Summary
Background
In old age, reduction in physical function leads to loss of independence, the need for hospital and long-term nursing-home care, and premature death. We did a systematic review to assess the effectiveness of community-based complex interventions in preservation of physical function and independence in elderly people.
Methods
We searched systematically for randomised controlled trials assessing community-based multifactorial interventions in elderly people (mean age at least 65 years) living at home with at least 6 months of follow-up. Outcomes studied were living at home, death, nursing-home and hospital admissions, falls, and physical function. We did a meta-analysis of the extracted data.
Findings
We identified 89 trials including 97 984 people. Interventions reduced the risk of not living at home (relative risk [RR] 0·95, 95% CI 0·93–0·97). Interventions reduced nursing-home admissions (0·87, 0·83–0·90), but not death (1·00, 0·97–1·02). Risk of hospital admissions (0·94, 0·91–0·97) and falls (0·90, 0·86–0·95) were reduced, and physical function (standardised mean difference −0·08, −0·11 to −0·06) was better in the intervention groups than in other groups. Benefit for any specific type or intensity of intervention was not noted. In populations with increased death rates, interventions were associated with reduced nursing-home admission. Benefit in trials was particularly evident in studies started before 1993.
Interpretation
Complex interventions can help elderly people to live safely and independently, and could be tailored to meet individuals' needs and preferences.
Introduction
In old age, reduction in physical function can lead to loss of independence, the need for hospital and long-term nursing-home care, and premature death. The importance of physical, functional, psychological, and social factors in realising a healthy old age is recognised by elderly people,1,2 health-care professionals,3 and policy makers.4
The risk factors for reduced physical function in elderly people, as identified in longitudinal studies,5,6 relate to comorbidities, physical and psychosocial health, environmental conditions, social circumstances, nutrition, and lifestyle. The need for a preventive strategy based around identification and treatment of diverse risk factors was identified more than 40 years ago,7 and many trials of complex intervention packages have been reported and reviewed. In this context, a complex intervention can be regarded as a combination of interdisciplinary teamwork for health and social problems. Trials have focused on general and frail elderly populations,8–11 elderly people discharged from hospital,12 and those at risk of falling.13–15 However, the development of risk factors, admission to hospital, and risk of falling represent a common chain of experiences for many elderly people.16 Likewise, multifactorial interventions in these populations have common characteristics and, in addition to targeting specific outcomes relating to hospital readmissions and falls, share the common aims of physical function maintenance, disability limitation, and promotion of independence.
In the UK, yearly multidimensional assessments of physical and cognitive health for all individuals aged at least 75 years became a necessity in primary care in 1989,17 with guidelines on content and implementation provided for England.18 In due course, a targeted approach to assessment and care was developed and promoted with community nurse-led case management of elderly people with medical conditions identified from hospital admissions and general practice records.19 The report20 stresses the importance of a team-based approach incorporating appropriate skills to meet the health and care needs of elderly people.
Geriatric screening and multidimensional assessment are recognised in modernised health-care systems in Germany, Italy, France, the Netherlands, and Denmark.21 In US-managed care organisations, the focus of care is on frail elderly people and those discharged from hospital.22 Care is coordinated by case managers and this model has been applied in other countries, including England.23
The systematic reviews cited above examined the effectiveness of interventions in specific groups of elderly people or clinical settings. To guide new preventive and anticipatory care efforts, we intended to answer the question of the effectiveness of all community-based complex interventions used to preserve physical function and independence in elderly people. We did a systematic review of randomised controlled trials with outcomes of independent living, hospital and nursing home admissions, physical function, and falls.
Methods
Search strategy and selection criteria
We used Cochrane systematic review methods24 to identify randomised controlled trials that met our inclusion criteria. We included trials that compared community-based multifactorial intervention with usual care or minimum intervention, with follow-up for at least 6 months. Interventions were eligible for the review if individuals received personalised assessment and provision of or referral for appropriate specialist medical and social care. Mean age of eligible study populations was at least 65 years at baseline, with individuals living at home or preparing for hospital discharge to home.
The search strategy covered issues related to: randomised controlled trials; elderly people; community and home setting; health, social, behavioural, and occupational therapy interventions; and hospital and nursing home admissions, physical function, and disability. Searches were tailored to individual computerised databases; Medline strategy is shown in the webappendix.
Searches were made in CENTRAL (issue 4, 2004) and updated to January, 2005, with searches of Medline and Embase from 2003 to January, 2005. Further searches were done of CINAHL from 1982 to January, 2005, PsycINFO from 1972 to January, 2005, and ISI Science and Social Science Citation Index from 1945 to January, 2005. Reference lists of trials and previous reviews were searched and follow-up reports of previously unfinished trials were sought. Additional trials reported after 2004 and before December, 2006, were identified by the Web of Science citation search facility with focus on previous reviews and key trials.
One reviewer (KR) scanned abstracts and titles. Potentially relevant articles were acquired and data were extracted in duplicate from most (64%) reports and recorded on a piloted form and Excel spreadsheet. All outcome data were further checked with original articles. Information was extracted on study characteristics (randomisation procedure, blind assessment at baseline and follow-up, follow-up period, intention-to-treat analysis, and losses to follow-up), participants (inclusion criteria, numbers of individuals in randomised groups, age of participants, baseline comparisons, and country and date of recruitment), intervention (aims, content, carer involvement, contributors, format, duration, and intensity), and outcomes. Disagreements in extracted data were resolved by discussion among reviewers. We did not exclude trials from the review once they had been included. Trials that were not intention to treat were not included in meta-analyses.
The outcomes studied were living at home at follow-up, death, nursing-home and hospital admissions, falls, and physical function.
Potential sources of heterogeneity that were investigated were context of intervention (geriatric assessment in general or frail elderly populations, community-based care after hospital discharge, fall prevention, or group education and counselling); quality of studies (losses to follow-up); mortality rate in study population; date recruitment commenced; mean age of participants; intensity of intervention; and extent of control group intervention activity. Frail populations typically included people with limitations in activities of daily living and chronic conditions, and those thought to be at risk of functional deterioration or hospital admission.
The intensity of interventions was calculated by addition of three measures of intervention intensity: multidisciplinary input (one discipline=1, two disciplines or two or more similar disciplines=2, and three or more different disciplines=3), number of scheduled visits (one to four=1, five to nine=2, ten or more=3), and the duration of the intervention (0–1 month=1, 2–6 months=2, more than 6 months=3). To create groups with similar numbers, scores of 1–4 were regarded as low, 5–6 as medium, and 7–9 as high intensity. Analyses were also done for every feature of intensity separately.
Statistical analysis
Meta-analyses were done with Review Manager and additional statistics with SPSS (version 12.0.1). Authors of articles published after 1991 were contacted for information that was not available in the published material.
We chose to use fixed-effects meta-analysis a priori because the complex interventions for elderly people we have defined had common characteristics and aims. For dichotomous outcomes, relative risks (RRs) were summarised with Mantel-Haenszel fixed-effects meta-analyses.24 However, for results showing significant heterogeneity (I2>50%), random-effects meta-analysis was also done with the method of DerSimonian and Laird.24 Meta-regression was done with Stata (version 10.0). By convention not living at home was used instead of living at home. For physical function, data were summarised as the standardised mean difference (SMD). Only intention-to-treat analyses were included, which for physical function mainly represented available case analyses. Results were summarised descriptively for those studies with insufficient data.
