Abstract
The hypothesis is proposed that the central dynamics of the action–perception cycle has five steps: emergence from an existing macroscopic brain state of a pattern that predicts a future goal state; selection of a mesoscopic frame for action control; execution of a limb trajectory by microscopic spike activity; modification of microscopic cortical spike activity by sensory inputs; construction of mesoscopic perceptual patterns; and integration of a new macroscopic brain state. The basis is the circular causality between microscopic entities (neurons) and the mesoscopic and macroscopic entities (populations) self-organized by axosynaptic interactions. Self-organization of neural activity is bidirectional in all cortices. Upwardly the organization of mesoscopic percepts from microscopic spike input predominates in primary sensory areas. Downwardly the organization of spike outputs that direct specific limb movements is by mesoscopic fields constituting plans to achieve predicted goals. The mesoscopic fields in sensory and motor cortices emerge as frames within macroscopic activity. Part 1 describes the action–perception cycle and its derivative reflex arc qualitatively. Part 2 describes the perceptual limb of the arc from microscopic MSA to mesoscopic wave packets, and from these to macroscopic EEG and global ECoG fields that express experience-dependent knowledge in successive states. These macroscopic states are conceived to embed and control mesoscopic frames in premotor and motor cortices that are observed in local ECoG and LFP of frontoparietal areas. The fields sampled by ECoG and LFP are conceived as local patterns of neural activity in which trajectories of multiple spike activities (MSA) emerge that control limb movements. Mesoscopic frames are located by use of the analytic signal from the Hilbert transform after band pass filtering. The state variables in frames are measured to construct feature vectors by which to describe and classify frame patterns. Evidence is cited to justify use of linear analysis. The aim of the review is to enable researchers to conceive and identify goal-oriented states in brain activity for use as commands, in order to relegate the details of execution to adaptive control devices outside the brain.
Keywords: Beta activity β, Brain–computer interface BCI, Electrocorticogram ECoG, Epsilon activity ε, Gamma activity γ, Hilbert transform, Local field potential LFP, Multiple spike activity MSA, Stationarity
Appendix 1 The Hilbert Transform
Brain waves are commonly treated as if they were the sum of the outputs of a set of neural oscillators, each of which has a constant frequency and variable amplitude. This treatment is based on the assumption that brain dynamics is linear and time-invariant, which is clearly not the case. The advantage conveyed by this assumption is the ease with which Fourier analysis can be applied to brain waves using the Fast Fourier Transform (FFT) to decompose segments of brain waves into frequency components. The disadvantage is the inability of linear analysis to capture and display the nonlinear state transitions by which brains operate. An alternative linear transform is the Hilbert transform, which when applied to a brain wave recording in effect calculates the rate of change in the amplitude at each time step of the digitized signal. This operation effectively re-expresses an oscillation as a vector that rotates counterclockwise in the complex plane. The amplitude is expressed by the length of the vector, A(t), and the rate of change is expressed by the angular velocity of the rate of rotation of the vector about the origin of the complex plane. The rate of rotation is expressed as a rate of change in phase in degrees/second, radians/second (rad/s), or cycles/second (Hz). The immediate advantage is that the Hilbert transform decomposes a brain wave into an analytic amplitude, A(t), and an analytic phase, φ(t). The change in phase in rad with each time step divided by the digitizing interval in s approximates an instantaneous frequency that can vary, unlike the frequencies that are extracted by Fourier decomposition. The disadvantage is that the Hilbert transform is very sensitive to noise of many kinds; it only works well after band pass filtering of a brain wave. Criteria for optimal band pass filtering have been described elsewhere (Freeman 2004a, b; 2005; 2006).
The application of the Hilbert transform to each intracranial recording from an array of microelectrodes is a multi-step procedure. First, a high pass filter set at ˜400 Hz extracts the MSA, and a low pass filter set at ˜400 Hz extracts the LFP from the same n microelectrode recordings. Second, the low pass data are down-sampled from ˜40,000/s to 200/s and normalized to zero mean for every channel and unit standard deviation (SD) for all channels, trials and data sets to give the normalized LFP. Third, the demeaned, normalized LFP are band pass filtered in the classic empirical ranges: theta (3–7 Hz), alpha (7–12 Hz), beta (12–30 Hz), gamma (30–60 Hz), and high gamma (60–200 Hz), and the n channels in each pass band are segmented to save the data from each trial with ˜3 s preceding and ˜3 s following each CS onset (Fig. 6A). Fourth, the Hilbert transform is applied to get the analytic signal, Vj(t), with a real part (blue curve), the filtered LFP, vj(t), and an imaginary part (red curve), iuj(t), the output of the Hilbert transform:
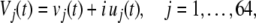 |
1 |
where the Hilbert transform of vj(t) in the time segment, t′,
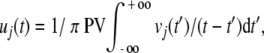 |
2 |
where PV signifies the Cauchy Principal Value. The imaginary part is also known as the quadrature of the signal, because each cosine component in the recorded signal is transformed to a sine component; taking the derivative by the transform is equivalent to shifting the phase of v(t) by 90° (π/2 rad) to get u(t).
