Abstract
G-protein coupled receptors (GPCRs) comprise one of the largest classes of signalling molecules. A wide diversity of activating ligands induce the active conformation of GPCRs and lead to signalling via heterotrimeric G-proteins and downstream effectors. In addition, a complex series of reactions participate in the ‘turn-off’ of GPCRs in both physiological and pharmacological settings. Some key players in the inactivation or ‘desensitization’ of GPCRs have been identified, whereas others remain the target of ongoing studies. G-protein coupled receptor kinases (GRKs) specifically phosphorylate activated GPCRs and initiate homologous desensitization. Uncoupling proteins, such as members of the arrestin family, bind to the phosphorylated and activated GPCRs and cause desensitization by precluding further interactions of the GPCRs and G-proteins. Adaptor proteins, including arrestins, and endocytic machinery participate in the internalization of GPCRs away from their normal signalling milieu. In this review we discuss the roles of these regulatory molecules as modulators of GPCR signalling.
A broad spectrum of extracellular signals, such as hormones, neurotransmitters, chemokines, odorants and light, are detected by members of the family of G-protein coupled receptors (GPCRs). More than a thousand members of this receptor family are predicted to exist. All GPCRs identified to date share the typical structural motif of seven membrane-spanning helices, and all convert extracellular signals into intracellular signals by activating heterotrimeric G-proteins. In addition to the ligands that activate the GPCRs, there are a number of other regulatory factors that influence the activation and deactivation of GPCR signalling, and hence play important roles in the specificity and spatiotemporal patterns of physiological responses to extracellular signals.
In the simplest model, GPCRs can be viewed as proteins that can exist in an inactive or active state (Gether & Kobilka, 1998). While increasing evidence is emerging that some GPCRs exhibit constitutive activity in the absence of ligand (Leurs et al. 1998), under normal conditions, and in the absence of their respective activating ligands, the inactive conformation is favoured (Gether & Kobilka, 1998). Upon activation by ligands, GPCRs are converted into the active conformation and are able to complex with and activate heterotrimeric G-proteins. The heterotrimeric G-proteins are composed of three subunits: the α-subunit which carries the guanine-nucleotide binding site, and the β- and γ-subunits which form a tightly bound dimer. Inactive G-proteins are heterotrimers composed of a GDP-bound α-subunit associated with the Gβγ-dimer (Fig. 1). The activated GPCRs function as GDP/GTP exchange factors and promote the release of GDP and the binding of GTP to the α-subunits. This leads to dissociation of the α-subunit and the Gβγ-dimer (Fig. 1). Both GTP-Gα and Gβγ can interact with a variety of effector systems in order to modulate cellular signalling pathways (Hamm, 1998). The deactivation of GPCR signalling occurs at several levels. Importantly, the G-protein Gα-subunit hydrolyses GTP to GDP and in turn reassociates with Gβγ to form the inactive heterotrimer (Fig. 1). In addition, ligand dissociation from the GPCRs converts the receptors back to their inactive state.
Figure 1. Schematic model of G-protein signalling cycle.
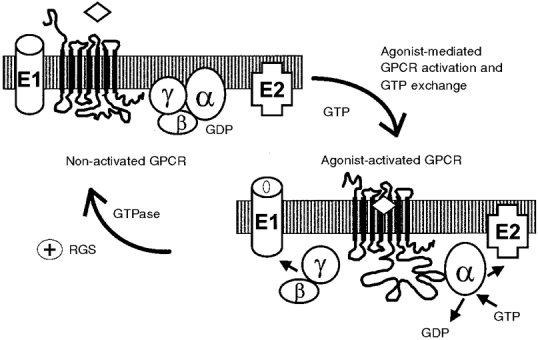
In the non-activated state, the GPCR is in the inactive conformation, and GDP is bound to the heterotrimeric G-protein. Upon agonist binding, the GPCR undergoes a conformational change to the activated state and is able to bind to the heterotrimeric G-proteins and act as a guanine nucleotide exchange factor. This causes the release of GDP and allows GTP to bind to the G-protein α-subunit. Following the nucleotide exchange the G-protein can dissociate into the GTP bound α-subunits and the βγ-dimer. Both the α-subunit and the βγ-dimer can interact with effector molecules such as ion channels (E1) or membrane bound enzymes (E2) and modulate their activity. The deactivation of signalling is initiated by the hydrolysis of GTP by the α-subunit. This reaction can be accelerated by proteins termed regulators of G-protein signalling (RGS) which have been shown to directly bind to the α-subunit of G-proteins. In the GDP bound state the α-subunit reassembles with the βγ-dimer to form the inactive heterotrimer.
Several mechanisms exist to regulate the length and strength of GPCR signals. In many cases a time-dependent decrease of the cellular response to the external signal occurs despite the continued presence of the signalling ligand. This attenuation of signalling is known as desensitization, and is important in physiological and pharmacological settings. In principal, desensitization of a G-protein-mediated signal can be achieved due to attenuation of signalling at the level of the receptor, the G-protein or the effector system, and examples of each are known. Indeed, there has been much interest recently in proteins termed the regulators of G-protein signalling (RGS proteins) (Berman & Gilman, 1998) that accelerate the hydrolysis of Gα-bound GTP and promote the deactivation of G-proteins (Fig. 1). RGS proteins may be important for the turn-off of many physiological responses; however, our understanding of their roles is only now being elucidated. The most extensively studied mechanism of desensitization is that occurring at the level of the GPCRs and is the main focus of this review.
Desensitization of G-protein coupled receptors
Many GPCRs have been shown to desensitize during the course of exposure to agonist (Krupnick & Benovic, 1998; Pitcher et al. 1998a; Bünemann et al. 1999). The time course and extent of receptor desensitization vary depending on the type of receptor and cellular background. Two general forms of desensitization have been described. Homologous desensitization refers to the situation whereby only the activated GPCRs desensitize, while heterologous desensitization refers to the situation whereby activation of one GPCR leads to the desensitization of responses initiated by another, heterologous GPCR. Multiple mechanisms of receptor desensitization exist, and not all are understood. However, agonist-dependent phosphorylation of the GPCRs appears to play an important role in initiating the desensitization of many GPCRs (Freedman & Lefkowitz, 1996; Pitcher et al. 1998a). While classical second-messenger-activated protein kinases may participate in the desensitization of various types of GPCRs, a unique family of protein kinases known as the G-protein coupled receptor kinases (GRKs) has been proposed to be responsible for agonist-dependent phosphorylation of GPCRs and to play a key role in initiating homologous desensitization.
A GRK-dependent pathway of desensitization was elucidated first in studies of the visual receptor rhodopsin (Kuhn, 1974; Weller et al. 1975; Zhao et al. 1995). Subsequently, a similar pathway was identified in studies of desensitization of β2-adrenergic receptors (β2AR). Because the β2AR has been the most extensively studied GPCR in terms of desensitization (Krupnick & Benovic, 1998; Lefkowitz, 1998; Pitcher et al. 1998a), the pathway that leads to desensitization of the β2AR has been viewed as a paradigm for other GPCRs. Indeed, in the past several years, other GPCRs have been shown to be regulated by GRKs in a manner similar to β2ARs (Krupnick & Benovic, 1998; Pitcher et al. 1998a). However, it is also becoming increasingly clear that there are many variations in the molecular events associated with desensitization of GPCRs and that the model developed for the β2AR does not apply in all respects to all GPCRs. In this review, we will focus on the roles of GRKs and the mechanisms of desensitization that have been elucidated for GPCRs other than the β2AR, as many excellent reviews already exist that describe the events associated with the desensitization of the β2AR (Freedman & Lefkowitz, 1996; Lefkowitz, 1998; Pitcher et al. 1998a). The general model developed to describe GRK-dependent desensitization is shown in Fig. 2. After activation of GPCRs by their respective agonists, GRKs specifically phosphorylate the agonist-activated receptors, leaving the non-activated receptors unaffected. This specificiy of GRKs for agonist-activated GPCRs is critical for their role in initiating homologous desensitization. Desensitization is thought to be achieved when ‘uncoupling’ proteins such as the arrestins (Krupnick & Benovic, 1998) bind to the GRK-phosphorylated receptors and cause receptor- G-protein uncoupling by preventing further interactions of the phosphorylated GPCRs with G-proteins (Fig. 2). Interestingly, phosphorylation by other kinases does not necessarily lead to arrestin binding; for example, arrestins do not bind to β2AR phosphorylated by protein kinase A (Lohse et al. 1992). Thus a second level of specificity is built into the GRK-arrestin-dependent mechanism of homologous desensitization. Four arrestins have been identified; visual and cone arrestins are localized to the visual system, whereas arrestin 2 and arrestin 3 (also referred to as β-arrestin 1 and β-arrestin 2) are ubiquitously distributed and thought to play a role in the uncoupling of many GPCRs (Krupnick & Benovic, 1998). Whether or not other uncoupling proteins exist is a topic that needs to be explored.
Figure 2. Scheme of GRK-dependent modulation of GPCR-mediated signalling.