If not living at home was unavailable, the sum of deaths and nursing home admissions was used, which led to a potential overestimation by double counting of people admitted to a nursing home and who subsequently died. Analyses were done with or without estimates. For nursing-home admissions as an outcome, some trials reported permanent admission whereas others reported individuals living in a nursing home at follow-up. Results were analysed separately and combined.
Several measures of physical function were reported and we classified these as pertaining to severity of disability such as limitations in activities of daily living or generic physical function. Differences in activities of daily living and generic physical function at follow-up were analysed separately and combined. If SDs were unavailable, values were calculated as described in the Cochrane handbook for systematic reviews of interventions.25 Otherwise, baseline values were used, either those from a trial in a similar population or from appropriate population statistics. For all outcomes, scales were recoded such that high values indicated poor physical function. Funnel plots were inspected at all stages of the review to identify possible publication bias.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
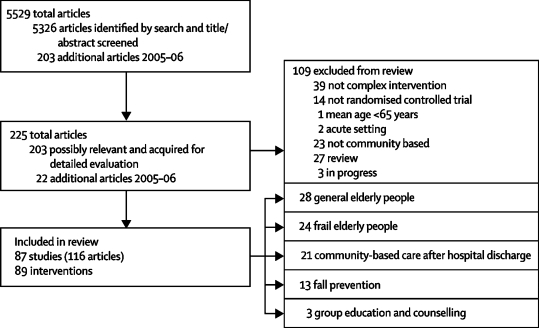
The review process is summarised in figure 1 according to QUOROM guidelines.26 89 intervention trials meeting our inclusion criteria are summarised in webtable 1 with full details available from the authors. Trials assessed geriatric assessment in elderly people representing the general population (n=28)27–53 or those selected as frail (n=24),54–77 community-based care after hospital discharge (n=21),78–98 fall prevention (n=13),51,99–110 or group education and counselling (n=3).111–113 All trial interventions were complex and many individuals would have been eligible for any of the them.
Figure 1.
QUOROM flow diagram
Randomisation was by individual or household (n=80) or by clusters of general practices, community groups, or municipalities (n=9). The total number of people randomised was 97 984 with a median of 321 (range 54–43 219) in trials. One large study randomised 43 219 people.33 Mortality rates ranged from 0 to 60·8% per year, with a median of 6·8%. Trials assessing geriatric assessment in general and frail populations had median mortality rates of 5·4% (0–10·5%) and 6·1% (1·1–60·8%), respectively, suggesting that the frail category was often subject to selection, probably indicating eligibility issues. In trials of community-based care after hospital discharge, the median mortality rate was 16·2% (6·3–53·0%); for fall prevention and group education, it was 4·3% (0–11·6%) and 3·4% (2·7–4·4%), respectively.
Losses to follow-up were used as a marker of study quality. In trials with death as an outcome, 40 (48%) of 84 had losses to follow-up of 1% or less (range 0–27·6%). For physical function, few trials included people who had died or moved to nursing homes in their analyses; exceptions were Close100 and Gagnon61 and their colleagues' trials. 15 (35%) of 43 trials had losses of participants to follow-up for interview of 5% or less (0–33%).
The allocation process was described in 61 (69%) of 89 trials, but difficulties of assessing concealment and masking in complex intervention trials are unlikely to have been fully addressed.114 Intervention activity in control groups was evident in 40 (45%) of 89 trials.
Data for variability between clusters were insufficient and the effect of analysis errors arising from inclusion of cluster randomised trials was explored by sensitivity analysis. Inspection of funnel plots at all stages of the review gave no indication of selection bias in studies included in the analysis (data not shown).
Outcomes are summarised by type of intervention in the table. The outcome of living at home at follow-up was available for 51 interventions; in a further nine trials death and nursing-home admission were used, with the consequent inclusion of the large Medical Research Council (MRC) trial.33 Overall, 60 (67%) of 89 trials reported living at home at follow-up or an estimate. However, this outcome was reported in only 4 [31%] of 13 trials.
Table.
Relative risk (95% CIs) of outcome by intervention context (standardised mean difference for physical function) and I2 heterogeneity statistic
| Study context | Not living at home N=79 578 | Death N=93 754 | Nursing home admission N=79 575 | Hospital admission N=20 047 | People with falls N=15 607 | Physical function N=21 651 | |
|---|---|---|---|---|---|---|---|
| Geriatric assessment of general elderly people | 0·95 (0·93 to 0·98) | 1·00 (0·98 to 1·03) | 0·86 (0·83 to 0·90) | 0·98 (0·92 to 1·03) | 0·76 (0·67 to 0·86) | −0·12 (−0·16 to −0·08) | |
| I2 | 35·3% | 39·7% | 47·5% | 61·4% | 0 | 0 | |
| Geriatric assessment of elderly people selected as frail | 1·00 (0·87 to 1·15) | 1·03 (0·89 to 1·19) | 1·01 (0·83 to 1·23) | 0·90 (0·84 to 0·98) | 0·99 (0·89 to 1·10) | −0·01 (−0·06 to 0·04) | |
| I2 | 43·3% | 0 | 28·8% | 11·0% | 0 | 57·9% | |
| Community-based care after hospital discharge | 0·90 (0·82 to 0·99) | 0·97 (0·89 to 1·05) | 0·77 (0·64 to 0·91) | 0·95 (0·90 to 0·99) | 0·82 (0·61 to 1·08) | −0·05 (−0·15 to 0·04) | |
| I2 | 2·2% | 5·2% | 0 | 57·0% | 40·3% | 0 | |
| Fall prevention | 0·86 (0·63 to 1·19) | 0·79 (0·66 to 0·96) | 1·26 (0·70 to 2·27) | 0·84 (0·61 to 1·16) | 0·92 (0·87 to 0·97) | −0·25 (−0·36 to −0·13) | |
| I2 | 0 | 0 | 0 | 0 | 65·8% | 4·1% | |
| Group education and counselling | 0·62 (0·43 to 0·88) | 0·80 (0·42 to 1·55) | 0·50 (0·05 to 5·49) | 0·75 (0·51 to 1·09) | n/a | 0·05 (−0·20 to 0·30) | |
| I2 | 0 | 0 | n/a | n/a | n/a | n/a | |
| All complex interventions | 0·95 (0·93 to 0·97) | 1·00 (0·97 to 1·02) | 0·87 (0·83 to 0·90) | 0·94 (0·91 to 0·97) | 0·90 (0·86 to 0·95) | −0·08 (−0·11 to–0·06) | |
| I2 | 29·3% | 10·6% | 29·0% | 43·0% | 52·8% | 45·9%* | |
n/a=not applicable.
Activities of daily living −0·08 (−0·11 to −0·04, I2=37·5%) and generic physical function −0·09 (−0·13 to −0·05, I2=64·0%).
