Abstract
Using whole-cell patch-clamp recording techniques, we have examined voltage-gated ion currents in a cultured human intestinal smooth muscle cell line (HISM). Experiments were performed at room temperature on cells after passages 16 and 17.
Two major components of the whole-cell current were a tetraethylammonium-sensitive (IC50 = 9 mm), iberiotoxin-resistant, delayed rectifier K+ current and a Na+ current inhibited by tetrodotoxin (IC50 = 100 nm). No measurable inward current via voltage-gated Ca2+ channels could be detected in these cells even with 10 mm Ca2+ or Ba2+ in the external solution. No current attributable to calcium-activated K+ channels was found and no cationic current in response to muscarinic receptor activation was present.
In divalent cation-free external solution two additional currents were activated: an inwardly rectifying hyperpolarization-activated current, IHA, and a depolarization-activated current, IDA.
IHA and IDA could be carried by several monovalent cations; the sizes of currents in descending order were: K+ > Cs+ > Na+ for IHA and Na+ > K+ >> Cs+ for IDA. IHA was activated and deactivated instantaneously and showed no inactivation whereas IDA was activated, inactivated and deactivated within tens of milliseconds. These currents were inhibited by external calcium with an IC50 of 0.3 μm for IDA and an IC50 of 20 μm for IHA.
Cyclopiazonic acid (CPA) induced an outward, but not an inward current. SK&F 96365, a blocker of store-operated Ca2+ channels, suppressed IDA with a half-maximal inhibitory concentration of 9 μm but was ineffective in inhibiting IHA at concentrations up to 100 μm. Gd3+ and La3+ strongly suppressed IDA at 1 and 10 μm, respectively and were less effective in blocking IHA (complete inhibition required a concentration of 100 μm for both). Carbachol at 10–100 μm evoked about a 3-fold increase in IHA amplitude and completely abolished IDA.
We conclude that IHA and IDA are Ca2+-blockable cationic currents with different ion selectivity profiles that are carried by different channels. IDA shows novel voltage-dependent properties for a cationic current.
It is well known that several distinct cellular phenotypes may exist during normal smooth muscle ontogenesis; in contrast to skeletal and cardiac muscle cells, which exhibit much more restricted cellular plasticity, mature smooth muscle cells can readily dedifferentiate from the contractile phenotypic state to a non-contractile (highly proliferative, migratory and synthetic) phenotype where synthesis of a connective tissue matrix and proliferation occur (for reviews see Chamley-Campbell et al. 1979; Owens, 1995), thus reverting to a more primitive phenotype in culture. This process involves considerable morphological, electrophysiological and biochemical changes and is thought to be crucial for normal smooth muscle myogenesis as well as for pathogenesis. It has recently been shown that cultured gastrointestinal smooth muscle cells also may retain distinct molecular phenotypes which, depending on the initial cell density, substrate specificity and serum supplementation, have been suggested to be identical to those in vivo (Brittingham et al. 1998). It thus appears that cultured cells recapitulate a significant part of in vivo smooth muscle ontogenesis. Substantial electrophysiological changes possibly relevant to the proliferation process have also been described (e.g. Snetkov et al. 1996).
In the present study we examined the properties of voltage-dependent inward and outward currents in cultured human intestinal smooth muscle (HISM) cells (American Type Culture Collection, ATCC no. 1692-CRL). This established cell line is widely used in biochemical and molecular biology research but data concerning the electrophysiological properties of HISM cells are scarce. The major inward current, which we identified as a ‘classical’ fast, TTX-sensitive Na+ current with properties similar to those of cells freshly dispersed from the adult human colon (Xiong et al. 1993), was previously erroneously referred to as Ca2+ current via L-type channels despite its very rapid inactivation kinetics (Bielefeldt et al. 1996). Also, we found two distinct Ca2+-blockable inward cationic currents to be present in these cells, which could be elicited in divalent cation-free external solution. One of these, IHA, was further potentiated by the muscarinic agonist carbachol via muscarinic receptor/G-protein activation since the effect was blocked by atropine or intracellular GDP-βS application. The other current, which we termed IDA, showed a pronounced U-shaped I-V relationship at negative potentials with a peak at about −30 mV. However, in contrast to the muscarinic cationic current widely present in differentiated gastrointestinal smooth muscles, IDA also showed pronounced inactivation. These two currents also had distinct pharmacological profiles.
