Met is of interest both as a gene with probable involvement in JH action and also in JH-insecticide resistance.
Isolation and characterization of Met27, a null allele, showed the consequences of loss of MET from flies. In addition to JH resistance, oogenesis in Met27 females is reduced to about 20% of wild-type (Wilson and Ashok, 1998). Males have reduced protein accumulation in the male accessory glands, and they court and mate wild-type females much less avidly than do Met+ or Met27; Met+ transgenic males (Wilson et al., 2003). Thus, the Met27 mutation has provided insight into the roles of both Met+ and JH in this insect.
Studies with D. melanogaster having overexpressed Met+ should give insight into the consequences of excess MET in the physiology of this insect. Therefore, genetic construction of transgenic Met+ flies showing overexpression driven by different promoters was carried out. Analysis of the resultant strains showed enhanced sensitivity to methoprene. High overexpression resulted in larval death, and in the presence of methoprene, dramatically advanced both the methoprene-sensitive period and resultant lethal period in development. However, oogenesis was not appreciably affected, suggesting that MET is not a limiting factor in this physiology in wild-type flies.
2. Materials and methods
2.1. Drosophila strains
Isolation and molecular analysis of the Met alleles have been previously described (Wilson and Ashok, 1998; Wilson et al., 2006a). The w; p[cDNA] and w; p[EN 71] are Met+ transgenic strains carrying either a Met+ cDNA or Met+ genomic fragment (termed EN 71) whose construction has been described (Ashok et al., 1998).
Two novel Met+ transgenic stocks, w; p[V2] and w; UAS-Met+, were constructed. Genomic DNA was extracted from Oregon-RC flies (Qiao and Raymond, 1995). Genomic Met+ was amplified by PCR following synthesis of a forward primer 5′-AGG CGC CAA TTA AAG GGG AA-3′, located 92 bp upstream of the ATG start codon, and a reverse primer 5′-GTG AGC TAC CAA TTA CGT CCA-3′, located 66 bp downstream from the TGA stop codon. The amplification procedure was as follows: The initial denaturation step was at 94°C for 3 min. Amplifications were achieved through 35 cycles at 94°C for 30 s, 55°C for 45 s, and 72°C for 150 s. A final extension reaction was carried out for 10 min at 72°C. The obtained 2.3kb PCR-fragment was cloned into the pCR2.1-TOPO vector (Invitrogen), sequenced and inserted into EcoRI sites (since Met does not contain an EcoRI site) of the pUAST transformation vector (Brand and Perrimon, l993). Proper orientation of the fragment in the vector was verified by restriction site analysis and sequencing.
Germline transformation of w or w MetW3 embryos with purified plasmid together with “wings clipped” helper plasmid pπ25.7 (Rubin and Spradling, 1982) in a ratio of 2-3:1 was carried out by injection into dechorionated embryos. Transformant flies were recognized by partial restoration of eye color and (if w embryos were transformed) crossed into a w MetW3 line to test for functionality of the Met+ transgene, which rescues the eye defect phenotype (Wilson et al., 2006a) produced by this Met allele. 18 independent transformant lines were isolated and tested for Met+ function. When grown at 25 °C, most of the lines weakly expressed Met+, which was not unexpected since the pUAST vector is designed for GAL4 expression and has only a weak hsp70 promoter (Brand and Perrimon, l993). However, one of the transformant lines, p[V2], strongly expressed Met+ independent of a GAL4 driver, presumably because the Met+ transgene inserted downstream of a strong promoter of an unknown gene.
For RT-PCR, total RNA was extracted from whole flies using TRIzol (Invitrogen) and treated with RNase-free DNase-I (Invitrogen). Aliquots of 5 μg total RNA were reverse transcribed with 200 U/ml of MMLV reverse transcriptase (Invitrogen) and 1 μg random hexamers (Invitrogen) in a 25 μl reaction mixture. The cDNA was amplified by PCR in a 25 μl reaction mixture consisting of 2.5 μl of 10X PCR buffer, 1 μl Taq DNA polymerase (Invitrogen), 1 μl of 10 mM dNTP mix, 1μl of 50 mM mM MgCl2, 1 μl each of 10 mM primer, 1 μl of cDNA, and 16 μl of H20. PCR primers used were for Met were 5′-CAGAGCAGCAGTCCCGATTT and 3′-CCATCGTCCATTAGGCTTTCCA. The primers for Rp49 were 5′-CCGCTTCAAGGGACAGTATC and 3′-ATCTCGCCGCAGTAAACG. Ten μl of each PCR product was electrophoresed on a 1% agarose gel and stained with ethidium bromide. An image of the gel was capture with the ImageQuant 400 (GE Healthcare) and then analyzed using the ImageQuant TL software (Amersham Biosciences). PCR products were cloned into TOPO vector (Invitrogen) and sequenced with M13 forward primer.