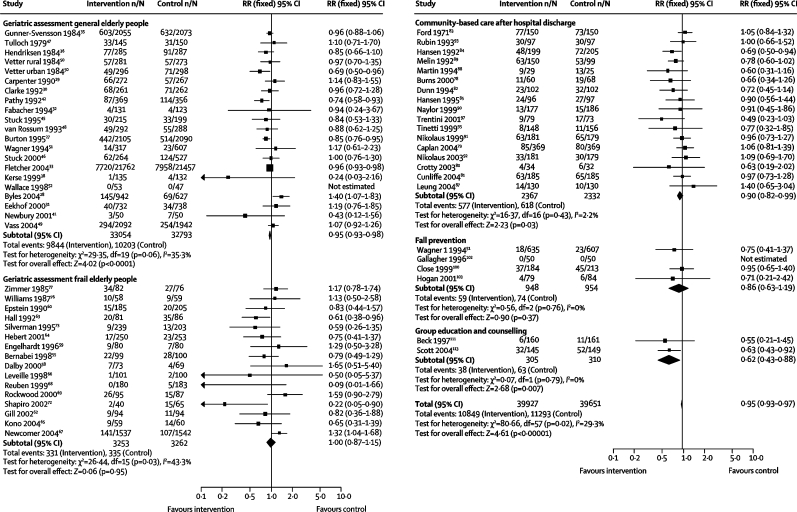
In a meta-analysis of 60 trials with 79 578 individuals (figure 2), the overall risk of not living at home was lower in the intervention group (RR 0·95, 95% CI 0·93–0·97) than in the control group. Geriatric assessment of general elderly people and community-based care after hospital discharge were the only types of intervention that had a significant effect on the risk of not living at home (figure 2). Removal of trials with estimated values had little effect (0·95, 0·90–1·00). Heterogeneity was only manifest in trials of geriatric assessment in general populations and those selected as frail (figure 2).
Figure 2.
Relative risk (RR) of not living at home
If typical rates of not living at home of about 7·6% (median in trials; range 0–12·1) per year for the general population are used, and the reduction in risk from intervention is 5%, a number needed to treat of 263 is obtained. For the increased rates of not living at home in people receiving community-based care after hospital discharge of about 25% per year with an RR reduction of 9%, the number needed to treat is 40.
Data for death were available for 84 (94%) of 89 interventions including 93 754 people (webfigure 1). Interventions had no overall benefit (RR 1·00, 95% CI 0·97–1·02) and the only appreciable benefit by type of intervention was noted in 11 trials targeting fall prevention (0·79, 0·66–0·96; webfigure 1). Slight heterogeneity (I2=10·6%) was almost exclusively limited to trials of geriatric assessment in general elderly populations (I2=39·7%; webfigure 1).
Data for nursing-home admission (31 trials) or for residence at follow-up (23 trials) were available for 79 575 people (webfigure 2) and were widely reported in trials of geriatric assessment in general (20 [71%] of 28) or populations selected as frail (16 [67%] of 24) and community-based care after hospital discharge (14 [67%] of 21), but not in trials of fall prevention (3 [23%] of 13).
For combined nursing-home outcomes, risk of admission was reduced in the intervention group (RR 0·87, 95% CI 0·83–0·90; webfigure 2). Only a marginal effect was seen for residence at follow-up (0·93, 0·79–1·09). Geriatric assessment and community-based care after hospital discharge were the only types of intervention to have a significant effect on the combined outcome (webfigure 2). Some heterogeneity was recorded in trials (I2=29·0%), mainly in geriatric assessment in general populations (I2=47·5%; webfigure 2).
For nursing-home care, typical median rates for trial populations were 2·2% (range 0·1–5·4) per year for the general population and 11·1% (2·1–40·2) per year for people receiving community-based care after hospital discharge, generating numbers needed to treat of 354 and 39, respectively.
Hospital admissions were reported in between 5 (38%) of 13 (falls prevention) and 18 (86%) of 21 (community-based care after hospital discharge) trials. The most commonly reported outcome—number of people having an admission (41 trials with 20 047 people [webfigure 3])—was used in the meta-analysis. Risk of hospital admission was reduced by interventions (RR 0·94, 95% CI 0·91–0·97; webfigure 3). Geriatric assessment in elderly people selected as frail and community-based care after hospital discharge were the only types of interventions to show significant effect on this outcome. Heterogeneity (I2=43·0%) was largely restricted to geriatric assessment in general elderly patients and community-based care after hospital discharge (61·4% and 57·0%, respectively; webfigure 3). In random-effects meta-analysis, the overall RR was similar (0·94, 95% CI 0·89–0·99).
Trials with data that were incompatible with the meta-analysis were inconsistent, with hospital admissions reduced in seven,33,40,42,56,94,100,113 similar in four,68,70,80,87 and increased in five trials.46,53,54,58,61 The large MRC trial reported slightly reduced total admissions in the intervention group (RR 0·96, 99% CI 0·79–1·16).33
All 13 studies targeting fall prevention reported individuals who had fallen, whereas falls were less likely to be reported in trials in general elderly people (6 [21%] of 28 trials), frail elderly people (5 [21%] of 24), and those with community-based care after hospital discharge (5 [24%] of 21). No trials of group education reported falls. An overall benefit was noted in 25 trials including 15 607 people (RR 0·90, 95% CI 0·86–0·95; webfigure 4). Interventions targeting fall prevention contributed 66% of the weight. Only trials of geriatric assessment in general elderly populations and those of fall prevention showed significantly reduced falls (webfigure 4). Heterogeneity (I2=52·8%; webfigure 4) was restricted to trials of community-based care after hospital discharge (I2=40·3%) and fall prevention(I2=65·8%). Use of random-effects meta-analysis led to wide CIs including unity for the interventions targeting fall prevention (0·91, 0·82–1·00), but the overall effect including all trials was broadly similar (0·89, 0·83–0·96). The four trials that did not report individuals who had fallen showed benefit with reduced total falls in intervention groups.29,66,91,102
Physical function outcome was measured in 73 trials. The Barthel index of activities of daily living restrictions (n=14) and SF-36 physical function dimension (n=7) were frequently reported. Information on change and functional deterioration was available for only nine and 16 studies, respectively, and we used the widely available physical function at follow-up in our analyses.
Meta-analysis included 43 interventions with 21 651 individuals (webfigure 5). Sources of variance data are available from the authors. In Reuben and colleagues' trial,68 substantial differences at baseline between randomised groups were reported, and analyses were done both with and without these data. In 36 trials with activities of daily living outcome, an overall benefit for interventions was noted (SMD −0·08, 95% CI −0·11 to −0·04) and in 14 trials with a generic physical function outcome that did not specifically focus on disability, the effect was similar (−0·09, −0·13 to −0·05). Exclusion of the trial with baseline differences had little effect on the SMD, and heterogeneity was evident for both outcomes (I2=37·5% and I2=57·5%, respectively).
When SF-36 physical function means and variances from the 1992 Office for National Statistics survey were used,115 an SMD of 0·09 translated as an improvement in a representative elderly population of between 3·3% and 7·2% dependent on age. For the Barthel index, in the trials included in the review, the SMD of 0·08 equated to about half a point improvement in the 20-point score.
Combination of activities of daily living and generic outcomes (only used if no activities of daily living outcome reported) showed a similar benefit (SMD −0·08, 95% CI −0·11 to −0·06; webfigure 5). Heterogeneity was little different in the combined analysis to that seen when activities of daily living and generic measures were analysed separately and was mainly restricted to trials of geriatric assessment in elderly people selected as frail. However, RRs were much the same in random-effects meta-analysis (−0·08, −0·13 to −0·04). Geriatric assessment in general populations and falls interventions showed benefit for physical function at follow-up when grouped by context.