We could not identify in HISM cells a muscarinic cationic current with a typical U-shaped voltage dependence as seen in all studied gastrointestinal smooth muscle cells from various non-human mammalian species (e.g. Benham et al. 1985; Inoue & Isenberg, 1990; Zholos & Bolton, 1994). From the above considerations it remains to be established whether such a current, although normally expressed in mature human smooth muscle cells, is lost during the dedifferentiation process in culture. Arresting cell growth by using serum-free conditions for up to 19 days also failed to induce such a current in HISM cells.
METHODS
Cell culture
HISM cells (ATCC no. 1692-CRL) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (Gibco-BRL), supplemented with 10 % fetal calf serum (FCS), 100 u ml−1 penicillin (Gibco-BRL) and 100 u ml−1 streptomycin. For electrophysiology, cells were treated with 0.25 % trypsin (Gibco-BRL) for 1 min at 37°C, collected by centrifugation, resuspended in fresh medium, plated on glass coverslips and left to attach for about 10 min. Cells were used after passages 16 and 17.
Electrical recordings and data analysis
Whole-cell membrane current was recorded at room temperature using low-resistance borosilicate patch pipettes (1-3 MΩ) and an Axopatch 200A (Axon Instruments Inc.) voltage-clamp amplifier. Voltage-clamp pulses were generated and data were captured using a Digidata 1200 interfaced to a computer running the pCLAMP 6 program (Axon Instruments Inc.). Series resistance was compensated (usually by about 80 %) to produce the fastest transient without oscillations. For sodium current (INa) recordings the signal was filtered (4-pole low-pass Bessel filter) at 5 kHz and sampled at 40 kHz; for cationic current these settings were 1 and 5 kHz, respectively. No leak correction was used in either case. Data were analysed and plotted using MicroCal Origin version 5.0 software (MicroCal Software, Inc., Northampton, MA, USA). Values are given as means ± s.e.m.
Solutions
The basic external physiological salt solution (PSS) used to maintain the cells before giga-seal formation consisted of (mm): NaCl 120, KCl 6, CaCl2 2.5, MgCl2 1.2, glucose 12 and Hepes 10, pH adjusted to 7.4 with NaOH. For INa recordings KCl was replaced by NaCl (total Na+ concentration, 130 mm) and MgCl2 was removed. Cationic currents were measured in solutions to which CaCl2 was not added. In ion substitution experiments NaCl was replaced with equimolar KCl, CsCl or N-methyl-d-glucamine chloride (NMDG-Cl; pH adjusted with KOH, CsOH or HCl, respectively). To study the dependency of the cationic current on [Ca2+]o, extracellular calcium was strongly buffered to 36 and 487 nm using Ca2+/EGTA mixtures (4 mm/10 mm and 9 mm/10 mm, respectively). For higher concentrations, 250 μm or 1 mm CaCl2 was added to nominally divalent cation-free solution.
Complete exchange of the external solution was achieved within about 1 s as described previously (Zholos & Bolton, 1995).
Pipettes were filled with the following solution (mm): CsCl 80, MgATP 1, creatine 5, glucose 20, Hepes 10, BAPTA 10 and CaCl2 4.6 ([Ca2+]i = 100 nm), pH adjusted to 7.4 with CsOH (total Cs+ concentration, 124 mm). GTP (1 mm) was added in some experiments where the effects of carbachol were studied. NaCl (10 mm) was added in experiments where INa was measured to define the sodium equilibrium potential (ENa). In experiments to measure K+ currents, the pipette solution contained (mm): KCl 130, MgATP 1, creatine 5, glucose 20, Hepes 10 and EGTA 0.05, pH adjusted to 7.4 with NaOH.
Chemicals used
Tetrodotoxin (TTX), tetraethylammonium chloride (TEA-Cl), adenosine 5′-triphosphate (ATP, magnesium salt), guanosine 5′-triphosphate (GTP, sodium salt), guanosine-5′-O-(2-thiodiphosphate (GDPβS, trilithium salt), cyclopiazonic acid (CPA), creatine, Hepes, BAPTA, EGTA, NMDG-Cl, carbamylcholine chloride (carbachol) and atropine were obtained from Sigma Chemical Co. SK&F 96365 was obtained from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, USA). All other chemicals were from BDH Laboratory Supplies (AnalaR grade; Poole, Dorset, UK).