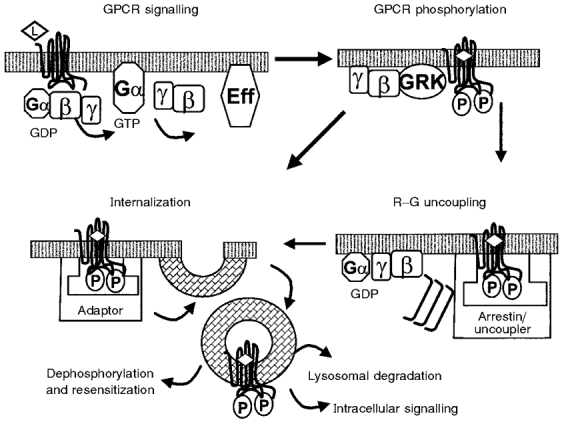
After activation of GPCRs by ligands (L), the receptors activate heterotrimeric G-proteins as described in Fig. 1. In the case of GRK2/3, the βγ-subunits of G-proteins may recruit GRKs to the membrane to allow for GPCR phosphorylation (P) of activated GPCRs. Phosphorylation by GRKs allows the GPCRs to interact with uncoupling proteins such as arrestins which cause uncoupling of the GPCRs from G-proteins. In addition to the uncoupling reaction, many GPCRs undergo internalization. This can be achieved by targeting of the phosphorylated receptor by an adaptor protein to endocytotic pathways. Internalization of the β2AR proceeds subsequent to the R-G-uncoupling step; the adaptor is a non-visual arrestin that targets the β2AR to clathrin-coated pits. For some GPCRs, internalization may proceed in a manner that is independent of the uncoupling reaction and may involve yet to be defined adaptors and endocytic machinery. After endocytosis, the GPCRs may undergo lysosomal degradation or recycle to the surface membrane. Eff, effector molecule.
A second event associated with desensitization of GPCRs is internalization of GPCRs from the plasma membrane (Figs 2 and 3). Recently it has become evident that multiple pathways of internalization of GPCRs exist (Fig. 3). The most well understood is GRK and arrestin dependent. The non-visual arrestins (arrestin 2 and 3) bind to clathrin and act as adaptors to facilitate the clathrin-mediated endocytosis of select GPCRs, such as β2ARs (Ferguson et al. 1996; Goodman et al. 1996; Zhang et al. 1996a). The arrestin- and clathrin-dependent internalization is a dynamin-dependent event (Zhang et al. 1996a) (Fig. 3). Dynamin is a GTPase that forms the necks of clathrin-coated pits and is essential for pinching off the vesicles from the plasma membrane (Urrutia et al. 1997). This pathway is not observed with all GPCRs, since other GPCRs are internalized via arrestin- and/or clathrin-independent pathways via proteins that can be dynamin dependent or independent (Fig. 3). Notably, these newly appreciated arrestin- and dynamin-independent pathways represent novel pathways of internalization whose components have yet to be identified. To illustrate the variations in the mechanisms of internalization, consider the multiple pathways of internalization that have been detected in studies of muscarinic cholinergic receptors (mAChRs) (Pals-Rylaarsdam & Hosey, 1997; Lee et al. 1998; Vogler et al. 1998). It is not yet clear if these pathways are modulated by GRK-mediated phosphorylation of the mAChR subtypes, but it is clear that there are at least two distinct pathways of internalization of mAChR subtypes that differ from the arrestin-, dynamin- and clathrin-dependent pathway used by the β2AR. One pathway, used by the M2 mAChR in HEK cells, is arrestin and dynamin independent (Fig. 3) (Pals-Rylaarsdam et al. 1997). This pathway is greatly facilitated by agonist-dependent phosphorylation of the receptors but is not blocked by dominant negative (DN)-GRK2 (Pals-Rylaarsdam et al. 1995, 1997; Pals-Rylaarsdam & Hosey, 1997). Another pathway is arrestin independent but dynamin dependent and is used by the M1, M3 and M4 mAChRs in HEK cells (Lee et al. 1998; Vogler et al. 1998). In considering this information, one might ask: if a receptor internalizes in an arrestin-independent manner, might the receptor-G-protein uncoupling step also be arrestin independent? Alternatively, if a receptor interacts with arrestin to cause receptor-G-protein uncoupling, why doesn't arrestin target the receptor to clathrin-coated pits for internalization? Answers to such questions will undoubtedly be forthcoming in the future and provide insights into the complex pathways mediating desensitization and internalization of GPCRs.
Figure 3. Multiple pathways of endocytosis for GPCRs.
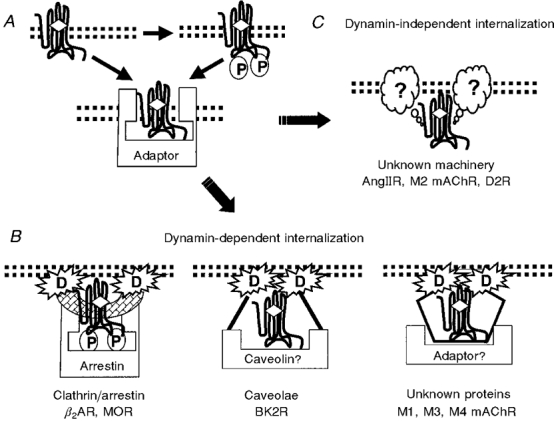
A, after agonist binding and activation of GPCRs, many GPCRs may undergo phosphorylation (P) by GRKs or other protein kinases. Phosphorylation can serve as a signal for the subsequent binding of certain adaptor proteins, but other mechanisms might also be operative, and might involve phosphorylation-independent binding to adaptors or direct association of the GPCRs with endocytic machinery. Subsequently, the GPCRs can be directed to different endocytic pathways. B, certain pathways are dynamin (D) dependent including internalization pathways that use arrestin as the adaptor and clathrin-coated pits for endocytosis as has been demonstrated for β2-adrenergic (β2AR) and μ-opioid receptors (MOR) (Zhang et al. 1997; Whistler & von Zastrow, 1998). Localization and/or internalization of GPCRs via caveolae may also occur and require dynamin for fission (Oh et al. 1998; Henley et al. 1998). For example, it has been inferred that the bradykinin 2 receptor (BK2R) is sequestered by caveolae (de Weerd & Leeb-Lundberg, 1997). It is not known if caveolin might serve as an adaptor in this process. In addition, some GPCRs, such as the M1, M3 and M4 muscarinic receptors (M1, M3, M4 mAChR), may be endocytosed via a dynamin-dependent process that uses as yet unknown adaptors and/or unknown endocytic machinery (Lee et al. 1998). C, internalization of GPCRs via dynamin-independent pathways has been demonstrated for angiotensin II-1A (AngIIR), M2-muscarinic (M2 mAChR) and D2 dopamine (D2R) receptors (Zhang et al. 1996a; Pals-Rylaarsdam et al. 1997; Vickery & von Zastrow, 1999). The cellular machinery for this process is not yet known and needs to be identified in the future.
The roles of endocytosis in the desensitization of GPCRs also are not completely understood, and different consequences of internalization have been noted depending on the GPCR being studied and the cellular background. In certain cases, such as with the M2 mAChRs studied in HEK cells, internalization and receptor-G-protein uncoupling have been dissociated and in these cells internalization does not cause desensitization (receptor-G-protein uncoupling) (Pals-Rylaarsdam et al. 1995, 1997; Pals-Rylaarsdam & Hosey, 1997). However, in Chinese hamster ovary cells, other data with M2 mAChRs suggest that internalization is necessary for receptor-G-protein uncoupling (Tsuga et al. 1998a). These contrasting results may reflect differences in cellular machinery and different molecular pathways of desensitization. For the M4 mAChR, internalization has been suggested to prolong desensitization (Bogatkewitsch et al. 1996). In marked contrast, internalization of the β2AR appears to allow for dephosphorylation, resensitization and recycling to the plasma membrane (Pitcher et al. 1995; Krueger et al. 1997). However, not all of the internalized β2ARs may undergo the recycling process, but rather some may undergo degradation in lysosomes, i.e. downregulation (Gagnon et al. 1998). The events that allow for the decision to recycle versus destroy are poorly understood. Recently, yet another role for internalization of GPCRs has been proposed. It has been suggested that GRK- and arrestin-dependent internalization is necessary for the β2AR to activate the mitogen activated protein (MAP) kinase pathway via a pertussis toxin sensitive reaction (Daaka et al. 1998). Clearly, more studies are necessary to unravel the multiple complex mechanisms and roles of receptor internalization.
GRK structure and regulation
GRKs are 57-80 kDa proteins that are members of the large family of serine/threonine kinases. Six mammalian GRKs have been cloned so far (Palczewski, 1997; Krupnick & Benovic, 1998; Pitcher et al. 1998a). GRK1 (rhodopsin kinase) and GRK4 have been shown to be expressed in a tissue specific manner (retina and testis, respectively), and thus are expected to regulate a limited number of substrates (Palczewski, 1997). In contrast, the other GRKs are more widely distributed and evidence exists to suggest that these GRKs are likely to be involved in desensitization of multiple types of GPCRs (Palczewski, 1997).
While GRKs were initially thought to be constitutively active soluble enzymes, it is now recognized that each of the GRKs is regulated via one or more different mechanisms (for a detailed review see Palczewski, 1997), and a significant amount of the GRKs are localized to membranes (Aragay et al. 1998b; Murga et al. 1998). A common theme that has emerged suggests that lipid modification or interactions may be important for the membrane localization and activity of GRKs. This is achieved through several distinct mechanisms, including C-terminal palmitoylation for GRK4 and GRK6 (Loudon & Benovic, 1997), isoprenylation for GRK1 (Inglese et al. 1992), electrostatic binding for GRK5 (Kunapuli et al. 1994a), and by binding to membrane phospholipids (DebBurman et al. 1995b) and/or Gβγ-subunits for GRK2 and GRK3 (Koch et al. 1993).