Physical function was reported in a form unsuitable for meta-analysis in 30 trials. Two interventions showed improvement in activities of daily living76,97 and five showed weak evidence of benefit.33,50,73,81 However, no improvement was noted in 19 trials,30,31,38,42,44,47,48,59,71,77,83,86,93,95,98,102,108,111,113 and in four trials generic physical function was largely unaffected by interventions.51,59,86
Study quality in terms of losses to follow-up (webtable 2) and randomisation process did not affect our findings—eg, RR of not living at home in 19 trials in which the randomisation process was not clear was 0·92 (95% CI 0·85–0·98), similar to that reported in trials with a clear description of randomisation (0·95, 0·93–0·98).
Analysis of results excluding trials with cluster randomisation had little effect on the overall RRs and variances. The contribution of the cluster randomised MRC trial to the meta-analyses was large, providing 71%, 58%, and 73% of not living at home, death, and nursing-home admission events, respectively. However, after exclusion of the trial, the results were reasonably consistent with RRs of 0·94 (95% CI 0·90–0·98) and 0·90 (0·83–0·97) for not living at home and nursing home admissions, respectively. When the MRC trial was excluded, the RR of death was reduced, although the 95% CI included unity (0·96, 0·92–1·00).
In trials with increased death rates, the RR of not living at home was reduced (second quartile of death rate 0·91, 95% CI 0·84–0·98, p=0·02; third quartile 0·96, 0·93–0·98, p=0·01; and fourth quartile 0·88, 0·79–0·96, p=0·05; webtable 3). Similarly, nursing-home admissions were reduced after intervention in populations with high death rates, which differed significantly from one for the two highest quartiles (third quartile of death rate 0·86, 0·82–0·90, p<0·0001, and fourth quartile 0·75, 0·63–0·89, p=0·01; webtable 3).
In trials with recruitment dates before the median of 1993, interventions showed benefit with a combined RR of not living at home of 0·89 (95% CI 0·84–0·93; webtable 4), whereas in trials from 1993 onwards the RR was 0·97 (0·94–0·99). Removal of the MRC trial from the analysis made this difference even more pronounced (1·04, 0·96–1·12) in trials from 1993 onwards. In meta-regression, the outcomes of not living at home, death, and nursing home admission all showed increased risk reduction in studies before 1993 (webtable 4). This increased risk reduction was also apparent for specific contexts—eg, for community-based care after hospital discharge, RR of not living at home in studies started before 1993 was 0·82 (0·73–0·93) compared with 1·01 (0·87–1·17) in later studies. Younger participants (≤74 years) tended to benefit more than elderly participants for all outcomes except hospital admission (webtable 5) and nursing-home admission.
Evidence did not suggest that interventions with an increased intensity were more effective in improving any outcome than those that had less direct health-professional involvement, shorter duration, and number of visits (webtable 6). Similarly, evidence did not exist for benefit of those interventions with multidimensional assessment compared with those with one discipline (one discipline: RR 0·95, 95% CI 0·93–0·97; at least three disciplines: 0·97, 0·89–1·07). No benefit for intense interventions was evident when interventions were grouped by type, including those after hospital discharge. Intervention activity in the control group did not affect outcomes (webtable 7).
Discussion
Our systematic review and meta-analysis showed that complex interventions can help elderly people to continue living at home, largely through prevention of the need for nursing-home care, and can help to reduce the rate of falls. Within the broad context of complex interventions, substantial variation in the format of care, involvement of health-care professionals, and site of care provision and intensity was reported. Evidence suggested that all elderly people might benefit from assessment and appropriate health and social interventions.
However, meta-analysis including trials done since 1993 suggested that modification of care beyond that achieved after earlier developments has been of little additional value. The 1980s to 1990s was a dynamic period in the specialty of care of elderly people. In the UK, the 1990 General Medical Services contract and the commission of the MRC trial of assessment and management of elderly people in the community affected the care of elderly people. Overall, care probably improved during this period because some of the principles of effective care became incorporated in normal practice.
The need for assessment of interventions was highlighted by the perceived ineffectiveness of the UK Evercare pilot programmes in relation to hospital admissions and death.23 This model involved nurse-led assessment and case management for people with long-term conditions. The UK assessment did not use a randomised approach and we identified no randomised controlled studies of the model in our widespread searches of published work. On the basis of our systematic review, we would not have expected reductions in hospital admissions or deaths in those people receiving assessment and case management.
In our review, the eligibility for care covered the broad experience of elderly people and in general the results were consistent with previous meta-analyses. Elkan and colleagues9 reported an overall benefit for home-visiting programmes in prevention of death and nursing-home admission. Stuck and coworkers11 noted benefit for home-visiting programmes, which was restricted to improvements in physical function with multidimensional assessment and many follow-ups, reduction in nursing-home admissions with increased numbers of follow-up visits, and reductions in mortality with application in younger populations (72·7–77·5 years). These reviews assessed 15 and 18 interventions, respectively. Assessment and multifactorial intervention have also shown benefit with reduction in rate of falls in three to 13 trials.13–15 Review of nine trials targeting people after hospital discharge showed some benefits with regard to living at home and institutionalisation.12
We used the principle that interventions relating to different aspects of care can be judged together as complex interventions. The interventions in this review had input from a wide range of health-care disciplines with different intensity and duration of care, but all addressed issues of preventive visits for elderly people with care based on assessment of medical and social need. Intensity, indicating direct multidisciplinary input, number of scheduled visits, and the duration of the intervention, might not capture the effective characteristics of the intervention. Inclusion of a qualitative element in trials would have helped to understand the care actually received by individuals.
Trials specifically targeting falls prevention included interventions that were more strongly focused on home safety and physical health than other trials included in this review. However, all interventions included in the review addressed diverse issues of medical and social care. Exclusion of trials specifically targeting fall prevention made little difference to overall outcomes, including risk of falling (RR 0·88, 95% CI 0·81–0·95).
The outcome of living at home might be an over simplistic marker for independent living. In Byles and colleagues' study,28 increased admissions to nursing homes in the intervention group were attributed to the assessment process and advice given. The intervention might have led to improved understanding of the limitations of home-based care and increased awareness of alternative care available in nursing homes. Conversely, if limitation of health-care use and costs are the main objectives, unfavourable care patterns for both the individual and carers might arise.
Interpretation of results related to physical function is restricted by selective reporting in people readily available for interview follow-up and by the large losses to follow-up in trials. Previous reviews have reported the number of people with functional deterioration, but this outcome was only available for a small number of trials. A further limitation in reporting changes in physical function is the large number of different outcome measures reported.
Other outcomes, including empowerment, autonomy, independent decision making, improved self esteem, and self confidence might accurately describe the effect of an intervention to the individual.116 Close and colleagues100 measured ability to go out alone as an outcome, perhaps a better marker of independence; and Kerse and colleagues38 obtained information on how often people did something they really enjoyed and the frequency of interactions with family and friends. Rockwood and colleagues69 used goal attainment scaling as part of the intervention and follow-up.117 This method aimed to assess specific outcomes based on personal goals set during intervention. Various other outcome measures related to health and psychosocial status and satisfaction with care and health-service use were reported, but their diversity and application in only a few trials restricted their value in a systematic overview.