Fifth, the square root of the sum of squares of the real and imaginary parts gives the analytic amplitude, Aj(t), for each channel, j = 1,...,n,
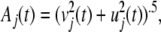 |
3 |
and the arctangent of the ratio of the imaginary part divided by the real part gives the analytic phase, φj(t) (Fig. 6B):
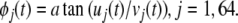 |
4 |
The mean of the square of amplitude, A2 j(t) over n gives the mean power, A2(t) (Fig. 6D), and the set of n scalar values of Aj(t) divided by A(t) gives the normalized feature vector at each time step, A(t). The feature vector provides a measure of the order parameter of the ensemble of cortical neurons that is under observation. A(t) specifies the normalized spatial pattern formed in the pass band by the signals from the n channels, and it designates a point in n-space that is occupied by the tip of the feature vector as it describes a trajectory through infinite brain state space that is projected into n-space by measurement.
Sixth, the rate of change in the normalized order parameter, De(t), is calculated from the analytic power by calculating the Euclidean distance between the tips of the feature vectors in n-space at each successive digitizing step:
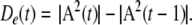 |
5 |
De(t) is a measure of the stability and stationarity of the normalized spatial pattern. Successive points in time specified by A(t) form clusters, whereas epochs of rapid change are manifested by a wide trajectory through n-space. The ratio of the rate of energy dissipation estimated by mean analytic power, A(t), divided by the rate of change in the order parameter, De(t), gives a quantity called the “pragmatic information”, He(t), which is maximal when the LFP amplitude peaks and when concomitantly the spatial pattern of the LFP is optimally stabilized.
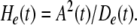 |
6 |
Seventh, the analytic phase, φj(t). is unwrapped by adding π radians at each break point where the arctangent goes to infinity (Fig. 6D), and the analytic frequency, ωj(t), is estimated by calculating the phase difference between successive digitizing steps in the unwrapped analytic phase, Δφj(t), time series and dividing that difference by the duration of the digitizing step, Δt. The mean analytic frequency, ω(t), and its spatial standard deviation, SDX, are calculated over the n channels at each time step. Typically in neocortical data the values of ω(t) and SDX are nearly constant for time periods of 60–120 ms indicating stationarity, and they fluctuate over the n channels in brief time periods that demarcate sudden transitions in analytic frequency, power, and spatial pattern. The implication is that areas of neocortex function in near-linear, stationary dynamics most of the waking state, but undergo brief state transitions 3–10 times each second. During the transitions the analytic amplitude, A(t), drops to a low level, and the variances of the analytic frequency, ω(t), given by SDX(t) increases briefly but dramatically (Fig. 1) in what is known as “phase slip” (Pikovsky et al. 2001). A state transition appears to be required to initiate the formation of a new spatial pattern, A(t), which is the order parameter manifesting a nonconvergent “chaotic” attractor in the landscape of basins of attraction sustained by an area of cortex.
Footnotes
http://sulcus.berkeley.edu
References
- Barrie JM, Freeman WJ, Lenhart M (1996) Modulation by discriminative training of spatial patterns of gamma EEG amplitude and phase in neocortex of rabbits. J Neurophysiol 76:520–539 [DOI] [PubMed]
- Barrie JM, Holcman D, Freeman WJ (1999) Statistical evaluation of clusters derived by nonlinear mapping of EEG spatial patterns. J Neurosci Meth 90:87–95 [DOI] [PubMed]
- Basar E (1998) Brain function and oscillations. 1. Principles and approaches. Springer-Verlag, Berlin
- Carmena JM, Lebedev MA, Henriquez CA, Nicolelis MAL (2005) Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci 25(46):10712–10716 [DOI] [PMC free article] [PubMed]
- Chapin JK, Moxon KA, Markowitz RS, Nicolelis MAL (1999) Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci 2:664–670 [DOI] [PubMed]
- Freeman WJ (1974) Relation of glomerular neuronal activity to glomerular transmission attenuation. Brain Res 65:91–107 [DOI] [PubMed]
- Freeman WJ (1975) Mass action in the nervous system. Academic, New York. Electronic 2004: http://sulcus.berkeley.edu/MANSWWW/MANSWWW.html