Many studies have shown that GRK1, GRK2 and GRK3 ‘translocate’ upon activation of GPCRs (Inglese et al. 1994; Krupnick & Benovic, 1998). However, it is not clear whether pools of GRKs are constantly membrane associated and undergo two-dimensional rather than three-dimensional translocation upon activation of GPCRs (Palczewski, 1997). Multiple factors are likely to affect the membrane association of GRKs, such as interactions between the kinases and receptors, as the GPCRs have been shown to modulate significantly the activities of certain GRKs (Palczewski, 1997). Prenylation has been demonstrated to be required for translocation of GRK1 (Inglese et al. 1992), whereas translocation of GRK2 and GRK3 may involve interaction with Gβγ (Daaka et al. 1997; Krupnick & Benovic, 1998). Since GRK2 and GRK3 require the presence of negatively charged phospholipids like PIP2 or PIP in order to be active (Onorato et al. 1995; DebBurman et al. 1996; Pitcher et al. 1996), it may be that Gβγ provides a signal for docking GRK2 and GRK3 to the plasma membrane where the kinases can bind to activating phospholipids.
The activities of GRKs appear to be inhibited by intracellular Ca2+ by direct interaction of GRKs with calcium-binding proteins. GRK1 can be inhibited by the calcium-binding protein recoverin (Gray-Keller et al. 1993; Chen et al. 1995), while GRK2, 5 and 6 appear to be negatively regulated by Ca2+-calmodulin (Pronin et al. 1997). In vitro, the Ca2+- calmodulin sensitivity of GRK5 and GRK6 is much higher than that of GRK2, suggesting that regulation by Ca2+- calmodulin may be of greater physiological relevance for these kinases (Levay et al. 1998). In a search for additional proteins that may regulate GRKs, actin was found to bind to GRK5 (Freeman et al. 1998) and to inhibit the kinase activity of GRK5 in a concentration-dependent manner. Ca2+-calmodulin and actin compete for inhibiting GRK5 and therefore may bind to an overlapping region on GRK5 (Freeman et al. 1998). Despite the biochemical evidence that Ca2+-calmodulin can interact with GRKs and inhibit the phosphorylation of GPCRs, there is no direct evidence for in vivo regulation of GRKs by calcium. In fact, the physiological role of Ca2+-dependent regulation of GRK1 has been questioned in a recent study, where no decrease in light-dependent phosphorylation of rhodopsin was found in retinas treated with α-toxin, which forms Ca2+-permeable pores in membranes (Otto-Bruc et al. 1998). GRK2 and GRK5 are substrates for PKC (Chuang et al. 1995; Pronin & Benovic, 1997). However, PKC-mediated phosphorylation of GRK2 results in an activation of this kinase (Pronin & Benovic, 1997), whereas GRK5 is negatively regulated by PKC (Chuang et al. 1995).
Specificity of GRKs
While six members of the GRK family have been identified so far, hundreds to thousands of GPCRs are predicted to exist. Thus, one interesting question concerns the specificity of the regulation of GPCRs by GRKs. Since GRK1 and GRK4 are expressed only in retina (Zhao et al. 1998) and testis/kidney (Virlon et al. 1998), respectively, the specialized localization of these GRKs is likely to contribute to their specificities. However, the other members of the GRK family are more widely distributed, and other factors must contribute to specificity of kinase-substrate interactions. A few GPCRs appear to be selectively phosphorylated by certain GRKs. For example, endothelin receptors (ETA and ETB) were shown to be preferentially phosphorylated by GRK2 when expressed in HEK293 cells (Freedman et al. 1997), while thrombin receptors (Ishii et al. 1994; Iaccarino et al. 1998a), as well as odorant receptors (Peppel et al. 1997), have been shown to be specifically phosphorylated and desensitized by GRK3. On the other hand, there are several receptors that appear to be phosphorylated equally well by GRK2, GRK3 and GRK5 in vitro, including the angiotensin II type 1a receptors (Ishizaka et al. 1997; Oppermann et al. 1996), β2ARs (Premont et al. 1995; Menard et al. 1996) and muscarinic M2 and M3 receptors (Richardson et al. 1993; Debburman et al. 1995a). For these and other receptors that are substrates for multiple GRKs, the specificity of GRK- GPCR interaction may be defined in part by the cellular and subcellular distribution of GRKs. Some insights into this issue have been obtained in studies of transgenic mice. For example, in transgenic mice targeted to overexpress GRK2 in cardiac myocytes, a reduced responsiveness to βAR agonists and angiotensin II was observed, while mice overexpressing the C-terminal portion of GRK2 (that inhibits GRK2 by virtue of its ability to bind Gβγ) exhibited increased sensitivity to βAR agonists (Koch et al. 1995; Rockman et al. 1996). Interestingly, mice overexpressing GRK3 showed no difference in β-adrenergic or angiotensin II signalling, while they did exhibit a decrease in thrombin receptor signalling (Iaccarino et al. 1998a). As the studies of the GRK-transgenic mice progress, it will be of interest to determine what other known substrates of the GRKs are affected by the targeted overexpression of the GRKs. In addition, it would also be of interest to determine if the effects of overexpressing the C-terminus of GRK2 are due specifically to an inhibition of GRK2 versus a generalized sequestration of Gβγ-subunits and a consequent inhibition of Gβγ-dependent signalling pathways. In other studies, long-term stimulation with β-adrenergic agonists or antagonists in mice caused upregulation or downregulation, respectively, of GRK2, but not of any other GRK, in cardiac myocytes (Iaccarino et al. 1998b), indicating that the degree of GRK specificity can be high in vivo, even if there is no clear specificity in vitro. This suggests that other, as yet undefined, factors contribute to the specificity of GRK- GPCR interactions in intact cells.
How important are GRKs for desensitization of GPCRs other than the β2AR?
GRKs have been implicated in the initiation of agonist-induced desensitization of a variety of GPCRs (Table 1). In order to characterize the causes of desensitization, it is important to measure the desensitization of G-protein-activated signalling pathways in intact cells. However, under these conditions it is difficult to distinguish between desensitization that occurs at the level of the receptor versus G-proteins or effectors. To overcome this problem and to gain direct insights into the roles of GRKs in receptor desensitization, alternative approaches have been adopted to assess receptor desensitization, including the use of purified and reconstituted systems (Benovic et al. 1987b; Kwatra et al. 1989a; Lohse et al. 1992; Richardson et al. 1993), where GRK-mediated phosphorylation and its functional consequences can be monitored directly, as well as the use of isolated cell membranes to assess the ability of GRKs to modulate receptor stimulated G-protein activity (Oppermann et al. 1996; Whistler & von Zastrow, 1998). In addition, a large number of studies have used overexpression of GRKs and/or arrestins to assess the participation of these proteins in desensitization and internalization of GPCRs. Notably, the measurement of agonist-induced internalization of GPCRs has frequently been used as an ‘indicator’ of receptor desensitization, and, most recently, many investigators have asked if receptor internalization is promoted by GRKs (Tsuga et al. 1994, 1998c; Zhang et al. 1998). However, caution needs to be exercised in those cases where receptor internalization is used to reflect receptor desensitization for several reasons. First, the actual desensitization of GPCRs, i.e. the uncoupling of receptors from G-proteins, often precedes sequestration and internalization of these receptors (Roth et al. 1991). In addition, there are cases where receptor-G-protein uncoupling appears to be mediated by distinct mechanisms from those that promote receptor internalization (Pals-Rylaarsdam et al. 1995, 1997; Pals-Rylaarsdam & Hosey, 1997). Indeed, internalization can occur in the absence of receptor-G-protein uncoupling (Pals-Rylaarsdam & Hosey, 1997). It is important to keep in mind that the mechanisms and roles of internalization have not been completely defined and cannot be assumed to reflect directly the mechanisms promoting receptor- G-protein uncoupling.
Table 1.
Evidence for GRK-dependent regulation of G-protein coupled receptors
The first column provides a partial fist of GPCRs that have been analysed as targets of GRK-dependent regulation. Studies that demonstrated agonist-dependent phosphorylation of G-protein coupled receptors either in cell-free assays (in vitro) or in intact cells (in vivo) are listed in column 2. GRKs that have been demonstrated to phosphorylate receptors in cell-free assays are listed in column 3, whereas effects of heterologously expressed GRKs on agonist-induced desensitization (DES), phosphoryktion (P) and internalization (I) of individual GPCRs in intact cells are shown in column 4. The last two columns cite studies that have analysed the effects of coexpression of arrestins or a dominant negative mutant of dynamin on agonist-induced internalization of given GPCRs. If coexpression of arrestins increased, or if dominant negative dynamin inhibited, agonist-induced internalization of receptors it is stated with ‘yes’, if not it is stated with ‘no’. Studies that observed an increase of desensitization of the GPCR upon coexpression of arrestins are indicated as (DES). GnRH, gonadotropin-releasing hormone.
GRKs can phosphorylate a variety of GPCRs in vitro and/or in native systems or in heterologous expression systems (Table 1). Overexpression of GRKs was shown to enhance internalization and/or desensitization of many GPCRs, but this does not necessarily mean that these receptors normally utilize the GRK pathway defined for the β2AR for their desensitization and/or internalization. It is important to distinguish between desensitization events that occur with endogenous cellular machinery and those that may be ‘forced’ to occur by overexpression of GRKs and/or arrestins, as multiple pathways of desensitization and internalization are likely to exist.
In order to gain insights into the type of pathway that is utilized for desensitization some useful tools and approaches are available. The availability of purified GRKs allows one to ask if a given receptor is a substrate for GRKs in vitro. Typically such assays are performed using purified GRKs and purified and reconstituted receptors (Benovic et al. 1987a,b; Richardson & Hosey, 1992; Richardson et al. 1993; Haga et al. 1996), although other approaches using membranes have also been introduced (Pei et al. 1994; Debburman et al. 1995a). If the receptor is found to be stoichiometrically phosphorylated in vitro with ‘reasonable’ concentrations of receptor and GRK, then this receptor may be a potential target of GRK in intact cells. Unfortunately there are no specific pharmacological inhibitors of GRKs available that can be used in intact cells to corroborate results that have obtained from in vitro phosphorylation studies.