RESULTS
Whole-cell membrane currents
Experiments were performed on 122 human cultured intestinal cells from passages 16-17. In 11 cells studied using basic external PSS and K+-based, low-EGTA pipette solution, voltage pulses applied from either −50 or −100 mV evoked time-independent currents the size of which was a linear function of the test potential between −90 and about 0 mV. These presumably ‘leak’ currents indicated an input resistance of about 3 GΩ. At more positive potentials an outward current was activated and reached a peak in about 20-100 ms in the voltage range 0-90 mV, respectively (Fig. 1). The current was reversibly inhibited by TEA+ with an IC50 of 9.0 ± 0.1 mm(n = 3) and it was also abolished when K+ was replaced by Cs+ in the pipette solution. Thus, the current could be tentatively identified as a delayed rectifier K+ current. No spontaneous transient outward current (STOC)-like activity (Bolton et al. 1999), characteristic of smooth muscle cells of various origins, was seen in HISM cells. Moreover, caffeine (10 mm) or carbachol (50 μm) application even at depolarized or positive potentials (e.g. +10 mV) did not activate any outward currents. Since ryanodine- and carbachol-sensitive intracellular Ca2+ stores are functional in these cells (Bielefeldt et al. 1997), these results suggest the absence or a very low expression level of Ca2+-activated K+ channels. Furthermore, iberiotoxin (IbTX), a potent and selective inhibitor of large conductance Ca2+-activated K+ (BKCa) channels, had no effect on IK (Fig. 1, bottom left). In four cells IK amplitude at +100 mV in the presence of 300 nm IbTX was 94 ± 10 % of control (P < 0.05).
Figure 1. Whole-cell membrane currents in HISM cells under quasiphysiological conditions.
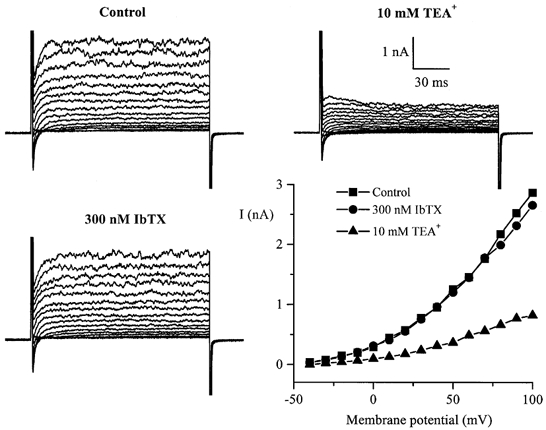
Families of superimposed whole-cell currents in a single cultured HISM cell evoked by 150 ms voltage pulses applied at 10 s intervals from a holding potential of −100 mV to test potentials between −40 and +100 mV with a 10 mV increment in control and in the presence of 10 mm TEA+ or 300 nm IbTX. I-V relationships were constructed by measuring the peak amplitude of the outward current at each test potential. High-K+ pipette solution and normal PSS in the bath were used.
The outward current evoked from a holding potential of −50 mV was not affected by shifting the holding potential to −100 mV. However, an initial fast transient inward current appeared. To isolate and characterize this current in the experiments described below, K+ in the pipette and external solutions was replaced by Cs+ and Na+, respectively.
TTX-sensitive Na+ current
An inward current with rapid activation/inactivation kinetics was seen in all cells studied. However, its maximal amplitude greatly varied (from −670 to −5315 pA at +10 mV from a holding potential of −100 mV) with no obvious relation to cell size. The mean value was 2.3 ± 0.2 nA (n = 24), which corresponded to a current density of 20 ± 2 pA pF−1. The cell membrane capacitance was on average 127 ± 8 pF (n = 67).
Replacing external Na+ with Cs+ completely abolished the inward current even though either 10 mm Ca2+ or 10 mm Ba2+ was present in the external solution (Fig. 2A and B). The current was also abolished by replacing Na+ with K+ or NMDG+ (not shown).
Figure 2. Inward currents in a single cultured HISM cell.