2.2. Met+ overexpression in GAL4-UAS transgenic strains
The GAL4-UAS system in D. melanogaster allows directed expression of a UAS-linked transgene by a specific selectable promoter that is ligated to the yeast GAL4 transcriptional regulator (Brand and Perrimon, l993). Simple genetic crosses serve to bring the two transgenes together to control expression. The four independent Met+ transformant lines were made homozygous for the UAS Met+ transgene. Each of these lines represents independent insertions of the transgene into an autosome. Each line was crossed with flies carrying either an actin-GAL4 or tubulin-GAL4 transgene heterozygous to a TM3 or TM6 balancer chromosome carrying either a Tubby (Tb) or Stubble (Sb) dominant mutation, allowing ready identification of adults (Sb) or larvae (Tb) carrying the balancer chromosome. Both tubulin and actin genes are expressed widely in D. melanogaster tissues, so either GAL4 transgene should drive UAS-Met+ expression throughout development in perhaps all tissues.
A similar cross with tubulin-GAL4/TM6, Sb allows this promoter to drive UAS-Met+ expression. Note that F1 progeny carrying the TM3 balancer chromosome lack actin-GAL4 and therefore serve as control flies.
2.3. Methoprene resistance
For determination of the median effective doses (ED50), cultures were assayed for methoprene sensitivity on each of 4 or 5 doses of methoprene (isopropyl-(2E,4E)-11-methoxy-3,7,11-tri-methyl-2,4-dodecadienonate, ChemService, PA), applied as ethanolic solutions (25 μl) to the surface of each culture (food surface area 3.8 cm2) in diagnostic amounts consisting of 0.27, 0.54, 2.7, 5.4, and 26.8 μg/vial. Mortality occurs typically during the pharate adult stage; survivors were examined for methoprene-induced morphogenetic defects commonly seen in dipteran insects consisting of malrotated male genitalia and defective sternal bristles, particularly on the posterior sternites (Wilson and Fabian, 1986). The survival data were used to calculate the ED50 values using the PriProbit program (Sakuma, 1998).
2.4. Oviposition
Females were isolated within 8 h after eclosion and allowed to age for 6 days in the presence of wild-type (Oregon-RC) males and baker’s yeast sprinkled on the food surface to maximize oogenesis. They were then placed individually in 28 × 95-mm plastic food vials (Capitol, Fonda, NY) in the continued presence of yeasted food and males. Eggs were counted during the next 4 days, a time period when the ovipositional rate reaches a maximal steady-state value and allows strain comparison (Wilson and Ashok, 1998). Egg fertility was noted, and vials having unfertilized eggs were discarded because unmated females are less fecund.
3. Results
3.1. Verification of transgenic stocks
Three transgenic stocks were created in w MetW3genetic backgrounds following P-element-mediated transformation with the Met+ constructs. The first construct, termed p[EN71], includes a genomic fragment carrying sequence comprising the Met+ 5.5 kb transcript plus 0.3 kb of upstream and 1.7 kb of downstream sequence and showing autonomous expression, as expected (Ashok et al., 1998). Two Met+ cDNA transgenic stocks, termed p[V2] and p[cDNA], also showed autonomous expression, probably resulting from insertion of each transgene into an unidentified promoter region. All three stocks were verified as expressing functional Met+ by (1) loss of resistance following methoprene treatment of cultures (Ashok et al., 1998) and (2) rescue of the non-conditional Met eye phenotype readily expressed in the MetW3 allele (Wilson et al., 2006a).
3.2. Expression of Met+ in w; p[Met+] transgenic strains
To maximize Met+ expression, the Met+ transgene in each strain was genetically inserted into a w genetic background by backcrosses with w flies, which express a Met+ allele (Ashok et al., 1998) and therefore contribute endogenous Met+ transcript in addition to that expressed by the transgene. Following the creation of Met+ transgenic homozygotes by standard chromosomal manipulation, Met+ transcript levels in each stock were analyzed by RT-PCR. As shown in Table 1 and Figure 1, both w; p[EN71] and w; p[cDNA] express levels as high as 3.4-fold that of w, and w; p[V2] shows higher levels, 5.9-fold in larvae and 3.0-fold in adults. Therefore, the transgenic stocks offer a range of expression levels to evaluate overexpression of both genomic and cDNA Met+.
Table 1.