Another approach to determine if GRKs participate in receptor regulation is to ask if a loss of GRK activity causes a loss of receptor phosphorylation, desensitization and/or internalization. For example, one can ask if overexpression of a dominant negative (DN) GRK (Kong et al. 1994) inhibits the phosphorylation, desensitization and/or internalization that is observed in response to agonist. If the answer is yes, this is considered to be a good indication for a GRK-dependent desensitization process. However, there are several caveats to be considered. Overexpression of DN GRK2 or 3 can cause a sequestering of Gβγ in these cells and therefore will inhibit signalling mediated by the G-protein βγ-subunits (Inglese et al. 1994). In fact, overexpression of the C-terminus (βγ-binding domain) of GRK2 has been demonstrated to disrupt G-protein-mediated signalling such as MAP kinase activation via stimulation of M1 and M2 mAChRs (Crespo et al. 1994) or M2 mAChR-mediated activation of Gβγ-activated GIRK channels (Bünemann & Hosey, 1998). Therefore it is very important to also use as a control overexpressed wild-type (WT) GRK2 or 3 and to compare and contrast the effects of the WT and DN GRKs to differentiate between effects due to the kinase activity of GRK2 or 3 and their Gβγ-sequestering activity. Overexpression of WT GRKs might increase phosphorylation, desensitization and/or internalization of a GPCR. In this scenario, the results would suggest that the GPCR under study is capable of undergoing regulation by GRKs, but it would not necessarily suggest that this occurs physiologically. Such results could suggest that a particular cell has a low complement of GRKs, or that the GPCR is a relatively poor substrate for GRKs. Another approach to explore the function of GRKs in the regulation of GPCRs in intact cells is to use strategies that knock out or at least reduce the amount of GRKs. The development of GRK knock-out mice (discussed below) and antisense approaches are of great potential. These approaches are less prone to the problems that overexpression of signalling molecules can cause, i.e. pushing a signalling pathway in a new direction.
In order to test whether arrestins are important for desensitization and internalization of GPCRs, several useful arrestin constructs have been developed that may help to clarify this issue. Increased internalization due to the overexpression of WT arrestins would indicate that a given GPCR can be internalized in an arrestin-dependent manner, but would not indicate that the endogenous internalization pathway is arrestin dependent. However, overexpression of the clathrin-binding domain of arrestin 2 or 3 blocks internalization of β2AR and may be a useful tool to determine if other GPCRs are internalized via arrestin- clathrin interaction (Fig. 2) (Krupnick et al. 1997; Zhang et al. 1997). On the other hand, there are instances where overexpression of neither WT nor DN arrestins affected internalization (Lee et al. 1998), suggesting that the internalization of certain GPCRs is insensitive to arrestins. Similar problems as discussed for overexpression of GRK constructs may occur with overexpression of arrestins, and therefore knock-out or antisense approaches may help to determine which role arrestins play in vivo. Recently an antisense approach has yielded selected clones of HEK293 cells with reduced expression of arrestin 2 and arrestin 3 (J. L. Benovic, personal communication). In these cells, β2ARs exhibited less internalization than in control cells.
Another important tool to dissect the pathway of receptor internalization is a dominant negative mutant of dynamin, dynamin K44A (Damke et al. 1994), which is deficient in GTPase activity and unable to catalyse the fission of clathrin-coated pits from the membrane. This protein has been shown to inhibit endocytosis via clathrin-coated pits in many different systems (van der Bliek et al. 1993; Baba et al. 1995; Zhang et al. 1996a; Altschuler et al. 1998). In addition, the DN dynamin mutant also has been recently reported to inhibit the pinching off of caveolae (Henley et al. 1998; Oh et al. 1998), thus demonstrating that caveolae also use a dynamin-dependent process (see Fig. 3). The B2 bradykinin receptor has been reported to be sequestered in caveolae in response to agonist treatment (de Weerd & Leeb-Lundberg, 1997). The M1, M3 and M4 mAChRs are internalized in HEK cells in a dynamin-dependent manner (Lee et al. 1998; Vogler et al. 1998), but the internalization of these receptors is not inhibited by dominant negative arrestin mutants (Lee et al. 1998). Therefore these receptors do not appear to be internalized via the arrestin-clathrin pathway used by β2ARs, but they could conceivably be internalized via caveolae or, alternatively, may use other adaptor proteins that may target these receptors to clathrin-coated pits or as yet unidentified endocytic vesicles (Fig. 3).
Importance of GRKs in desensitization in vivo
Studies in heterologous expression systems have provided insights into the mechanisms involved in desensitization and internalization. However, in many cases it is difficult to test whether the desensitization pathway that is utilized in heterologous expression systems is comparable to that utilized in native tissues. Technical limitations, such as difficulties in transfecting native cells with plasmids carrying the cDNAs for various proteins of interest, hamper our current understanding of the mechanisms of desensitization in native systems and in vivo. However, recent insights into the roles of GRKs in vivo have been provided from studies of transgenic mice. Homozygous GRK2 knock-out mice were embryonic lethal at embryonic day 15. The embryos had severe defects in cardiac development and most probably died of cardiac failure (Jaber et al. 1996), suggesting an unknown but important role for GRKs in cardiac development. In contrast the heterozygous GRK2(+/-) mice were normal and these mice, as well as GRK2(+/-) mice overexpressing the C-terminus of GRK2 (‘GRK2 inhibitor’), exhibited increased contractile function compared with control mice (Rockman et al. 1998b). Furthermore, as discussed above, mice overexpressing GRK2 showed a reduced responsiveness to β2AR stimulation, while animals overexpressing the C-terminal portion of GRK2 (‘βARK-inhibitor’) exhibited increased βAR responsiveness (Koch et al. 1995). Recently, the decreased myocardial βAR responsiveness that was observed in animal models of chronic heart failure, as well as in human hearts (Bristow et al. 1990), has been correlated with increased GRK activity (most likely to be GRK2 and GRK5) (Ungerer et al. 1993, 1994). In addition, targeted overexpression of the ‘GRK2 inhibitor’ has been demonstrated to prevent the development of cardiomyopathy in a murine model of heart failure (Rockman et al. 1998a). These and other studies that demonstrated that changes of the activity of GRKs in myocytes resulted in alteration of the βAR responsiveness suggest that GRKs might actually play important roles in regulating cardiac contractility in vivo (Koch et al. 1995; Ungerer et al. 1996; Akhter et al. 1997; Drazner et al. 1997). Reducing GRK activity in vivo, for example by gene transfer, could be a potential new therapeutic approach for patients with chronic heart failure (Drazner et al. 1997; Rockman et al. 1998a).
Another successful strategy has been to document the pathophysiology caused by naturally occurring mutations in the GRK1 gene. Mutations in the GRK1 gene have been correlated with Oguchi disease, an autosomal recessive form of stationary night blindness in man characterized in part by delayed photoreceptor recovery (Yamamoto et al. 1997). Biochemical studies have confirmed that these mutations lead to gene products that virtually lack GRK1 activity (Khani et al. 1998). Thus the lack of GRK1 results in lack of the normal ‘turn-off’ of rhodopsin and the consequent disease state.
Role of GRKs in regulating M2 mAChRs
While it is not possible to review extensively in this article the evidence for a role of GRKs in the regulation of very many GPCRs, a non-visual, non-adrenergic GPCR that has been analysed fairly extensively in terms of desensitization is the M2 mAChR. GRKs have been implicated in the phosphorylation and desensitization of M2 mAChR, and in vitro these receptors are comparable as substrates for GRK2, 3 and 5 to the β2AR (Kwatra et al. 1989a; Richardson & Hosey, 1992; Kunapuli et al. 1994b; Tsuga et al. 1994; Pals-Rylaarsdam et al. 1995). Agonists are known to induce phosphorylation and desensitization of the M2 mAChR in intact cardiac myocytes (Kwatra & Hosey, 1986; Kwatra et al. 1987, 1989b). While no direct evidence links GRKs to this reaction, the properties of agonist-induced phosphorylation of the M2 mAChR in cardiac myocytes closely resemble the GRK-mediated phosphorylation and regulation of these receptors that is observed in heterologous expression systems and in reconstituted systems (Kwatra et al. 1989a; Richardson & Hosey, 1992; Richardson et al. 1993; Pals-Rylaarsdam et al. 1995). In addition, in HEK cells, DN GRK2 reduced agonist-dependent phosphorylation of the M2 mAChR by 50 % and eliminated desensitization of the Gi-mediated attenuation of adenylyl cyclase (Pals-Rylaarsdam et al. 1995), suggesting that an endogenous GRK regulates the M2 mAChR in these cells. On the other hand, in the same cell system, overexpression of DN GRK2 or WT GRK2 had no effect on internalization of the M2 mAChR, suggesting that GRKs may not play a role in the internalization of these receptors in HEK cells. In similar studies a small effect of WT GRK on internalization was observed in CHO-K1 cells, but only at low concentrations of agonist (Tsuga et al. 1994).
The M2 mAChR also activates G-protein gated K+ (KACh) channels in cardiac atrial myocytes, and this Gβγ-mediated response rapidly desensitizes (Yamada et al. 1998). Treatment of isolated atrial myocytes with muscarinic agonists for seconds to several minutes causes heterologous desensitization of the ability of other endogenous Gi-coupled receptors, such as the A1 adenosine or sphingosylphosphorylcholine receptors, to activate this current (Bünemann et al. 1997). In addition, homologous desensitization of ACh-activated KACh channels occurs in atrial myocytes with a time course of several minutes to many hours (Shui et al. 1995, 1998; Bünemann et al. 1996). When freshly isolated atrial myocytes are cultured in the absence of ACh, an increase in sensitivity to ACh is observed, suggesting that atrial myocytes might be partially desensitized in vivo (Bünemann et al. 1997). Further support for this suggestion is that the time course of the development of the increase in agonist sensitivity during agonist-free culture was similar to that of the time course of recovery of the M2 mAChR following long-term desensitization in vitro (Bünemann et al. 1997).