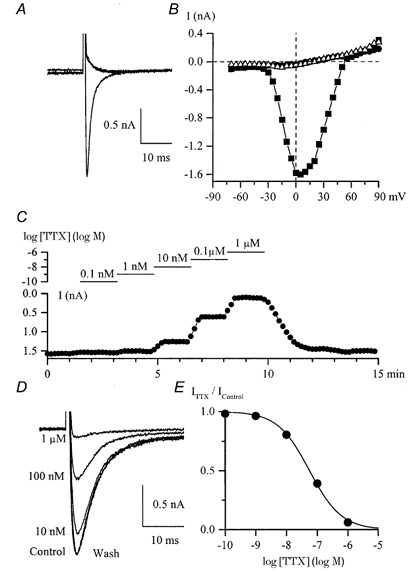
A, superimposed current traces elicited by depolarizing pulses to 0 mV from −100 mV in control (135 mm Na+, 2.5 mm Ca2+) and after replacement of Na+ by Cs+ (125 mm) with 10 mm CaCl2 or BaCl2 added to the external solution. B, I-V relationships for the peak inward current in control (bull) and high-Cs+ external solution with 10 mm Ca2+ (•) or 10 mm Ba2+ (▵) in the same cell. C, peak inward Na+ current was measured at +10 mV by applying voltage steps from −100 mV every 10 s upon cumulative application of ascending concentrations of TTX as shown by the horizontal lines on the logarithmic concentration scale. External solutions contained Na+ and Ca2+ but not Mg2+. D, examples of INa from the same experiment. The concentrations of TTX applied are indicated. E, concentration-effect curve for the experiment illustrated in C and D. Data points represent relative current (ITTX/IControl) fitted by the logistic function with an IC50 value of 56 nm. ITTX is the current in TTX.
There are two major classes of voltage-dependent Na+ channel, TTX-sensitive and TTX-resistant channels. TTX was added to the external solution in a cumulative manner at concentrations ranging from 0.1 nm to 1 μm while INa was measured at 10 s intervals (Fig. 2C). TTX at 1 nm slightly reduced the current; at 1 μm the current was inhibited almost completely (superimposed current traces in Fig. 2D). The IC50 value was 56 nm (Fig. 2E; mean 96 ± 21 nm, n = 3).
Superimposed TTX-sensitive currents obtained by subtracting current traces in the presence of 1 μm TTX from those in control are shown in Fig. 3A. Voltage pulses were applied from −100 mV to test potentials ranging from −70 to +90 mV in 5 mV increments. Measurable inward current was activated at about −30 mV, reached a peak value at +10 mV and reversed at +68 mV, very close to the ENa of +65 mV ([Na+]i was 10 mm; Fig. 3B). The activation and inactivation kinetics accelerated with membrane depolarization. Thus, the time to peak exponentially decreased from 12 to 2 ms in the range −30 to +30 mV. The decay time constant decreased from about 10 to 2.5 ms at potentials from −10 to +30 mV, respectively. At more negative potentials where the current was too small for reliable fitting or could not be measured directly, a conventional double-pulse protocol revealed an inactivation process with time constants in the range 25-14 ms at potentials from −50 to −20 mV, respectively (not shown). Overall the time constant decreased e-fold with depolarization by 37 mV.
Figure 3. Voltage-dependent properties of TTX-sensitive inward Na+ current in external solution with Ca2+ but no Mg2+.
A, superimposed current traces obtained by paired subtraction of currents after 1 μm TTX application from those in control. The holding potential was −100 mV; 30 ms voltage steps were applied to test potentials ranging from −70 to +5 mV (left) and from +10 to +90 mV (right) in 5 mV increments. B, I-V relationship for the peak INa shown in A. C, activation curve obtained by dividing the peak current amplitude by the driving force at each test potential (E – ENa, where ENa = +65 mV) and fitted by a Boltzmann function with the following best fit parameters: maximal conductance, Gmax = 34 nS; potential of half-maximal activation, V1/2 = 0 mV; slope factor, k = −7.3 mV. D, superimposed current traces elicited by 25 ms voltage steps to +10 mV applied from various holding potentials (−120 to −15 mV in 5 mV increments). E, steady-state inactivation of INa for the experiment illustrated in D. Data points were fitted by a Boltzmann function with a potential of half-maximal inactivation of −57.4 mV and a slope factor of 9.8 mV.