RT-PCR values for Met and Rp49 transcript levels in each indicated strains. Each value is normalized to that of w larvae or adults set at the value 1. The strain UAS-Met+/actin expresses UAS-Met+ transgene driven by GAL4-actin, and the strain UAS-Met+/TM3 is a TM3-balancer control. ND=not determined; NA=not applicable.
| Strain Genotype | Larval | Adult | ||||
|---|---|---|---|---|---|---|
| Met | Rp49 | Met/Rp49 | Met | Rp49 | Met/Rp49 | |
| w | 1 | 1 | 1 | 1 | 1 | 1 |
| w;p[cDNA] | 2.4 | 0.7 | 3.4 | 0.8 | 0.6 | 1.3 |
| w; p[EN71] | 1.7 | 1.1 | 1.5 | 3.4 | 1.1 | 3.1 |
| w; p[V2] | 7.1 | 1.2 | 5.9 | 3.6 | 1.2 | 3.0 |
| UAS-Met+/actin | 12.5 | 1.1 | 11.4 | 13.4 | 1.1 | 12.2 |
| UAS-Met+/TM3 | 1.1 | 0.8 | 0.8 | 3.1 | 1.0 | 3.1 |
| w; Met27 | 0 | 0.9 | NA | ND | ND | NA |
Figure 1.
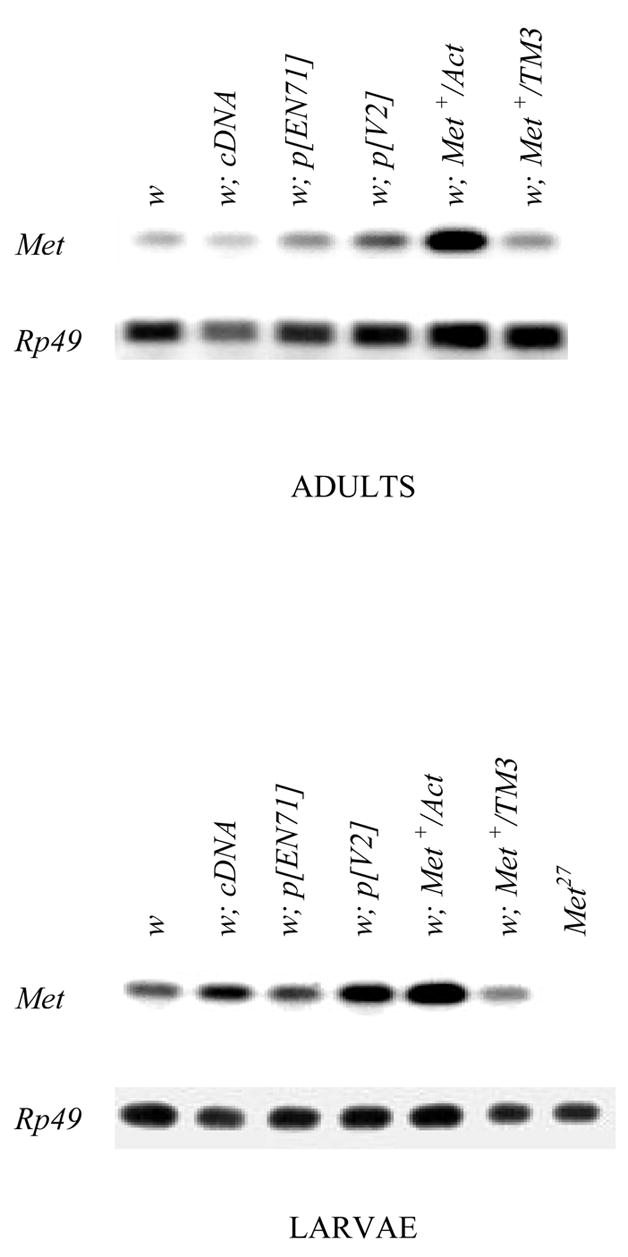
RT-PCR determinations of Met transcripts from late third-instar larvae and adults of the Met+ overexpressing strains. The strain labeled w; Met+/actin expresses the UAS-Met+ transgene driven by GAL4-actin, and the strain labeled w; Met+/TM3 is a sibling TM3-balancer control.
3.2. Evaluation of Met+ transgenic strain phenotypes
Resistance to toxicity
Each of the three transgenic strains was evaluated for levels of methoprene susceptibility following exposure to various amounts of methoprene applied to the surface of the larval diet, previously shown to be an effective method of exposure (Wilson, 1996). w; p[EN71] and w; p[cDNA] showed higher susceptibility than either w or the wild-type strain Oregon-RC, and, in agreement with the Met+ transcript levels, w; p[V2] showed the highest level (Table 2). The increases in susceptibility were reflected not only by the survival values but also by the increased fraction of adults from cultures exposed to a lower sublethal dose that showed methoprene-induced morphogenetic defects, such as sternite bristle defects or malrotated male genitalia. The susceptibility value for w; p[V2] represents the highest susceptibility seen in our laboratory or reported for any D. melanogaster strain. Therefore, the overexpression of Met+ transcript by these strains is also reflected by overexpression of functional Met+ gene product.
Table 2.