A strength of our review is the inclusion of the large MRC trial of assessment and management of elderly people in the community. Recruitment to the trial commenced in 1995 and in the context of our review is a late trial. However, the authors note that annual assessments, as promoted in the UK, were poorly implemented at this time. Although the cluster design was associated with reduced study power and the study lacked an untreated control group, the MRC trial served to support the overall meta-analysis. Although not significant at the prespecified 1% level, the reported RR for institutional admissions was 0·83 (99% CI 0·66–1·06), which was reasonably similar to that in our meta-analysis (0·87, 0·82–0·91). The outcome of living at home was not available, but an estimate based on the sum of deaths and institutional admissions again suggested similar benefit in the large trial and the meta-analysis. Neither approach showed benefit with regard to death.
Because the evidence did not suggest that one format of care provision was better than another, the possibility might exist to tailor different formats of care to the needs and preferences of the individual, a conclusion similar to that drawn from the UK assessment of an expert patient programme.118 Provision of alternative intervention formats and intensities could lead to better uptake and adherence with care without compromising potential benefit.
Our interpretation of the benefits of complex interventions that identify elderly people who have a high chance of reduction in ability for targeted specialist care differs from the conclusions of the MRC trial investigators who reported that, “The different forms of multidimensional assessment offered almost no differences in patient outcome”,33 which is certainly true in the context of the trial and the specific targeted versus universal interventions being assessed. We believe that our general conclusion, drawn from all the available randomised evidence, and a wide contextual understanding of the changes that have taken place in health care for elderly people during the last four decades, is of relevance in situations with less developed services for elderly people, and suggests that a withdrawal of existing well developed services would be inappropriate.
Acknowledgments
The study was funded by the MRC Health Services Research Collaboration. We are especially grateful to the authors of trials for the provision of additional information for our review and meta-analysis, and thank Margaret Burke for help in undertaking the searches of published work.
Contributors
SE conceived and designed the review and SE and PD provided supervision. ADB and KR identified and acquired reports of trials and extracted data. ADB contacted authors of trials for additional information and analysed and interpreted the data. SA provided statistical advice and input. PD, SA, RG-H, JH, and SE contributed to the interpretation of the data. ADB and SE drafted the manuscript. PD, SA, RG-H, and JH critically reviewed the manuscript. All authors saw and approved the final version of the manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Web Extra Material
Relative risk (RR) of death
Relative risk (RR) of nursing home admission or residence at follow-up
Relative risk (RR) of hospital admission
Relative risk (RR) of falling
Standardised mean difference (SMD) of physical function at follow up
Characteristics of randomised controlled trials included in the systematic review
Relative risk (95% CIs) of outcome by quality of studies (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by death rate (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by date recruitment started (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by mean age at recruitment (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk of outcome (95% CIs) by intensity of intervention (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk of outcome (95% CIs) by activity in control group (standardised mean difference for physical function) and I2 heterogeneity statistic
References
- 1.Age Concern . Adding quality to quantity: older people's views on quality of life and its enhancement. Age Concern; London: 2003. [Google Scholar]
- 2.Phelan EA, Anderson LA, LaCroix AZ, Larson EB. Older adults' views of successful aging—how do they compare with researchers' definitions? J Am Geriatr Soc. 2004;52:211–216. doi: 10.1111/j.1532-5415.2004.52056.x. [DOI] [PubMed] [Google Scholar]
- 3.British Geriatrics Society Primary and Continuing Care Special Interest Group . The specialist health needs of older people outside an acute hospital setting. British Geriatrics Society; London: 2005. [Google Scholar]
- 4.WHO . Active ageing: a policy framework. World Health Organisation; Geneva: 2002. [Google Scholar]
- 5.Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 6.Ayis S, Gooberman-Hill R, Bowling A, Ebrahim S. Predicting catastrophic decline in mobility among older people. Age Ageing. 2006;35:382–387. doi: 10.1093/ageing/afl004. [DOI] [PubMed] [Google Scholar]
- 7.Williamson J, Stokoe IH, Gray S. Old people at home; their unreported needs. Lancet. 1964;1:1117–1120. doi: 10.1016/s0140-6736(64)91803-3. [DOI] [PubMed] [Google Scholar]
- 8.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032–1036. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 9.Elkan R, Kendrick D, Dewey M. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719–724. doi: 10.1136/bmj.323.7315.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Haastregt JCM, Diederiks JPM, van Rossum E, de Witte LP, Crebolder HFJM. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ. 2000;320:754–758. doi: 10.1136/bmj.320.7237.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 12.Hyde CJ, Robert IE, Sinclair AJ. The effects of supporting discharge from hospital to home in older people. Age Ageing. 2000;29:271–279. doi: 10.1093/ageing/29.3.271. [DOI] [PubMed] [Google Scholar]
- 13.Hill-Westmoreland EE, Soeken K, Spellbring AM. A meta-analysis of fall prevention programs for the elderly: how effective are they? Nurs Res. 2002;51:1–8. doi: 10.1097/00006199-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;4 doi: 10.1002/14651858.CD000340. CD000340. [DOI] [PubMed] [Google Scholar]
- 15.Chang JT, Morton SC, Rubenstein LZ. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ. 2004;328:680–683. doi: 10.1136/bmj.328.7441.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health . Health Survey for England 2000: the general health of older people and their use of health services. The Stationery Office; London: 2002. [Google Scholar]
- 17.Department of Health . Terms of service for doctors in general practice. Department of Health; London: 1989. [Google Scholar]
- 18.Department of Health . National service framework for older people. Department of Health; London: 2001. [Google Scholar]
- 19.Department of Health . Supporting people with long term conditions: liberating the talents of nurses who care for people with long term conditions. Department of Health; London: 2005. [Google Scholar]
- 20.Department of Health . A recipe for care—not a single ingredient. Department of Health; London: 2007. [Google Scholar]
- 21.Leichsenring K. Developing integrated health and social care services for older persons in Europe. Int J Integr Care. 2004;4:1–15. doi: 10.5334/ijic.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglass C. The development and evolution of geriatric assessment teams over the past 25 years: a cross-cultural comparison of the US and the UK. J Interprofl Care. 2001;15:267–280. doi: 10.1080/13561820120063156. [DOI] [PubMed] [Google Scholar]