Other studies have implicated GRKs in these processes of desensitization. One study has shown a loss of desensitization of KACh channels in outside-out patches of rat atrial myocytes that can be restored by application of purified GRK2 (Shui et al. 1998). Further studies using membrane patches of CHO-K1 cells which heterologously expressed KACh channels showed that coexpression of GRK2 greatly increased the M2 mAChR-induced desensitization of KACh channels. However, expression of the DN GRK2 also enhanced desensitization compared with cells not expressing GRK2 (Shui et al. 1998). Since very little desensitization was observed when neither WT nor DN GRK2 was expressed (Shui et al. 1998), the ‘desensitization’ might have been due to sequestration of Gβγ. In addition, in these studies one cannot distinguish between desensitization of the M2 mAChR and desensitization of G-proteins or channels. However, support for a role for GRKs in the desensitization of M2 mAChR-induced KACh channels came from observations that when DN GRK2 was overexpressed with phosphorylation-deficient mutants of the M2 mAChR in CHO cells, agonist-dependent desensitization was reduced compared with that observed in cells expressing WT M2 mAChR and either WT or DN GRK2 (Shui et al. 1998). When all the evidence is reviewed about desensitization of M2 mAChR responses in cardiac myocytes, the suggestion that GRKs and receptor phosphorylation may participate in desensitization is still at the correlative stage, and more studies will be required to elucidate the exact pathway(s) that lead to homologous and heterologous desensitization, as well as internalization of the M2 mAChR in native systems.
Other functions of GRKs
Since GRKs are believed to be activated by agonist-activated GPCRs and downstream events, it is conceivable that the activated GRKs may play unknown roles in signal transduction pathways by phosphorylating non-GPCR substrates. In this sense the GRKs may function like other protein kinases that are activated by receptor and G-protein signalling. Recently several groups independently detected that GRK2 and GRK5 (Carman et al. 1998) can bind and phosphorylate tubulin (Carman et al. 1998; Haga et al. 1998; Pitcher et al. 1998b). The physiological consequence of the interaction of GRK2 with tubulin is not yet understood, but GRK2 does colocalize with microtubules in intact cells (Pitcher et al. 1998b) and the colocalization of GRK2 and tubulin is facilitated by activation of various GPCRs with agonist. Since tubulin is an important protein for cytoskeletal function, it is easy to speculate that GRKs may play a role in a GPCR-induced modulation of cytoskeletal arrangement. Furthermore, since receptor-induced activation of GRKs, at least for GRK1, 4, 5, and 6, seems to be independent of the activation of G-proteins, these GRKs may work themselves as signal transducers for GPCRs.
One interesting structural feature of GRKs is an RGS homology domain (Berman & Gilman, 1998) at the N-terminus of these proteins. Since RGS proteins can bind to Gα-subunits and accelerate GTP hydrolysis (i.e. they are GAPs), they play an important role in modulating G-protein signalling (Berman & Gilman, 1998; Zerangue & Jan, 1998). So far there is no experimental evidence that GRKs themselves exhibit GAP activity towards Gα-subunits, but the presence of RGS-like domains in GRKs leads to the speculation that GRKs may have additional functions in G-protein signalling. In this regard, a novel GRK-interacting protein (GIT1) has been discovered in a yeast two-hybrid screen and can bind to GRK2, 3, 5 and 6 (Premont et al. 1998). Overexpression of GIT1 increased GRK2-dependent phosphorylation of β2ARs and caused receptor-G-protein uncoupling, but GIT1 also inhibited agonist-induced sequestration and resensitization of these receptors (Premont et al. 1998). Interestingly GIT1 was found to act as a GAP for the ARF family of small G-proteins, which are implicated in the regulation of vesicular trafficking including endocytosis. Therefore, GIT1 might be an inhibitor of endocytic pathways. If this hypothesis is right it would explain why GIT1 decreased agonist-induced internalization of β2ARs despite the observed increase in phosphorylation (Premont et al. 1998). It is obvious that we have not learned all there is about the roles of GRKs in physiology and that the next years promise to provide new insights into the complex regulation of GPCRs and other functions by GRKs.
References
- Akhter SA, Skaer CA, Kypson AP, McDonald PH, Peppel KC, Glower DD, Lefkowitz RJ, Koch WJ. Restoration of β-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proceedings of the National Academy of Sciences of the USA. 1997;94:12100–12105. doi: 10.1073/pnas.94.22.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, Schmid SL. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. Journal of Cell Biology. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Patterson TA, Jin W, Chavkin C. Agonist-induced phosphorylation of the κ-opioid receptor. Journal of Neurochemistry. 1997;69:2405–2412. doi: 10.1046/j.1471-4159.1997.69062405.x. [DOI] [PubMed] [Google Scholar]
- Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez AC, Mayor F., Jr Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proceedings of the National Academy of Sciences of the USA. 1998a;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragay AM, Ruiz-Gomez A, Penela P, Sarnago S, Elorza A, Jimenez-Sainz MC, Mayor F., Jr G protein-coupled receptor kinase 2 (GRK2): mechanisms of regulation and physiological functions. FEBS Letters. 1998b;430:37–40. doi: 10.1016/s0014-5793(98)00495-5. [DOI] [PubMed] [Google Scholar]
- Aramori I, Zhang J, Ferguson SS, Bieniasz PD, Cullen BR, Caron MG. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO Journal. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged μ-opioid receptor expressed in HEK 293 cells. Journal of Neurochemistry. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- Baba T, Damke H, Hinshaw JE, Ikeda K, Schmid SL, Warnock DE. Role of dynamin in clathrin-coated vesicle formation. Cold Spring Harbor Symposia on Quantitative Biology. 1995;60:235–242. doi: 10.1101/sqb.1995.060.01.027. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Gomez J. Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family. Journal of Biological Chemistry. 1993;268:19521–19527. [PubMed] [Google Scholar]
- Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated β-adrenergic receptor by the β-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proceedings of the National Academy of Sciences of the USA. 1987a;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Onorato JJ, Arriza JL, Stone WC, Lohse M, Jenkins NA, Gilbert DJ, Copeland NG, Caron MG, Lefkowitz RJ. Cloning, expression, and chromosomal localization of β-adrenergic receptor kinase 2. A new member of the receptor kinase family. Journal of Biological Chemistry. 1991;266:14939–14946. [PubMed] [Google Scholar]
- Benovic JL, Regan JW, Matsui H, Mayor F, Jr, Cotecchia S, Leeb-Lundberg LM, Caron MG, Lefkowitz RJ. Agonist-dependent phosphorylation of the α2-adrenergic receptor by the β-adrenergic receptor kinase. Journal of Biological Chemistry. 1987b;262:17251–17253. [PubMed] [Google Scholar]
- Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. β-Adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proceedings of the National Academy of Sciences of the USA. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gate. Journal of Biological Chemistry. 1998;273:1269–1272. doi: 10.1074/jbc.273.3.1269. [DOI] [PubMed] [Google Scholar]
- Bogatkewitsch GS, Lenz W, Jakobs KH, Van Koppen CJ. Receptor internalization delays m4 muscarinic acetylcholine receptor resensitization at the plasma membrane. Molecular Pharmacology. 1996;50:424–429. [PubMed] [Google Scholar]
- Brass LF. Homologous desensitization of HEL cell thrombin receptors. Distinguishable roles for proteolysis and phosphorylation. Journal of Biological Chemistry. 1992;267:6044–6050. [PubMed] [Google Scholar]
- Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM. β-Adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation. 1990;82:I12–25. [PubMed] [Google Scholar]
- Bünemann M, Brandts B, Pott L. Downregulation of muscarinic M2 receptors linked to K+ current in cultured guinea-pig atrial myocytes. The Journal of Physiology. 1996;494:351–362. doi: 10.1113/jphysiol.1996.sp021497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Brandts B, Pott L. In vivo downregulation of M2 receptors revealed by measurement of muscarinic K+ current in cultured guinea-pig atrial myocytes. The Journal of Physiology. 1997;501:549–554. doi: 10.1111/j.1469-7793.1997.549bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Hosey MM. Regulators of G protein signaling (RGS) proteins constitutively activate Gβγ-gated potassium channels. Journal of Biological Chemistry. 1998;273:31186–31190. doi: 10.1074/jbc.273.47.31186. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Lee KB, Pals-Rylaarsdam R, Roseberry AG, Hosey MM. Desensitization of G-protein-coupled receptors in the cardiovascular system. Annual Review of Physiology. 1999;61:169–192. doi: 10.1146/annurev.physiol.61.1.169. [DOI] [PubMed] [Google Scholar]
- Carman CV, Som T, Kim CM, Benovic JL. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. Journal of Biological Chemistry. 1998;273:20308–20316. doi: 10.1074/jbc.273.32.20308. [DOI] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca2+-dependent interaction of recoverin with rhodopsin kinase. Journal of Biological Chemistry. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Yu QM, Wu YL, Ma L, Pei G. Selective interference of β-arrestin 1 with κ and δ but not μ opioid receptor/G protein coupling. Journal of Biological Chemistry. 1998;273:24328–24333. doi: 10.1074/jbc.273.38.24328. [DOI] [PubMed] [Google Scholar]
- Chu P, Murray S, Lissin D, von Zastrow M. δ and κ opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. Journal of Biological Chemistry. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- Chuang TT, LeVine H, III, De Blasi A. Phosphorylation and activation of β-adrenergic receptor kinase by protein kinase C. Journal of Biological Chemistry. 1995;270:18660–18665. doi: 10.1074/jbc.270.31.18660. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Saura C, Canela EI, Mallol J, Lluis C, Franco R. Ligand-induced phosphorylation, clustering, and desensitization of A1 adenosine receptors. Molecular Pharmacology. 1997;52:788–797. doi: 10.1124/mol.52.5.788. [DOI] [PubMed] [Google Scholar]