Effect of methoprene on the viability and morphology of Met overexpressing strains. The ED50 values are given in μg/food vial. Morphogenetic defects in posterior female sternite bristle morphology/patterning and male genitalia rotation were scored as either normal or abnormal (Wilson and Fabian 1986) after examination of 25 male and 25 female survivors at a low dose (0.054 μg/vial) chosen to demonstrate differential responses of the fly lines.
| Genotype | Methoprene ED50 (95% fiducial limits) | Progeny with bristle/male genitalia defects (%) |
|---|---|---|
| Oregon-RC | 1.4 (1.1–1.7) | 4 |
| w | 0.92 (0.76–1.1) | 8 |
| w; p[EN71] | 0.19 (0.16–0.23) | 88 |
| w; p[cDNA] | 0.11 (0.086–0.14) | 76 |
| w; p[V2] | 0.081 (0.030–0.36) | 92 |
| w; Met27 | 18 (13–33) | 0 |
Preadult development
The effect of Met+ overexpression on preadult development was examined in the transgenic strain showing the highest Met+ levels, w; p[V2]. Egg hatch was good for each strain (Table 3), showing little effect of Met+ overexpression on embryonic development. Newly hatched (0–2 hrs) larvae from cultures of either w or w; p[V2] were carefully transferred to new food vials, and survival and time-to-pupariation measured for each strain. Although a significant difference was found between the two strains for development time, no differences were evident for survival (Table 3). Therefore, this high level of Met+ overexpression does not appear to be markedly detrimental to preadult development.
Table 3.
Developmental parameters of the w; p[V2] strain compared with w. Egg hatch was determined for 50 eggs from each culture, and the remaining values were determined from 100 0–2 hour-old larvae carefully transferred to food vials without added yeast. Standard error of mean (SEM) given in parenthesis. Larval development time means compared by a Mann-Whitney test gave p=0.0104, a significant difference between the two strains. Chi-square tests for the egg hatch and survival values showed no difference (0.05 significance level, 1 df) between the two strains: χ= 0.044 for egg hatch, 0.05 for survival to pupariation, and 0.37 for survival to adult.
| Genotype | Egg Hatch (%) | Time of Larval Developmental (days) | Survival to Pupariation (%) | Survival to Adult (%) |
|---|---|---|---|---|
| w | 92 | 5.3 (0.066) | 94 | 90 |
| w; p[V2] | 88 | 5.6 (0.082) | 91 | 82 |
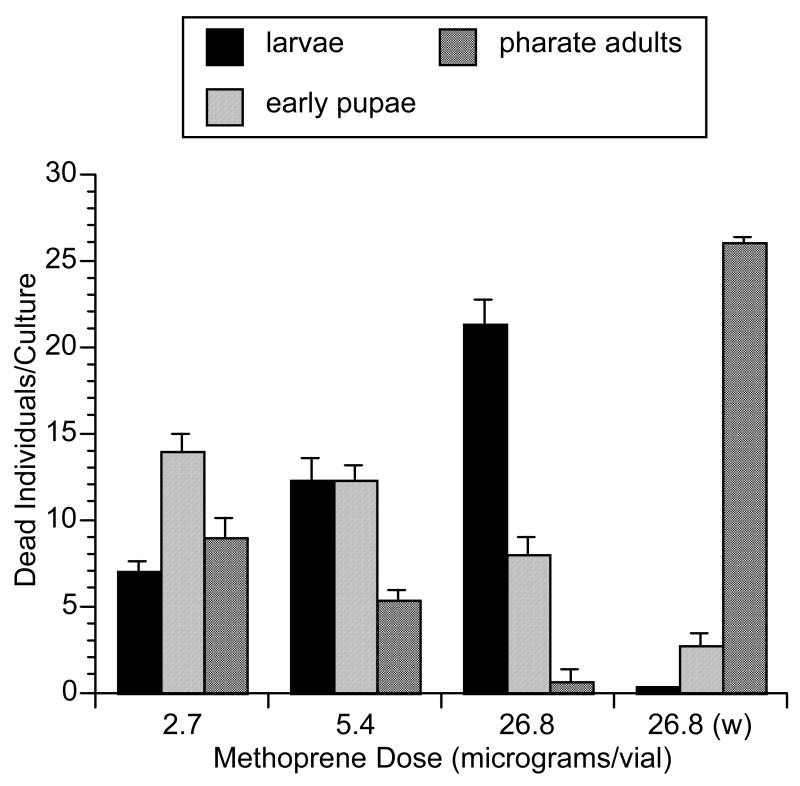
Previous studies have shown that the sensitive period in dipteran insects for applied methoprene or JH III is a 12-hr window centered at pupariation, but the morphogenetic and toxic effects are not evident until the end of pupal development, 4–5 days later at the pharate adult stage (Postlethwait, 1974). To determine if Met+ overexpression can alter this period of sensitivity and/or response to applied hormone, newly hatched (0–2 hrs) w; p[V2] larvae were exposed continuously to one of three diagnostic doses of methoprene that produced typical pharate adult lethality in control w animals treated in an identical manner. Cultures of w showed good larval survival and pupariation, and death at the pharate adult stage, as expected. However, w; p[V2] showed poorer larval survival, especially at the 26.8 μg/vial methoprene dose (Figure 2). Larval death was not rapid; some larvae died within a day of transfer as small larvae on the vial wall, whereas other larvae fed and increased in size for several days, then began dying as third-instar size. At all three doses, pupariation of surviving larvae occurred, but they usually did not survive to the pharate adult stage as did w pupae. At the 26.8 μg dose, prepupae were often misshaped and failed to pupate. At 2.7 μg, 25–30% of the pupae developed into abnormal-appearing pharate adults at this lowest methoprene dose. Therefore, when Met+ is grossly overexpressed, not only are the flies more sensitive to methoprene, but at higher doses the sensitive period for methoprene pathology is also altered, resulting at the 26.8 μg dose in larval death beginning within 1 day following the onset of exposure and continuing until late third-instar. At a lower lethal methoprene level (2.7 μg), pupariation usually occurred, and death was seen in both early pupae and pharate adults.