- 23.Gravelle H, Dusheiko M, Sheaff R. Impact of case management (Evercare) on frail elderly patients: controlled before and after analysis of quantitative outcome data. BMJ. 2007;334:31–34. doi: 10.1136/bmj.39020.413310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. John Wiley and Sons; Chichester: 2005. [Google Scholar]
- 25.Deeks JJ, Higgins JPT, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. John Wiley and Sons; Chichester: 2005. Analysing and presenting results. [Google Scholar]
- 26.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 27.Burton LC, Paglia MJ, German PS, Shapiro S, Damiano AM. The effect among older persons of a general preventive visit on three health behaviors: smoking, excessive alcohol drinking, and sedentary lifestyle. Prev Med. 1995;24:492–497. doi: 10.1006/pmed.1995.1078. [DOI] [PubMed] [Google Scholar]
- 28.Byles JE, Tavener M, O'Connell RL. Randomised controlled trial of health assessments for older Australian veterans and war widows. Med J Austr. 2004;181:186–190. doi: 10.5694/j.1326-5377.2004.tb06233.x. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter GI, Demopoulos GR. Screening the elderly in the community: controlled trial of dependency surveillance using a questionnaire administered by volunteers. BMJ. 1990;300:1253–1256. doi: 10.1136/bmj.300.6734.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke M, Clarke SJ, Jagger C. Social intervention and the elderly: a randomized controlled trial. Am J Epidemiol. 1992;136:1517–1523. doi: 10.1093/oxfordjournals.aje.a116473. [DOI] [PubMed] [Google Scholar]
- 31.Eekhof JAH, De Bock GH, Schaapveld K, Springer MP. Effects of screening for disorders among the elderly: an intervention study in general practice. Fam Pract. 2000;17:329–333. doi: 10.1093/fampra/17.4.329. [DOI] [PubMed] [Google Scholar]
- 32.Fabacher D, Josephson K, Pietruszka F, Linderborn K, Morley JE, Rubenstein LZ. An in-home preventive assessment program for independent older adults: a randomized controlled trial. J Am Geriatr Soc. 1994;42:630–638. doi: 10.1111/j.1532-5415.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher AE, Price GM, Ng ESW. Population-based multidimensional assessment of older people in UK general practice: a cluster-randomised factorial trial. Lancet. 2004;364:1667–1677. doi: 10.1016/S0140-6736(04)17353-4. [DOI] [PubMed] [Google Scholar]
- 34.Fox PJ, Breuer W, Wright JA. Effects of a health promotion program on sustaining health behaviors in older adults. Am J Prev Med. 1997;13:257–264. [PubMed] [Google Scholar]
- 35.Gunner-Svensson F, Ipsen J, Olsen J, Waldstrøm B. Prevention of relocation of the aged in nursing homes. Scand J Prim Health Care. 1984;2:49–56. doi: 10.3109/02813438409017704. [DOI] [PubMed] [Google Scholar]
- 36.Hendriksen C, Lund E, Strømgård E. Consequences of assessment and intervention among elderly people: a three year randomised controlled trial. BMJ. 1984;289:1522–1524. doi: 10.1136/bmj.289.6457.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jitapunkul S. A randomised controlled trial of regular surveillance in Thai elderly using a simple questionnaire administered by non-professional personnel. J Med Assoc Thai. 1998;81:352–356. [PubMed] [Google Scholar]
- 38.Kerse NM, Flicker L, Jolley D, Arroll B, Young D. Improving the health behaviours of elderly people: randomised controlled trial of a general practice education programme. BMJ. 1999;319:683–687. doi: 10.1136/bmj.319.7211.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwan RT, Davison N, Forster DP, Pearson P, Stirling E. Screening elderly people in primary care: a randomized controlled trial. Br J Gen Pract. 1990;40:94–97. [PMC free article] [PubMed] [Google Scholar]
- 40.Morrissey JP, Harris RP, Kincade-Norburn J. Medicare reimbursement for preventive care: changes in performance of services, quality of life, and health care costs. Med Care. 1995;33:315–331. [PubMed] [Google Scholar]
- 41.Newbury JW, Marley JE, Beilby JJ. A randomised controlled trial of the outcome of health assessment of people aged 75 years and over. Med J Aust. 2001;175:104–107. doi: 10.5694/j.1326-5377.2001.tb143541.x. [DOI] [PubMed] [Google Scholar]
- 42.Pathy MS, Bayer A, Harding K, Dibble A. Randomised trial of case finding and surveillance of elderly people at home. Lancet. 1992;340:890–893. doi: 10.1016/0140-6736(92)93294-w. [DOI] [PubMed] [Google Scholar]
- 43.Sahlen K-G, Dahlgren L, Hellner BM, Stenlund H, Lindholm L. Preventive home visits postpone mortality—a controlled trial with time-limited results. BMC Public Health. 2006;6:220. doi: 10.1186/1471-2458-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sørensen KH, Sivertsen J. Follow-up three years after intervention to relieve unmet medical and social needs of old people. Compr Gerontol [B] 1988;2:85–91. [PubMed] [Google Scholar]
- 45.Stuck AE, Aronow HU, Steiner A. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med. 1995;333:1184–1189. doi: 10.1056/NEJM199511023331805. [DOI] [PubMed] [Google Scholar]
- 46.Stuck AE, Minder CE, Peter-Wüest I. A randomized trial of in-home visits for disability prevention in community-dwelling older people at low and high risk for nursing home admission. Arch Intern Med. 2000;160:977–986. doi: 10.1001/archinte.160.7.977. [DOI] [PubMed] [Google Scholar]
- 47.Tulloch AJ, Moore V. A randomized controlled trial of geriatric screening and surveillance in general practice. J R Coll Gen Pract. 1979;29:733–742. [PMC free article] [PubMed] [Google Scholar]
- 48.van Rossum E, Frederiks CMA, Philipsen H, Portengen K, Wiskerke J, Knipschild P. Effects of preventive home visits to elderly people. BMJ. 1993;307:27–32. doi: 10.1136/bmj.307.6895.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vass M, Avlund K, Kvist K, Hendriksen C, Andersen CK, Keiding N. Structured home visits to older people. Are they only of benefit for women? A randomised controlled trial. Scand J Prim Health Care. 2004;22:106–111. doi: 10.1080/02813430410005829. [DOI] [PubMed] [Google Scholar]
- 50.Vetter NJ, Jones DA, Victor CR. Effect of health visitors working with elderly patients in general practice: a randomised controlled trial. BMJ. 1984;288:369–372. doi: 10.1136/bmj.288.6414.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner EH, LaCroix AZ, Grothaus L. Preventing disability and falls in older adults: a population-based randomized trial. Am J Public Health. 1994;84:1800–1806. doi: 10.2105/ajph.84.11.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace JI, Buchner DM, Grothaus L. Implementation and effectiveness of a community-based health promotion program for older adults. J Gerontol A Biol Sci Med Sci. 1998;53:M301–M306. doi: 10.1093/gerona/53a.4.m301. [DOI] [PubMed] [Google Scholar]
- 53.Yeo G, Ingram L, Skurnick J, Crapo L. Effects of a geriatric clinic on functional health and well-being of elders. J Gerontol. 1987;42:252–258. doi: 10.1093/geronj/42.3.252. [DOI] [PubMed] [Google Scholar]
- 54.Balaban DJ, Goldfarb NI, Perkel RL, Lepidus Carlson BL. Follow-up study of an urban family medicine home visit program. J Fam Pract. 1988;26:307–312. [PubMed] [Google Scholar]
- 55.Bernabei R, Landi F, Gambassi G. Randomised trial of impact of model of integrated care and case management for older people living in community. BMJ. 1998;316:1348–1351. doi: 10.1136/bmj.316.7141.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49:351–359. doi: 10.1046/j.1532-5415.2001.49076.x. [DOI] [PubMed] [Google Scholar]