- Freeman WJ (2000) Neurodynamics: an exploration of mesoscopic brain dynamics. Springer, London
- Freeman WJ (2004a) Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clin Neurophysiol 115:2077–2088 [DOI] [PubMed]
- Freeman WJ (2004b) Origin, structure, and role of background EEG activity. Part 2. Analytic phase. Clin Neurophysiol 115:2089–2107 [DOI] [PubMed]
- Freeman WJ (2005) Origin, structure, and role of background EEG activity. Part 3. Neural frame classification. Clin Neurophysiol 116(5):1118–1129 [DOI] [PubMed]
- Freeman WJ (2006) Origin, structure, and role of background EEG activity. Part 4. Neural frame simulation. Clin Neurophysiol 117:572–589 [DOI] [PubMed]
- Freeman WJ, Burke BC (2003) A neurobiological theory of meaning in perception. Part 4. Multicortical patterns of amplitude modulation in gamma EEG. Int J Bifurc Chaos 13:2857–2866 [DOI]
- Freeman WJ, Gaál G, Jornten R (2003) A neurobiological theory of meaning in perception. Part 3. Multiple cortical areas synchronize without loss of local autonomy. Int J Bifurc Chaos 13:2845–2856 [DOI]
- Freeman WJ, Rogers LJ (2002) Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEG. J Neurophysiol 87:937–945 [DOI] [PubMed]
- Freeman WJ, Rogers LJ (2003) A neurobiological theory of meaning in perception. Part 5. Multicortical patterns of phase modulation in gamma EEG. Int J Bifurc Chaos 13:2867–2887 [DOI]
- Freeman WJ, Viana Di Prisco G (1986) Relation of olfactory EEG to behavior: time series analysis. Behav Neurosci 100:753–763 [DOI] [PubMed]
- Gonzalez SL, Grave de Peralta R, Thut G, Millán J del R, Morier P, Landis T (2006) Very high frequency oscillations (VHFO) as a predictor of movement intentions. NeuroImage, Short communication 32:170–179 [DOI] [PubMed]
- Gordon E (2000) Integrative neuroscience. Harwood Academic, Sydney
- Haken H (1983) Synergetics: an introduction. Springer-Verlag, Berlin
- Haken H (2006) Synergetics of brain function. Int J Psychophysiol 60(2):110–124 [DOI] [PubMed]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP (2006) Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442:164–172 [DOI] [PubMed]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD (2000) Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97:1867–1872 [DOI] [PMC free article] [PubMed]
- Kozma R, Freeman WJ (2001) Chaotic resonance: methods and applications for robust classification of noisy and variable patterns. Int J Bifurc Chaos 10:2307–2322
- Liley DTJ, Alexander DM, Wright JJ, Aldous MD (1999) Alpha rhythm emerges from large-scale networks of realistically coupled multicompartmental model cortical neurons. Network-Comput Neural Syst 10:79–92 [DOI] [PubMed]
- Ohl FW, Scheich H, Freeman WJ (2001) Change in pattern of ongoing cortical activity with auditory category learning. Nature 412:733–736 [DOI] [PubMed]
- Pikovsky A, Rosenblum M, Kurths J (2001) Synchronization—a universal concept in non-linear sciences. Cambridge University Press, Cambridge UK
- Robinson PA, Loxley PN, O’Connor SC, Rennie CJ (2001) Modal analysis of corticothalamic dynamics, electroencephalographic spectra, and evoked potentials. #041909, Physical Ref E6304: N4PT1:1090. US15–US26 [DOI] [PubMed]
- Sammon JW (1969) A nonlinear mapping for data structure analysis. IEEE Trans Comput C-18:401–409
- Sanchez JS, Carmena JM, Lebede MA, Nicolelis MAL, Harris JG, Principe JC (2004) Ascertaining the importance of neurons to develop better brain–computer interfaces. IEEE Trans Biomed Eng 51:943–953 [DOI] [PubMed]
- Tass P, Kurths J, Rosenblum M, Weule J, Pikovsky A, Volkmann J, Schnitzler H, Freund H (1999) Complex phase synchronization in neurophysiological data. In: Uhl C. (ed) Analysis of neurophysiological brain functioning. Springer-Verlag, Berlin, pp 252–273
- Traub RD, Whittington MA, Stanford IM, Jefferys JGR (1996) A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383:421–424 [DOI] [PubMed]
- Tsuda I (2001) Toward an interpretation of dynamics neural activity in terms of chaotic dynamical systems. Behav Brain Sci 24:793–847 [DOI] [PubMed]
- Whittington MA, Faulkner HJ, Doheny HC, Traub RD (2000) Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Therap 86:171–190 [DOI] [PubMed]
- Wright JJ, Liley DTJ (1996) Dynamics of the brain at global and microscopic scales: Neural networks and the EEG. Behav Brain Sci 19:285–295