Figure 2.
Stage-specific death of w; p[V2] individuals raised on one of three lethal doses of methoprene compared with w raised on 26.8 μg/vial. Values for larval death were determined by subtracting the number of dead pupae from the total individuals in each of 3 separate cultures of 30. The predominant pharate adult stage of death of w is typical of the effect of methoprene on non-overexpressing Met+ strains. Error bars represent SEM values.
3.3. Met+ overexpression in GAL4 transgenic strains
Although Met+ is overexpressed in the three transgenic strains described above, the temporal and tissue specificity of expression is unknown and uncontrollable. The GAL4-UAS system allows expression of a UAS transgene by the specified promoter carried in a GAL4 transgene (Brand and Perrimon, l993). By the choice of promoter-GAL4 line that is genetically crossed to the UAS-transgene line, the temporal and/or tissue-specific expression of the UAS-transgene can be controlled. Three independent UAS-Met+ transgenic strains were constructed and crossed individually with lines carrying either actin or tubulin promoter GAL4 transgenic lines to express the UAS-Met+ transgene. Both actin and tubulin genes are widely expressed in tissues, thus their promoters should drive Met+ expression throughout development in perhaps all tissues.
Progeny from crosses of each line were examined for abnormal development that could be attributed to overexpressed Met+. In the w; UAS-Met+/tubulin-GAL4 progeny, egg hatch was good (84%), but larvae began dying a day later and rarely pupariated and survived to adulthood. Dead larvae were seen either on the vial walls (younger larvae) or on the surface of the food in older larvae. Three-to-four day-old w; UAS-Met+/tubulin-GAL4 larvae were identified by their non-Tubby phenotype, carefully transferred to new food vials, and observed daily for 3–4 days. They grew little and generally died within 2 days. Each of the three w; UAS-Met+ lines, as well as w; p[V2], responded in a similar manner when crossed with w; tubulin-GAL4 (data not shown). Control w; UAS-Met+/TM3 siblings transferred in a similar manner developed to typical third-instar size, pupariated, and eclosed with good survival, ruling out a transfer problem with w; UAS-Met+/tubulin-GAL4 larvae.
Overexpression was examined in a similar manner by crossing w; UAS-Met+/flies to flies carrying an actin-GAL4 transgenic driver. Survival to adulthood of larvae was found at low levels (<5%) in each of the three lines, indicating that the actin promoter does not drive expression of UAS-Met+ as strongly as the tubulin promoter. Since the w; p[V2] strain has the Met+transgene in the pUAS vector, these females were crossed with w;/actin-GAL4 males to examine overexpression driven by actin-GAL4 in this strain. No pupae were found in cultures of w; p[V2]/actin-GAL4progeny, indicating that these larvae responded more strongly to the actin-GAL4 driver than did the other w; UAS-Met+strains.
To examine functionality of the UAS-Met+ transgene expressed in this manner in adults, w MetW3; UAS-Met+ females from each of the three strains were crossed with w; actin-GAL4/TM3 males, and non-TM3 F1 progeny males (hemizygous for the MetW3 mutation) examined for rescue of the eye phenotype. Each w MetW3; UAS-Met+/actin-GAL4 male failed to display the eye phenotype shown by w MetW3; UAS-Met+/TM3 sibling males, demonstrating that functional MET, driven by actin-GAL4, was produced in the flies.
3.4. Oviposition
JH is necessary for vitellogenic oocyte development in D. melanogaster (Postlethwait and Weiser, 1973; Postlethwait and Handler, 1978). Previously, Met was shown to be involved in oogenesis as reflected by depressed oviposition and oogenesis in the Met27null mutant but not in transgenic Met27; Met+ sibs (Wilson and Ashok, 1998). To determine if elevated MET results in altered, perhaps elevated oogenesis, oviposition was measured in w; p[V2] females during a 4-day period beginning 8 days after eclosion and compared with that of w females. No significant differences could be detected between the strains (Table 4). To determine if the transgenic females were producing extra oocytes but accumulating instead of ovipositing them, ten 8-day-old females were dissected from each strain and the stage-14 (mature) oocytes censused (Table 4). Again, no significant differences between the strains could be found, showing no enhancement of oogenesis in the Met+ overexpressing strains.