- Cramer H, Muller-Esterl W, Schroeder C. Subtype-specific desensitization of human endothelin ETA and ETB receptors reflects differential receptor phosphorylation. Biochemistry. 1997;36:13325–13332. doi: 10.1021/bi9708848. [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. Journal of Biological Chemistry. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and Gβγ isoform-specific interactions with G protein-coupled receptor kinases. Proceedings of the National Academy of Sciences of the USA. 1997;94:2180–2185. doi: 10.1073/pnas.94.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. Journal of Cell Biology. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV. β-Adrenergic receptor kinase-2 and β-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- Debburman SK, Kunapuli P, Benovic JL, Hosey MM. Agonist-dependent phosphorylation of human muscarinic receptors in Spodoptera frugiperda insect cell membranes by G protein-coupled receptor kinases. Molecular Pharmacology. 1995a;47:224–233. [PubMed] [Google Scholar]
- DebBurman SK, Ptasienski J, Benovic JL, Hosey MM. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein βγ subunits. Journal of Biological Chemistry. 1996;271:22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- DebBurman SK, Ptasienski J, Boetticher E, Lomasney JW, Benovic JL, Hosey MM. Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. Journal of Biological Chemistry. 1995b;270:5742–5747. doi: 10.1074/jbc.270.11.5742. [DOI] [PubMed] [Google Scholar]
- de Weerd WF, Leeb-Lundberg LM. Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Gα subunits Gαq and Gαi in caveolae in DDT1 MF-2 smooth muscle cells. Journal of Biological Chemistry. 1997;272:17858–17866. doi: 10.1074/jbc.272.28.17858. [DOI] [PubMed] [Google Scholar]
- Diviani D, Lattion AL, Cotecchia S. Characterization of the phosphorylation sites involved in G protein-coupled receptor kinase- and protein kinase C-mediated desensitization of the α1B-adrenergic receptor. Journal of Biological Chemistry. 1997;272:28712–28719. doi: 10.1074/jbc.272.45.28712. [DOI] [PubMed] [Google Scholar]
- Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the α1B-adrenergic receptor. Journal of Biological Chemistry. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- Drazner MH, Peppel KC, Dyer S, Grant AO, Koch WJ, Lefkowitz RJ. Potentiation of β-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. Journal of Clinical Investigation. 1997;99:288–296. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Franci C, Gosling J, Tsou CL, Coughlin SR, Charo IF. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. Critical role of carboxyl-tail serines/threonines in receptor function. Journal of Immunology. 1996;157:5606–5612. [PubMed] [Google Scholar]
- Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human β2-adrenergic receptor. Journal of Biological Chemistry. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. Journal of Biological Chemistry. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Progress in Hormone Research. 1996;51:319–351. 352–353. discussion. [PubMed] [Google Scholar]
- Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. Phosphorylation and desensitization of the human β1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. Journal of Biological Chemistry. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- Freeman JL, De La Cruz EM, Pollard TD, Lefkowitz RJ, Pitcher JA. Regulation of G protein-coupled receptor kinase 5 (GRK5) by actin. Journal of Biological Chemistry. 1998;273:20653–20657. doi: 10.1074/jbc.273.32.20653. [DOI] [PubMed] [Google Scholar]
- Gagnon AW, Kallal L, Benovic JL. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the β2-adrenergic receptor. Journal of Biological Chemistry. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. Journal of Biological Chemistry. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Polans AS, Palczewski K, Detwiler PB. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 1993;10:523–531. doi: 10.1016/0896-6273(93)90339-s. [DOI] [PubMed] [Google Scholar]
- Haga K, Kameyama K, Haga T, Kikkawa U, Shiozaki K, Uchiyama H. Phosphorylation of human m1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 and protein kinase C. Journal of Biological Chemistry. 1996;271:2776–2782. doi: 10.1074/jbc.271.5.2776. [DOI] [PubMed] [Google Scholar]
- Haga K, Ogawa H, Haga T, Murofushi H. GTP-binding-protein-coupled receptor kinase 2 (GRK2) binds and phosphorylates tubulin. European Journal of Biochemistry. 1998;255:363–368. doi: 10.1046/j.1432-1327.1998.2550363.x. [DOI] [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. Journal of Biological Chemistry. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. Desensitization of the delta-opioid receptor correlates with its phosphorylation in SK-N-BE cells: involvement of a G protein-coupled receptor kinase. Journal of Neurochemistry. 1998;70:2129–2138. doi: 10.1046/j.1471-4159.1998.70052129.x. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Lohse MJ, Bouvier M, Liggett SB, Caron MG, Lefkowitz RJ. Two kinases mediate agonist-dependent phosphorylation and desensitization of the β2-adrenergic receptor. Symposia of the Society for Experimental Biology. 1990;44:225–240. [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. Journal of Cell Biology. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkin RW, Sanchez-Yague J, Ascoli M. Agonist-induced phosphorylation of the luteinizing hormone/chorionic gonadotropin receptor expressed in a stably transfected cell line. Molecular Endocrinology. 1993;7:823–832. doi: 10.1210/mend.7.7.8413307. [DOI] [PubMed] [Google Scholar]
- Hipkin RW, Wang Z, Ascoli M. Human chorionic gonadotropin (CG)- and phorbol ester-stimulated phosphorylation of the luteinizing hormone/CG receptor maps to serines 635, 639, 649, and 652 in the C-terminal cytoplasmic tail. Molecular Endocrinology. 1995;9:151–158. doi: 10.1210/mend.9.2.7776965. [DOI] [PubMed] [Google Scholar]
- Hsu MH, Chiang SC, Ye RD, Prossnitz ER. Phosphorylation of the N-formyl peptide receptor is required for receptor internalization but not chemotaxis. Journal of Biological Chemistry. 1997;272:29426–29429. doi: 10.1074/jbc.272.47.29426. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Rockman HA, Shotwell KF, Tomhave ED, Koch WJ. Myocardial overexpression of GRK3 in transgenic mice: evidence for in vivo selectivity of GRKs. American Journal of Physiology. 1998a;275:H1298–1306. doi: 10.1152/ajpheart.1998.275.4.H1298. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation. 1998b;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Franchetti R, Masini M, De Blasi A. GRK2 and β-arrestin 1 as negative regulators of thyrotropin receptor-stimulated response. Molecular Endocrinology. 1996;10:1138–1146. doi: 10.1210/mend.10.9.8885248. [DOI] [PubMed] [Google Scholar]
- Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- Inglese J, Luttrell LM, Iniguez-Lluhi JA, Touhara K, Koch WJ, Lefkowitz RJ. Functionally active targeting domain of the β-adrenergic receptor kinase: an inhibitor of Gβγ-mediated stimulation of type II adenylyl cyclase. Proceedings of the National Academy of Sciences of the USA. 1994;91:3637–3641. doi: 10.1073/pnas.91.9.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi H, Birnbaumer M. Transient phosphorylation of the V1a vasopressin receptor. Journal of Biological Chemistry. 1998a;273:7155–7161. doi: 10.1074/jbc.273.12.7155. [DOI] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi H, Eberle AN, Birnbaumer M. Phosphorylation of the V2 vasopressin receptor. Journal of Biological Chemistry. 1997;272:2486–2492. doi: 10.1074/jbc.272.4.2486. [DOI] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi HM, Tran NT, Birnbaumer M. A serine cluster prevents recycling of the V2 vasopressin receptor. Proceedings of the National Academy of Sciences of the USA. 1998b;95:2222–2226. doi: 10.1073/pnas.95.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii I, Saito E, Izumi T, Ui M, Shimizu T. Agonist-induced sequestration, recycling, and resensitization of platelet-activating factor receptor. Role of cytoplasmic tail phosphorylation in each process. Journal of Biological Chemistry. 1998;273:9878–9885. doi: 10.1074/jbc.273.16.9878. [DOI] [PubMed] [Google Scholar]
- Ishii K, Chen J, Ishii M, Koch WJ, Freedman NJ, Lefkowitz RJ, Coughlin SR. Inhibition of thrombin receptor signaling by a G-protein coupled receptor kinase. Functional specificity among G-protein coupled receptor kinases. Journal of Biological Chemistry. 1994;269:1125–1130. [PubMed] [Google Scholar]
- Ishizaka N, Alexander RW, Laursen JB, Kai H, Fukui T, Oppermann M, Lefkowitz RJ, Lyons PR, Griendling KK. G protein-coupled receptor kinase 5 in cultured vascular smooth muscle cells and rat aorta. Regulation by angiotensin II and hypertension. Journal of Biological Chemistry. 1997;272:32482–32488. doi: 10.1074/jbc.272.51.32482. [DOI] [PubMed] [Google Scholar]
- Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proceedings of the National Academy of Sciences of the USA. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January B, Seibold A, Whaley B, Hipkin RW, Lin D, Schonbrunn A, Barber R, Clark RB. β2-Adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. Journal of Biological Chemistry. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- Khani SC, Nielsen L, Vogt TM. Biochemical evidence for pathogenicity of rhodopsin kinase mutations correlated with the Oguchi form of congenital stationary night blindness. Proceedings of the National Academy of Sciences of the USA. 1998;95:2824–2827. doi: 10.1073/pnas.95.6.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the βγ subunits of heterotrimeric G proteins on the β-adrenergic receptor kinase. Journal of Biological Chemistry. 1993;268:8256–8260. [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Kong G, Penn R, Benovic JL. A β-adrenergic receptor kinase dominant negative mutant attenuates desensitization of the β2-adrenergic receptor. Journal of Biological Chemistry. 1994;269:13084–13087. [PubMed] [Google Scholar]