- 57.Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47:775–783. doi: 10.1111/j.1532-5415.1999.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 58.Dalby DM, Sellors JW, Fraser FD, Fraser C, van Ineveld C, Howard M. Effect of preventive home visits by a nurse on the outcomes of frail elderly people in the community: a randomized controlled trial. CMAJ. 2000;162:497–500. [PMC free article] [PubMed] [Google Scholar]
- 59.Engelhardt JB, Toseland RW, O'Donnell JC, Richie JT, Jue D, Banks S. The effectiveness and efficiency of outpatient geriatric evaluation and management. J Am Geriatr Soc. 1996;44:847–856. doi: 10.1111/j.1532-5415.1996.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 60.Epstein AM, Hall JA, Fretwell M. Consultative geriatric assessment for ambulatory patients: a randomized trial in a health maintenance organization. JAMA. 1990;263:538–544. [PubMed] [Google Scholar]
- 61.Gagnon AJ, Schein C, McVey L, Bergman H. Randomized controlled trial of nurse case management of frail older people. J Am Geriatr Soc. 1999;47:1118–1124. doi: 10.1111/j.1532-5415.1999.tb05238.x. [DOI] [PubMed] [Google Scholar]
- 62.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 63.Hall N, De Beck P, Johnson D, Mackinnon K, Gutman G, Glick N. Randomized trial of a health promotion program for frail elders. Can J Aging. 1992;11:72–91. [Google Scholar]
- 64.Hébert R, Robichaud L, Roy P-M, Bravo G, Voyer L. Efficacy of a nurse-led multidimensional preventive programme for older people at risk of functional decline. A randomized controlled trial. Age Ageing. 2001;30:147–153. doi: 10.1093/ageing/30.2.147. [DOI] [PubMed] [Google Scholar]
- 65.Kono A, Kai I, Sakato C, Harker JO, Rubenstein LZ. Effect of preventive home visits for ambulatory housebound elders in Japan: a pilot study. Aging Clin Exp Res. 2004;16:293–299. doi: 10.1007/BF03324554. [DOI] [PubMed] [Google Scholar]
- 66.Leveille SG, Wagner EH, Davis C. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. J Am Geriatr Soc. 1998;46:1191–1198. doi: 10.1111/j.1532-5415.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 67.Newcomer R, Maravilla V, Faculjak P, Graves MT. Outcomes of preventive case management among high-risk elderly in three medical groups. A randomized clinical trial. Eval Health Prof. 2004;27:323–348. doi: 10.1177/0163278704270011. [DOI] [PubMed] [Google Scholar]
- 68.Reuben DB, Frank JC, Hirsch SH, McGuigan KA, Maly RC. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc. 1999;47:269–276. doi: 10.1111/j.1532-5415.1999.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 69.Rockwood K, Stadnyk K, Carver D. A clinimetric evaluation of specialized geriatric care for rural dwelling, frail older people. J Am Geriatr Soc. 2000;48:1080–1085. doi: 10.1111/j.1532-5415.2000.tb04783.x. [DOI] [PubMed] [Google Scholar]
- 70.Rubenstein LV, Calkins DR, Young RT. Improving patient function: a randomized trial of functional disability screening. Ann Intern Med. 1989;111:836–842. doi: 10.7326/0003-4819-111-10-836. [DOI] [PubMed] [Google Scholar]
- 71.Schrijnemaekers VJJ, Haveman MJ. Effects of preventive outpatient geriatric assessment: short-term results of a randomized controlled study. Home Health Care Serv Q. 1995;15:81–97. doi: 10.1300/J027v15n02_06. [DOI] [PubMed] [Google Scholar]
- 72.Shapiro A, Taylor M. Effects of a community-based early intervention program on the subjective well-being, institutionalization, and mortality of low-income elders. Gerontologist. 2002;42:334–341. doi: 10.1093/geront/42.3.334. [DOI] [PubMed] [Google Scholar]
- 73.Silverman M, Musa D, Martin DC, Lave JR, Adams J, Ricci EM. Evaluation of outpatient geriatric assessment: a randomized multi-site trial. J Am Geriatr Soc. 1995;43:733–740. doi: 10.1111/j.1532-5415.1995.tb07041.x. [DOI] [PubMed] [Google Scholar]
- 74.Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med. 2000;160:1825–1833. doi: 10.1001/archinte.160.12.1825. [DOI] [PubMed] [Google Scholar]
- 75.Stewart S, Harvey I, Poland F, Lloyd-Smith W, Mugford M, Flood C. Are occupational therapists more effective than social workers when assessing frail older people? Results of CAMELOT, a randomised controlled trial. Age Ageing. 2005;34:41–46. doi: 10.1093/ageing/afh230. [DOI] [PubMed] [Google Scholar]
- 76.Williams ME, Williams TF, Zimmer JG, Hall WJ, Podgorski CA. How does the team approach to outpatient geriatric evaluation compare with traditional care: a report of a randomized controlled trial. J Am Geriatr Soc. 1987;35:1071–1078. doi: 10.1111/j.1532-5415.1987.tb04923.x. [DOI] [PubMed] [Google Scholar]
- 77.Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health. 1985;75:134–141. doi: 10.2105/ajph.75.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burns R, Nichols LO, Martindale-Adams J, Graney MJ. Interdisciplinary geriatric primary care evaluation and management: two-year outcomes. J Am Geriatr Soc. 2000;48:8–13. doi: 10.1111/j.1532-5415.2000.tb03021.x. [DOI] [PubMed] [Google Scholar]
- 79.Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department—the DEED II study. J Am Geriatr Soc. 2004;52:1417–1423. doi: 10.1111/j.1532-5415.2004.52401.x. [DOI] [PubMed] [Google Scholar]
- 80.Crotty M, Whitehead C, Miller M, Gray S. Patient and caregiver outcomes 12 months after home-based therapy for hip fracture: a randomized controlled trial. Arch Phys Med Rehabil. 2003;84:1237–1239. doi: 10.1016/s0003-9993(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 81.Cunliffe AL, Gladman JRF, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age Ageing. 2004;33:246–252. doi: 10.1093/ageing/afh076. [DOI] [PubMed] [Google Scholar]
- 82.Dunn RB, Lewis PA, Vetter NJ, Guy PM, Hardman CS, Jones RW. Health visitor intervention to reduce days of unplanned hospital re-admission in patients recently discharged from geriatric wards: the results of a randomised controlled study. Arch Gerontol Geriatr. 1994;18:15–23. doi: 10.1016/0167-4943(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 83.Ford AB, Katz S, Downs TD, Adams M. Results of long-term home nursing: the influence of disability. J Chronic Dis. 1971;24:591–596. doi: 10.1016/0021-9681(71)90047-6. [DOI] [PubMed] [Google Scholar]
- 84.Hansen FR, Spedtsberg K, Schroll M. Geriatric follow-up by home visits after discharge from hospital: a randomized controlled trial. Age Ageing. 1992;21:445–450. doi: 10.1093/ageing/21.6.445. [DOI] [PubMed] [Google Scholar]
- 85.Hansen FR, Poulsen H, Sørensen KH. A model of regular geriatric follow-up by home visits to selected patients discharged from a geriatric ward: a randomized controlled trial. Aging Clin Exp Res. 1995;7:202–206. doi: 10.1007/BF03324316. [DOI] [PubMed] [Google Scholar]
- 86.Hughes SL, Weaver FM, Giobbie-Hurder A. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284:2877–2885. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 87.Leung ACT, Liu CP, Chow NWS, Chi I. Cost-benefit analysis of a case management project for the community-dwelling frail elderly in Hong Kong. J Appl Gerontol. 2004;23:70–85. [Google Scholar]
- 88.Martin F, Oyewole A, Moloney A. A randomized controlled trial of a high support hospital discharge team for elderly people. Age Ageing. 1994;23:228–234. doi: 10.1093/ageing/23.3.228. [DOI] [PubMed] [Google Scholar]