Table 4.
Comparison of oviposition and oogenesis between w and w; p[V2] females. Eggs were censused from each of 25 8-day-old females over a 4-day period. Stage 14 oocytes (most mature) were censused from each of ten 8-day-old females. SEM values given in parentheses. Comparison of mean values of either oviposition or stage 14 oocytes did not differ significantly between the two strains (p= 0.42 for oviposition and 0.27 for stage 14 oocytes/female using an unpaired t-test).
| Genotype | Oviposition (eggs laid/female/4 days) | Stage 14 oocytes/female |
|---|---|---|
| w | 143.7 (4.09) | 13.3 (1.72) |
| w; p[V2] | 138.8 (4.39) | 10.9 (1.19) |
Likewise, exceptional survivor w; UAS-Met+/actin-GAL4 males and females were isolated shortly after eclosion and found to be fecund and fertile when set up on fresh food.
4. Discussion
This work has examined the effects of overexpression of the Met+ gene in D. melanogaster. This gene is involved in two related processes: action of endogenous JH, possibly as a component of a JH receptor (Shemshedini and Wilson, 1990; Miura et al., 2005), and resistance to JH analog insecticides (Wilson and Fabian, 1986; Konopova and Jindra, 2007). MET has been shown to bind JH (Shemshedini and Wilson, 1990; Miura et al., 2005), to be expressed in known JH target tissues (Pursley et al., 2000), and to have a structure (a bHLH PAS transcription factor) that is compatible with hormone action (Ashok et al., 1998). Met+ transcriptional control of a reporter gene has been demonstrated (Miura et al., 2005). A homologous gene, germ cell expressed (gce) has about 70% homology to Met and appears to be the sole Met-like gene in lower Diptera (Wang et al., 2007) and perhaps other insects. MET physically binds with GCE in D. melanogaster cells (Godlewski et al., 2006), suggesting a functional heterodimer. The Met27 null mutant does not show the lethality that one might expect from a JH receptor mutant (Wilson and Ashok, 1998); either gce (or some other gene) can rescue the lethality as a redundant gene or JH is not required for vital functions in D. melanogaster.
Recently, it was demonstrated in D. melanogaster L57 cells that MET interacts with two proteins, FKB39 and Chd64, that bind to a D. melanogaster JH response element (Li et al., 2007). It was further demonstrated that these proteins interact with USP and EcR, the component proteins of the ecdysone receptor. MET may facilitate cross-talk between these two hormonal signaling pathways through the ecdysone receptor. Perhaps the larval death seen when Met is overexpressed reflects the corruption of proper stoichiometric amounts of the signaling complex components necessary for molting or other larval physiology.
Since MET can bind JH (and presumably JH analog insecticides such as methoprene), the increase in methoprene susceptibility seen in the overexpressed strains is perhaps not surprising. The extent of this increase was somewhat proportional to the overexpression level in the various strains, and it reached a level of about 10-fold over w in the w; p[V2] strain. We believe that the toxicity and morphogenetic effects of JH agonist insecticides result from mis-expression of certain genes of unknown identity during metamorphosis (Wilson et al., 2006b). JH is present in low levels in D. melanogaster pupae (Bownes and Rembold, 1987; Sliter et al., 1987), and application of JH or methoprene serves to disrupt regulation of one or probably several genes at this time, resulting in the pathology, both vital and morphological, seen when it is exogenously applied. Since a JH receptor must be involved in the regulatory effect, then increasing the titer of a JH-binding component increases the susceptibility to the disruptive influence of JH. Another possible explanation relies on the observation of both MET:GCE and MET:MET dimer formation in D. melanogaster cells (Godlewski et al, 2006). Perhaps an increased titer of MET drives the equilibrium to form homodimers instead of heterodimers that may be required at a certain time(s) in development or disrupts the ecdysone-JH signaling complex proposed by Li et al (2007), and the consequences are detrimental to development and survival.
JH has been shown to be involved in oogenesis in many insects, including D. melanogaster. Oogenesis and oviposition in the Met27 mutant is decreased to about 20% of wild-type (Wilson and Ashok, 1998), reflecting the role of Met in these processes. A change in oogenesis (enhancement) in the overexpressing females might be anticipated, given the effect seen on methoprene susceptibility. Since little change was seen, then MET must not be the limiting factor in oogenesis. This physiology is complex, so it is perhaps not surprising that overexpressing only one component does not enhance the whole process.