- Kovoor A, Celver JP, Wu A, Chavkin C. Agonist induced homologous desensitization of μ-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Molecular Pharmacology. 1998;54:704–711. [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. Journal of Biological Chemistry. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annual Review of Pharmacology and Toxicology. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of β-arrestin dominant-negative mutants. Journal of Biological Chemistry. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- Kuhn H. Light-dependent phosphorylation of rhodopsin in living frogs. Nature. 1974;250:588–590. doi: 10.1038/250588a0. [DOI] [PubMed] [Google Scholar]
- Kunapuli P, Gurevich VV, Benovic JL. Phospholipid-stimulated autophosphorylation activates the G protein-coupled receptor kinase GRK5. Journal of Biological Chemistry. 1994a;269:10209–10212. [PubMed] [Google Scholar]
- Kunapuli P, Onorato JJ, Hosey MM, Benovic JL. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK5. Journal of Biological Chemistry. 1994b;269:1099–1105. [PubMed] [Google Scholar]
- Kwatra MM, Benovic JL, Caron MG, Lefkowitz RJ, Hosey MM. Phosphorylation of chick heart muscarinic cholinergic receptors by the β-adrenergic receptor kinase. Biochemistry. 1989a;28:4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra MM, Hosey MM. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. Journal of Biological Chemistry. 1986;261:12429–12432. [PubMed] [Google Scholar]
- Kwatra MM, Leung E, Maan AC, McMahon KK, Ptasienski J, Green RD, Hosey MM. Correlation of agonist-induced phosphorylation of chick heart muscarinic receptors with receptor desensitization. Journal of Biological Chemistry. 1987;262:16314–16321. [PubMed] [Google Scholar]
- Kwatra MM, Ptasienski J, Hosey MM. The porcine heart M2 muscarinic receptor: agonist-induced phosphorylation and comparison of properties with the chick heart receptor. Molecular Pharmacology. 1989b;35:553–558. [PubMed] [Google Scholar]
- Kwatra MM, Schwinn DA, Schreurs J, Blank JL, Kim CM, Benovic JL, Krause JE, Caron MG, Lefkowitz RJ. The substance P receptor, which couples to Gq/11, is a substrate of β-adrenergic receptor kinase 1 and 2. Journal of Biological Chemistry. 1993;268:9161–9164. [PubMed] [Google Scholar]
- Lattion AL, Diviani D, Cotecchia S. Truncation of the receptor carboxyl terminus impairs agonist-dependent phosphorylation and desensitization of the α1B-adrenergic receptor. Journal of Biological Chemistry. 1994;269:22887–22893. [PubMed] [Google Scholar]
- Lazari MF, Bertrand JE, Nakamura K, Liu X, Krupnick JG, Benovic JL, Ascoli M. Mutation of individual serine residues in the C-terminal tail of the lutropin/choriogonadotropin receptor reveal distinct structural requirements for agonist-induced uncoupling and agonist-induced internalization. Journal of Biological Chemistry. 1998;273:18316–18324. doi: 10.1074/jbc.273.29.18316. [DOI] [PubMed] [Google Scholar]
- Lee KB, Pals-Rylaarsdam R, Benovic JL, Hosey MM. Arrestin-independent internalization of the m1, m3, and m4 subtypes of muscarinic cholinergic receptors. Journal of Biological Chemistry. 1998;273:12967–12972. doi: 10.1074/jbc.273.21.12967. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. Journal of Biological Chemistry. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Leurs R, Smit MJ, Alewijnse AE, Timmerman H. Agonist-independent regulation of constitutively active G-protein- coupled receptors. Trends in Biochemical Sciences. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- Levay K, Satpaev DK, Pronin AN, Benovic JL, Slepak VZ. Localization of the sites for Ca2+-binding proteins on G protein-coupled receptor kinases. Biochemistry. 1998;37:13650–13659. doi: 10.1021/bi980998z. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Ostrowski J, Chesnut LC, Kurose H, Raymond JR, Caron MG, Lefkowitz RJ. Sites in the third intracellular loop of the α2A-adrenergic receptor confer short term agonist-promoted desensitization. Evidence for a receptor kinase-mediated mechanism. Journal of Biological Chemistry. 1992;267:4740–4746. [PubMed] [Google Scholar]
- Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of β-arrestin and arrestin in the β2-adrenergic receptor and rhodopsin systems. Journal of Biological Chemistry. 1992;267:8558–8564. [PubMed] [Google Scholar]
- Loudon RP, Benovic JL. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK6. Journal of Biological Chemistry. 1994;269:22691–22697. [PubMed] [Google Scholar]
- Loudon RP, Benovic JL. Altered activity of palmitoylation-deficient and isoprenylated forms of the G protein-coupled receptor kinase GRK6. Journal of Biological Chemistry. 1997;272:27422–27427. doi: 10.1074/jbc.272.43.27422. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Gαq/11, G-protein receptor kinase-2/3, and β-arrestin-1/2. Molecular Biology of the Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Barak LS, Bertrand L, Premont RT, Colapietro AM, Lefkowitz RJ, Caron MG. Members of the G protein-coupled receptor kinase family that phosphorylate the β2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35:4155–4160. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Benovic JL, Kelly E. A dominant negative mutant of the G protein-coupled receptor kinase 2 selectively attenuates adenosine A2 receptor desensitization. Molecular Pharmacology. 1997;51:991–998. doi: 10.1124/mol.51.6.991. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Luty JS, Willets J, Benovic JL, Kelly E. Enhanced expression of G protein-coupled receptor kinase 2 selectively increases the sensitivity of A2A adenosine receptors to agonist-induced desensitization. British Journal of Pharmacology. 1998;125:347–356. doi: 10.1038/sj.bjp.0702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga C, Penela P, Zafra F, Mayor F., Jr The subcellular and cellular distribution of G protein-coupled receptor kinase 2 in rat brain. Neuroscience. 1998;87:631–637. doi: 10.1016/s0306-4522(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Murray SR, Evans CJ, von Zastrow M. Phosphorylation is not required for dynamin-dependent endocytosis of a truncated mutant opioid receptor. Journal of Biological Chemistry. 1998;273:24987–24991. doi: 10.1074/jbc.273.39.24987. [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Tanaka K, Namba H, Yamashita S, Niwa M. Expression and regulation of G protein-coupled receptor kinase 5 and β-arrestin-1 in rat thyroid FRTL5 cells. Thyroid. 1996;6:627–631. doi: 10.1089/thy.1996.6.627. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Krupnick JG, Benovic JL, Ascoli M. Signaling and phosphorylation-impaired mutants of the rat follitropin receptor reveal an activation- and phosphorylation-independent but arrestin-dependent pathway for internalization. Journal of Biological Chemistry. 1998;273:24346–24354. doi: 10.1074/jbc.273.38.24346. [DOI] [PubMed] [Google Scholar]
- Neill JD, Duck LW, Musgrove LC, Sellers JC. Potential regulatory roles for G protein-coupled receptor kinases and β-arrestins in gonadotropin-releasing hormone receptor signaling. Endocrinology. 1998;139:1781–1788. doi: 10.1210/endo.139.4.5868. [DOI] [PubMed] [Google Scholar]
- Ng GY, Mouillac B, George SR, Caron M, Dennis M, Bouvier M, O'Dowd BF. Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. European Journal of Pharmacolgy. 1994;267:7–19. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Warabi K, Roush ED, Frederick J, Schwinn DA, Kwatra MM. Characterization of GRK2-catalyzed phosphorylation of the human substance P receptor in Sf9 membranes. Biochemistry. 1998;37:1192–1198. doi: 10.1021/bi972302s. [DOI] [PubMed] [Google Scholar]
- Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. Journal of Cell Biology. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato JJ, Gillis ME, Liu Y, Benovic JL, Ruoho AE. The β-adrenergic receptor kinase (GRK2) is regulated by phospholipids. Journal of Biological Chemistry. 1995;270:21346–21353. doi: 10.1074/jbc.270.36.21346. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ. Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. Journal of Biological Chemistry. 1996;271:13266–13272. doi: 10.1074/jbc.271.22.13266. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc AE, Fariss RN, Van Hooser JP, Palczewski K. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilized by α-toxin. Proceedings of the National Academy of Sciences of the USA. 1998;95:15014–15019. doi: 10.1073/pnas.95.25.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. GTP-binding-protein-coupled receptor kinases - two mechanistic models. European Journal of Biochemistry. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Benovic JL, Stiles GL. Agonist-dependent phosphorylation and desensitization of the rat A3 adenosine receptor. Evidence for a G-protein-coupled receptor kinase-mediated mechanism. Journal of Biological Chemistry. 1995;270:29607–29613. doi: 10.1074/jbc.270.49.29607. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. Identification of an A2a adenosine receptor domain specifically responsible for mediating short-term desensitization. Biochemistry. 1997;36:832–838. doi: 10.1021/bi962290v. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Gurevich VV, Lee KB, Ptasienski JA, Benovic JL, Hosey MM. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. Journal of Biological Chemistry. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Hosey MM. Two homologous phosphorylation domains differentially contribute to desensitization and internalization of the m2 muscarinic acetylcholine receptor. Journal of Biological Chemistry. 1997;272:14152–14158. doi: 10.1074/jbc.272.22.14152. [DOI] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Xu Y, Witt-Enderby P, Benovic JL, Hosey MM. Desensitization and internalization of the m2 muscarinic acetylcholine receptor are directed by independent mechanisms. Journal of Biological Chemistry. 1995;270:29004–29011. doi: 10.1074/jbc.270.48.29004. [DOI] [PubMed] [Google Scholar]
- Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse δ-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. Molecular Pharmacology. 1995;48:173–177. [PubMed] [Google Scholar]