- 89.Melin AL, Bygren LO. Efficacy of the rehabilitation of elderly primary health care patients after short-stay hospital treatment. Med Care. 1992;30:1004–1015. doi: 10.1097/00005650-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Naylor MD, Brooten D, Campbell R. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 91.Nikolaus T, Specht-Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age Ageing. 1999;28:543–550. doi: 10.1093/ageing/28.6.543. [DOI] [PubMed] [Google Scholar]
- 92.Nikolaus T, Bach M. Preventing falls in community-dwelling frail older people using a home intervention team (HIT): results from the randomized Falls-HIT trial. J Am Geriatr Soc. 2003;51:300–305. doi: 10.1046/j.1532-5415.2003.51102.x. [DOI] [PubMed] [Google Scholar]
- 93.Rubin CD, Sizemore MT, Loftis PA, de Mola NL. A randomized, controlled trial of outpatient geriatric evaluation and management in a large public hospital. J Am Geriatr Soc. 1993;41:1023–1028. doi: 10.1111/j.1532-5415.1993.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 94.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46:174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 95.Tinetti ME, Baker DI, Gottschalk M. Home-based multicomponent rehabilitation program for older persons after hip fracture: a randomized trial. Arch Phys Med Rehabil. 1999;80:916–922. doi: 10.1016/s0003-9993(99)90083-7. [DOI] [PubMed] [Google Scholar]
- 96.Townsend J, Piper M, Frank AO, Dyer S, North WRS, Meade TW. Reduction in hospital readmission stay of elderly patients by a community based hospital discharge scheme: a randomised controlled trial. BMJ. 1988;297:544–547. doi: 10.1136/bmj.297.6647.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trentini M, Semeraro S, Motta M. Effectiveness of geriatric evaluation and care. One-year results of a multicenter randomized clinical trial. Aging Clin Exp Res. 2001;13:395–405. doi: 10.1007/BF03351509. [DOI] [PubMed] [Google Scholar]
- 98.Williams EI, Greenwell J, Groom LM. The care of people over 75 years old after discharge from hospital: an evaluation of timetabled visiting by Health Visitor Assistants. J Public Health Med. 1992;14:138–144. [PubMed] [Google Scholar]
- 99.Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community-based program for reducing the incidence of falls in the elderly: a randomized trial. J Am Geriatr Soc. 2004;52:1487–1494. doi: 10.1111/j.1532-5415.2004.52411.x. [DOI] [PubMed] [Google Scholar]
- 100.Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–97. doi: 10.1016/S0140-6736(98)06119-4. [DOI] [PubMed] [Google Scholar]
- 101.Davison J, Bond J, Dawson P, Steen IN, Kenny RA. Patients with recurrent falls attending Accident & Emergency benefit from multifactorial intervention—a randomised controlled trial. Age Ageing. 2005;34:162–168. doi: 10.1093/ageing/afi053. [DOI] [PubMed] [Google Scholar]
- 102.Gallagher EM, Brunt H. Head over heels: impact of a health promotion program to reduce falls in the elderly. Can J Aging. 1996;15:84–96. [Google Scholar]
- 103.Hogan DB, MacDonald FA, Betts J. A randomized controlled trial of a community-based consultation service to prevent falls. CMAJ. 2001;165:537–543. [PMC free article] [PubMed] [Google Scholar]
- 104.Hornbrook MC, Stevens VJ, Wingfield DJ, Hollis JF, Greenlick MR, Ory MG. Preventing falls among community-dwelling older persons: results from a randomized trial. Gerontologist. 1994;34:16–23. doi: 10.1093/geront/34.1.16. [DOI] [PubMed] [Google Scholar]
- 105.Lightbody E, Watkins C, Leathley M, Sharma A, Lye M. Evaluation of a nurse-led falls prevention programme versus usual care: a randomized controlled trial. Age Ageing. 2002;31:203–210. doi: 10.1093/ageing/31.3.203. [DOI] [PubMed] [Google Scholar]
- 106.Lord SR, Tiedemann A, Chapman K, Munro B, Murray SM, Sherrington C. The effect of an individualized fall prevention program on fall risk and falls in older people: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1296–1304. doi: 10.1111/j.1532-5415.2005.53425.x. [DOI] [PubMed] [Google Scholar]
- 107.Steinberg M, Cartwright C, Peel N, Williams G. A sustainable programme to prevent falls and near falls in community dwelling older people: results of a randomised trial. J Epidemiol Community Health. 2000;54:227–232. doi: 10.1136/jech.54.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tinetti ME, Baker DI, McAvay G. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 109.van Haastregt JCM, Diederiks JPM, van Rossum E, de Witte LP, Voorhoeve PM, Crebolder HFJM. Effects of a programme of multifactorial home visits on falls and mobility impairments in elderly people at risk: randomised controlled trial. BMJ. 2000;321:994–998. doi: 10.1136/bmj.321.7267.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vetter NJ, Lewis PA, Ford D. Can health visitors prevent fractures in elderly people? BMJ. 1992;304:888–890. doi: 10.1136/bmj.304.6831.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beck A, Scott J, Williams P. A randomized trial of group outpatient visits for chronically ill older HMO members: the Cooperative Health Care Clinic. J Am Geriatr Soc. 1997;45:543–549. doi: 10.1111/j.1532-5415.1997.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 112.Clark F, Azen SP, Carlson M. Embedding health-promoting changes into the daily lives of independent-living older adults: long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci. 2001;56:P60–P63. doi: 10.1093/geronb/56.1.p60. [DOI] [PubMed] [Google Scholar]
- 113.Scott JC, Conner DA, Venohr I. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the Cooperative Health Care Clinic. J Am Geriatr Soc. 2004;52:1463–1470. doi: 10.1111/j.1532-5415.2004.52408.x. [DOI] [PubMed] [Google Scholar]
- 114.Campbell M, Fitzpatrick R, Haines A. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321:694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bowling A, Bond M, Jenkinson C, Lamping DL. Short form 36 (SF-36) health survey questionnaire: which normative data should be used? Comparisons between the norms provided by the omnibus survey in Britain, the health survey for England and the Oxford healthy life survey. J Public Health Med. 1999;21:255–270. doi: 10.1093/pubmed/21.3.255. [DOI] [PubMed] [Google Scholar]
- 116.Clark J. Preventive home visits to elderly people. Their effectiveness cannot be judged by randomised controlled trials. BMJ. 2001;323:708. doi: 10.1136/bmj.323.7315.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiresuk TJ, Smith A, Cardillo JE. Goal attainment scaling: applications, theory, and measurement. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. [Google Scholar]
- 118.Kennedy A, Reeves D, Bower P. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health. 2007;61:254–261. doi: 10.1136/jech.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative risk (RR) of death
Relative risk (RR) of nursing home admission or residence at follow-up
Relative risk (RR) of hospital admission
Relative risk (RR) of falling
Standardised mean difference (SMD) of physical function at follow up
Characteristics of randomised controlled trials included in the systematic review
Relative risk (95% CIs) of outcome by quality of studies (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by death rate (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by date recruitment started (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk (95% CIs) of outcome by mean age at recruitment (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk of outcome (95% CIs) by intensity of intervention (standardised mean difference for physical function) and I2 heterogeneity statistic
Relative risk of outcome (95% CIs) by activity in control group (standardised mean difference for physical function) and I2 heterogeneity statistic