The alteration of the methoprene pathology seen in the w; p[V2] strain at the diagnostic doses used differs from the methoprene sensitivity/pathology characteristic of wild-type (Postlethwait, 1974). The stage of sensitivity appears to be early first-instar larvae as opposed to the late larval/prepupal stage of sensitivity seen in wild-type. If newly hatched w; p[V2] larvae are allowed to feed on regular food for 24 hours, then transferred to methoprene food, they do not display the late larval-early pupal lethality seen for newly hatched larval exposure in this strain (data not shown). Newly hatched w; p[V2] larvae exposed to the lower, but still lethal, 2.7 μg dose of methoprene begins to show lethality at the pharate adult stage (Figure 2) that is characteristic of non-over-expressing strains. Therefore, the combination of a higher dose of methoprene coupled with Met+ overexpression alters the sensitive/lethal stage of development. Possibly, a similar toxicology hypothesized for the usual methoprene effect is occurring: the high titer of MET coupled with a relatively high methoprene dose (5.4–26.8 μg) is resulting in mis-expression of a vital gene(s) early in larval development that affects larval or early pupal development.
Overexpression using a tubulin/actin-GAL4 driver resulted in lethality during larval development even in the absence of methoprene. It is possible that the toxicity resulted from very high MET titers, which could not be accurately measured in this study due to the moribund condition of the larvae. Another possibility is that the actin/tubulin gene promoters resulted in Met+ overexpression in one or more tissues where it was especially toxic, perhaps in the presence of endogenous JH. The w; p[V2] strain also shows high overexpression, but without toxicity (unless driven by the tubulin-GAL4 transgene); perhaps Met+ is overexpressed only in noncritical tissues in this strain. Clearly, though, there is a limit to overexpression, either in quantity or tissue location, of Met+ that can be tolerated by the animal.
This work examined overexpression of an insecticide resistance gene. Insect overexpression of resistance genes encoding detoxifying proteins, such as a cytochrome P450, have long been shown to result in increased resistance to the insecticide (Feyereisen, 1995; ffrench-Constant et al., 2004). The present work shows that overexpression of an insecticide-binding protein can result in the opposite effect of higher sensitivity. Future agrochemical work that focuses on novel chemistries to overexpress insecticide target-site proteins in pest insects could result in increased susceptibility and lower insecticide usage to control these pests.
Acknowledgments
We thank Dr. Kuba Godlewski for his assistance with the RT-PCR. This work was supported by AI052290 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner M. Effects of juvenile hormone on adult differentiation of Drosophila melanogaster. Nature. 1970;227:187–189. doi: 10.1038/227187a0. [DOI] [PubMed] [Google Scholar]
- Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95:2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownes M. The roles of juvenile hormone, ecdysone and the ovary in the control of Drosophila vitellogenesis. J Insect Physiol. 1989;35:409–413. [Google Scholar]
- Bownes M, Rembold H. The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur J Biochem. 1987;164:709–712. doi: 10.1111/j.1432-1033.1987.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- Dubrovsky EB, Dubrovskaya VA, Berger EM. Juvenile hormone signaling during oogenesis in Drosophila melanogaster. Insect Biochem Mol Biol. 2002;32:1555–1565. doi: 10.1016/s0965-1748(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Dubrovsky EB, Dubrovskaya VA, Bilderback AL, Berger EM. The isolation of two juvenile hormone-inducible genes in Drosophila melanogaster. Dev Biol. 2000;224:486–495. doi: 10.1006/dbio.2000.9800. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Molecular biology of insecticide resistance. Toxicol Lett. 1995;82:83–90. doi: 10.1016/0378-4274(95)03470-6. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant RH, Daborn PJ, Le Goff G. The genetics and genomics of insecticide resistance. Trends Genet. 2004;20:163–170. doi: 10.1016/j.tig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Wang S, Wilson TG. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem Biophys Res Commun. 2006;342:1305–1311. doi: 10.1016/j.bbrc.2006.02.097. [DOI] [PubMed] [Google Scholar]
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: Sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The Aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Kambysellis MP, Heed WB. Juvenile hormone induces ovarian development in diapausing cave-dwelling Drosophila species. J Insect Physiol. 1974;20:1779–1786. doi: 10.1016/0022-1910(74)90207-8. [DOI] [PubMed] [Google Scholar]
- Kerr C, Ringo J, Dowse H, Johnson E. Icebox, a recessive X-linked mutation in Drosophila causing low sexual receptivity. J Neurogenet. 1997;11:213–229. doi: 10.3109/01677069709115097. [DOI] [PubMed] [Google Scholar]
- Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 2007 doi: 10.1074/jbc.M704595200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan K. Morphogenetic effects of juvenile hormone and juvenile hormone mimics on adult development of Drosophila. J Insect Physiol. 1973;19:441–453. doi: 10.1016/0022-1910(73)90119-4. [DOI] [PubMed] [Google Scholar]
- Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene-tolerant gene product. FEBS J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH. Juvenile hormone and the adult development of Drosophila. Biol Bull. 1974;147:119–135. doi: 10.2307/1540573. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Handler AM. Nonvitellogenic female sterile mutants and the regulation of vitellogenesis in Drosophila melanogaster. Dev Biol. 1978;67:202–213. doi: 10.1016/0012-1606(78)90309-3. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Jones J. Endocrine control of larval fat body histolysis in normal and mutant Drosophila melanogaster. J Exp Zool. 1978;203:207–214. doi: 10.1002/jez.1402030204. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Weiser K. Vitellogeneis induced by juvenile hormone in the female sterile mutant apterous-four in Drosophila melanogaster. Nature New Biol. 1973;244:284–285. doi: 10.1038/newbio244284a0. [DOI] [PubMed] [Google Scholar]
- Pursley S, Ashok M, Wilson TG. Intracellular localization and tissue specificity of the Methoprene-tolerant (Met) gene product in Drosophila melanogaster. Insect Biochem Mol Biol. 2000;30:839–845. doi: 10.1016/s0965-1748(00)00056-4. [DOI] [PubMed] [Google Scholar]
- Qiao CL, Raymond M. The same esterase B1 haplotype is amplified in insecticide resistant mosquitoes of the Culex pipiens complex from the Americas and China. Heredity. 1995;74:339–345. doi: 10.1038/hdy.1995.51. [DOI] [PubMed] [Google Scholar]
- Restifo LL, Wilson TG. A juvenile hormone agonist reveals distinct developmental pathways mediated by ecdysone-inducible Broad Complex transcription factors. Develop Genet. 1998;22:141–159. doi: 10.1002/(SICI)1520-6408(1998)22:2<141::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 899–939. [Google Scholar]
- Riddiford LM. Cellular and molecular actions of juvenile hormone I. General considerations and premetamorphic actions. Adv Insect Physiol. 1994;24:213–274. [Google Scholar]
- Riddiford LM. Molecular aspects of juvenile hormone action in insect metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis: Postembryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. Academic Press; San Diego: 1996. pp. 223–251. [Google Scholar]
- Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol. 1991;82:172–183. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
- Rubin G, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sakuma M. PROBIT analysis of preference data. Appl Entomol Zool. 1998;33:339–347. [Google Scholar]
- Shemshedini L, Lanoue M, Wilson TG. Evidence for a juvenile hormone receptor involved in protein synthesis in Drosophila melanogaster. J Biol Chem. 1990;265:1913–1918. [PubMed] [Google Scholar]
- Shemshedini L, Wilson TG. Resistance to juvenile hormone and an insect growth regulator in Drosophila is associated with an altered cytosolic juvenile hormone binding protein. Proc Natl Acad Sci USA. 1990;87:2072–2076. doi: 10.1073/pnas.87.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter TJ, Sedlak BJ, Baker FC, Schooley PA. Juvenile hormone in Drosophila melanogaster. Identification and titer determination during development. Insect Biochem. 1987;17:161–165. [Google Scholar]
- Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Staal GB. Insect growth regulators with juvenile hormone activity. Annu Rev Entomol. 1975;20:417–460. doi: 10.1146/annurev.en.20.010175.002221. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Wang S, Baumann A, Wilson TG. Drosophila melanogaster Methoprene-tolerant (Met) gene homologs from three mosquito species: Members of PAS transcriptional factor family. J Insect Physiol. 2007;53:246–253. doi: 10.1016/j.jinsphys.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG. A correlation between juvenile hormone deficiency and vitellogenic oocyte degeneration in Drosophila melanogaster. Wilhelm Roux Arch Entwicklungsmech Org. 1982;191:257–263. doi: 10.1007/BF00848413. [DOI] [PubMed] [Google Scholar]
- Wilson TG. Genetic evidence that mutants of the Methoprene-tolerant gene of Drosophila melanogaster are null mutants. Arch Insect Biochem Physiol. 1996;32:641–649. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<641::AID-ARCH35>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wilson TG. The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: how these compounds kill insects. J Insect Physiol. 2004;50:111–121. doi: 10.1016/j.jinsphys.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Ashok M. Insecticide resistance resulting from an absence of target-site gene product. Proc Natl Acad Sci USA. 1998;95:14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG, DeMoor S, Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant27 mutant phenotype. Insect Biochem Mol Biol. 2003;33:1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Develop Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Wang S, Beno M, Farkas R. Wide mutational spectrum of a gene involved in hormone action and insecticide resistance in Drosophila melanogaster. Mol Gen Genomics. 2006a;276:294–303. doi: 10.1007/s00438-006-0138-4. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Yerushalmi Y, Donnell DM, Restifo LL. Interaction between hormonal signaling pathways in Drosophila melanogaster as revealed by genetic interaction between Methoprene-tolerant and Broad-Complex. Genetics. 2006b;172:253–264. doi: 10.1534/genetics.105.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone II. Roles of juvenile hormone in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- Yamamoto K, Chadarevian A, Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988;239:916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]
- Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]