- Pei G, Tiberi M, Caron MG, Lefkowitz RJ. An approach to the study of G-protein-coupled receptor kinases: an in vitro-purified membrane assay reveals differential receptor specificity and regulation by Gβγ subunits. Proceedings of the National Academy of Sciences of the USA. 1994;91:3633–3636. doi: 10.1073/pnas.91.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. Journal of Biological Chemistry. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, Lefkowitz RJ. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. Journal of Biological Chemistry. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annual Review of Biochemistry. 1998a;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. Journal of Biological Chemistry. 1998b;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ. The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proceedings of the National Academy of Sciences of the USA. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JLR, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proceedings of the National Academy of Sciences of the USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB Journal. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. Journal of Biological Chemistry. 1996;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- Pronin AN, Benovic JL. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. Journal of Biological Chemistry. 1997;272:3806–3812. doi: 10.1074/jbc.272.6.3806. [DOI] [PubMed] [Google Scholar]
- Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. Journal of Biological Chemistry. 1997;272:18273–18280. doi: 10.1074/jbc.272.29.18273. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER. Desensitization of N-formylpeptide receptor-mediated activation is dependent upon receptor phosphorylation. Journal of Biological Chemistry. 1997;272:15213–15219. doi: 10.1074/jbc.272.24.15213. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Kim CM, Benovic JL, Ye RD. Phosphorylation of the N-formyl peptide receptor carboxyl terminus by the G protein-coupled receptor kinase, GRK2. Journal of Biological Chemistry. 1995;270:1130–1137. doi: 10.1074/jbc.270.3.1130. [DOI] [PubMed] [Google Scholar]
- Quintana J, Hipkin RW, Sanchez-Yague J, Ascoli M. Follitropin (FSH) and a phorbol ester stimulate the phosphorylation of the FSH receptor in intact cells. Journal of Biological Chemistry. 1994;269:8772–8779. [PubMed] [Google Scholar]
- Ramkumar V, Kwatra M, Benovic JL, Stiles GL, Stilesa GL. Functional consequences of A1 adenosine-receptor phosphorylation by the β-adrenergic receptor kinase. Biochimica et Biophysica Acta. 1993;1179:89–97. doi: 10.1016/0167-4889(93)90075-z. with erratum in Biochimica et Biophysica Acta, 1220, 229. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Hosey MM. Agonist-induced phosphorylation and desensitization of human m2 muscarinic cholinergic receptors in Sf9 insect cells. Journal of Biological Chemistry. 1992;267:22249–22255. [PubMed] [Google Scholar]
- Richardson RM, Kim C, Benovic JL, Hosey MM. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the β-adrenergic receptor kinase. Journal of Biological Chemistry. 1993;268:13650–13656. [PubMed] [Google Scholar]
- Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proceedings of the National Academy of Sciences of the USA. 1998a;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. Journal of Biological Chemistry. 1998b;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proceedings of the National Academy of Sciences of the USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth NS, Campbell PT, Caron MG, Lefkowitz RJ, Lohse MJ. Comparative rates of desensitization of β-adrenergic receptors by the β-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1991;88:6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlador ML, Nathanson NM. Synergistic regulation of m2 muscarinic acetylcholine receptor desensitization and sequestration by G protein-coupled receptor kinase-2 and β-arrestin-1. Journal of Biological Chemistry. 1997;272:18882–18890. doi: 10.1074/jbc.272.30.18882. [DOI] [PubMed] [Google Scholar]
- Schleicher S, Boekhoff I, Arriza J, Lefkowitz RJ, Breer H. A β-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proceedings of the National Academy of Sciences of the USA. 1993;90:1420–1424. doi: 10.1073/pnas.90.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui Z, Boyett MR, Zang WJ, Haga T, Kameyama K. Receptor kinase-dependent desensitization of the muscarinic K+ current in rat atrial cells. The Journal of Physiology. 1995;487:359–366. doi: 10.1113/jphysiol.1995.sp020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui Z, Khan IA, Tsuga H, Haga T, Boyett MR. Role of receptor kinase in short-term desensitization of cardiac muscarinic K+ channels expressed in Chinese hamster ovary cells. The Journal of Physiology. 1998;507:325–334. doi: 10.1111/j.1469-7793.1998.325bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ. Phosphorylation/dephosphorylation of the β-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proceedings of the National Academy of Sciences of the USA. 1986;83:9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RH, Sibley DR, Lefkowitz RJ. A novel catecholamine-activated adenosine cyclic 3′,5′-phosphate independent pathway for β-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986;25:1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- Takano T, Honda Z, Sakanaka C, Izumi T, Kameyama K, Haga K, Haga T, Kurokawa K, Shimizu T. Role of cytoplasmic tail phosphorylation sites of platelet-activating factor receptor in agonist-induced desensitization. Journal of Biological Chemistry. 1994;269:22453–22458. [PubMed] [Google Scholar]
- Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. Journal of Biological Chemistry. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- Tobin AB, Willars GB, Burford NT, Nahorski SR. Relationship between agonist binding, phosphorylation and immunoprecipitation of the m3-muscarinic receptor, and second messenger responses. British Journal of Pharmacology. 1995;116:1723–1728. doi: 10.1111/j.1476-5381.1995.tb16654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T. Desensitization of human muscarinic acetylcholine receptor m2 subtypes is caused by their sequestration/internalization. Journal of Biochemistry. 1998a;124:863–868. doi: 10.1093/oxfordjournals.jbchem.a022191. [DOI] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T, Honma T, Lameh J, Sadee W. Internalization and downregulation of human muscarinic acetylcholine receptor m2 subtypes. Role of third intracellular m2 loop and G protein-coupled receptor kinase 2. Journal of Biological Chemistry. 1998b;273:5323–5330. doi: 10.1074/jbc.273.9.5323. [DOI] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T, Kurose H, Nagao T. Sequestration of muscarinic acetylcholine receptor m2 subtypes. Facilitation by G protein-coupled receptor kinase (GRK2) and attenuation by a dominant-negative mutant of GRK2. Journal of Biological Chemistry. 1994;269:32522–32527. [PubMed] [Google Scholar]
- Tsuga H, Okuno E, Kameyama K, Haga T. Sequestration of human muscarinic acetylcholine receptor hm1-hm5 subtypes: effect of G protein-coupled receptor kinases GRK2, GRK4, GRK5 and GRK6. Journal of Pharmacology and Experimental Therapeutics. 1998c;284:1218–1226. [PubMed] [Google Scholar]
- Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of β-adrenergic receptor kinase during myocardial ischemia. Circulation Research. 1996;79:455–460. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circulation Research. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proceedings of the National Academy of Sciences of the USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. Journal of Cell Biology. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. Journal of Cell Biology. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virlon B, Firsov D, Cheval L, Reiter E, Troispoux C, Guillou F, Elalouf JM. Rat G protein-coupled receptor kinase GRK4: identification, functional expression, and differential tissue distribution of two splice variants. Endocrinology. 1998;139:2784–2795. doi: 10.1210/endo.139.6.6078. [DOI] [PubMed] [Google Scholar]
- Vogler O, Bogatkewitsch GS, Wriske C, Krummenerl P, Jakobs KH, van Koppen CJ. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. Journal of Biological Chemistry. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Auberger P, Pouyssegur J, Van Obberghen-Schilling E. Distinct mechanisms regulate 5-HT2 and thrombin receptor desensitization. Journal of Biological Chemistry. 1995;270:4813–4821. doi: 10.1074/jbc.270.9.4813. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu X, Ascoli M. Phosphorylation of the lutropin/choriogonadotropin receptor facilitates uncoupling of the receptor from adenylyl cyclase and endocytosis of the bound hormone. Molecular Endocrinology. 1997;11:183–192. doi: 10.1210/mend.11.2.9889. [DOI] [PubMed] [Google Scholar]
- Weller M, Virmaux N, Mandel P. Light-stimulated phosphorylation of rhodopsin in the retina: the presence of a protein kinase that is specific for photobleached rhodopsin. Proceedings of the National Academy of Sciences of the USA. 1975;72:381–385. doi: 10.1073/pnas.72.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by β-arrestin. Proceedings of the National Academy of Sciences of the USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacological Reviews. 1998;50:723–760. [PubMed] [Google Scholar]
- Yamamoto S, Sippel KC, Berson EL, Dryja TP. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nature Genetics. 1997;15:175–178. doi: 10.1038/ng0297-175. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB. μ-Opioid receptor phosphorylation, desensitization, and ligand efficacy. Journal of Biological Chemistry. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Current Biology. 1998;8:R313–316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for β-arrestins and clathrin-coated vesicle-mediated endocytosis in β2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. Journal of Biological Chemistry. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proceedings of the National Academy of Sciences of the USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SSG, Barak LS, Menard L, Caron MG. Dynamin and β-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. Journal of Biological Chemistry. 1996a;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. Differential μ opiate receptor phosphorylation and desensitization induced by agonists and phorbol esters. Journal of Biological Chemistry. 1996b;271:11449–11454. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- Zhao X, Huang J, Khani SC, Palczewski K. Molecular forms of human rhodopsin kinase (GRK1) Journal of Biological Chemistry. 1998;273:5124–5131. doi: 10.1074/jbc.273.9.5124. [DOI] [PubMed] [Google Scholar]
- Zhao X, Palczewski K, Ohguro H. Mechanism of rhodopsin phosphorylation. Biophysical Chemistry. 1995;56:183–188. doi: 10.1016/0301-4622(95)00031-r. [DOI] [PubMed] [Google Scholar]