Peak sodium conductance was estimated by dividing the peak current amplitude by the driving force (E – ENa, where E is the membrane potential) at each test potential (Fig. 3C). The half-activation potential (V1/2) was found to be −3.2 ± 1.4 mV and the slope factor (k) was −6.7 ± 1.0 mV (n = 19). The activation kinetics of the Na+ current was not analysed, but assuming an m3 process (e.g. G/Gmax = m3, where Gmax is the maximal conductance and the activation parameter m obeys the Boltzmann distribution), the V1/2 and k values could be estimated to be −13.1 and −9.4 mV, respectively, for the example shown in Fig. 3C.
The steady-state inactivation of the current was assessed by holding the membrane potential at different values between −120 and −15 mV for 10 s followed by a voltage step to +10 mV. Superimposed current traces obtained with this protocol are shown in Fig. 3D. Plotting the peak current as a function of the preceding holding potential (e.g. Fig. 3E) revealed a half-inactivation potential of −61.9 ± 2.2 mV and a slope factor of 9.5 ± 0.6 mV (n = 10).
Recovery from inactivation was measured by applying a 70 ms depolarizing pulse to +10 mV followed by a short test pulse to +10 mV with a variable delay (Fig. 4A, bottom trace). The recovery process could be well described by a single exponential function (dashed line in Fig. 4A; see also Fig. 4B). There was an e-fold change in the time constant per 15.7 mV in the example shown in Fig. 4B and C. On average the time constant describing the recovery process increased e-fold per 17.3 ± 4.2 mV (n = 4).
Figure 4. Recovery of INa from inactivation.
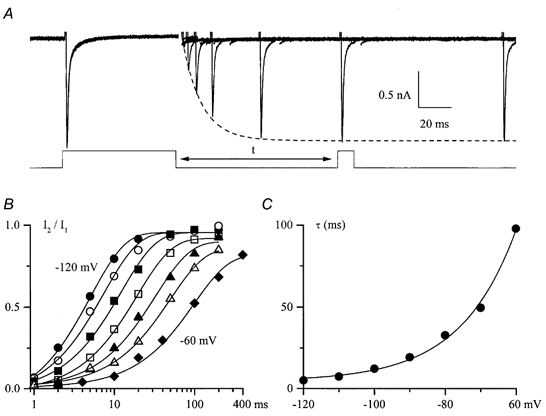
A, INa elicited by a 70 ms prepulse and a 10 ms test pulse to +10 mV applied from −100 mV with a variable interpulse interval, t (as shown below). Capacitance transients have been removed for clarity. Peak INa during the recovery process could be well fitted by a single exponential function with a time constant of 12.3 ms as shown by the dashed line. B, relative INa plotted against interpulse interval on a semilogarithmic scale at different membrane potentials (from left to right, −120 to −60 mV in 10 mV increments) and fitted by single exponential functions as shown by the continuous lines. C, voltage dependence of the time constant characterizing INa recovery from inactivation in a single experiment, fitted by a single exponential function with an e-fold increase in τ per 15.7 mV.
Cationic currents
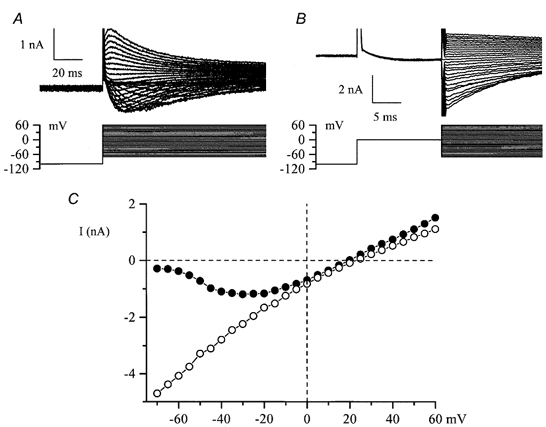
When the external solution was changed to a divalent cation-free, 130 mm Na+ solution two additional currents were observed. The voltage protocol used was either a series of steps from −40 mV to potentials between +100 and −180 mV as in Fig. 5A and B or a slow voltage ramp in the same range as shown schematically in Fig. 6A (bottom). The first current was inwardly rectifying with instantaneous activation and deactivation kinetics and no obvious inactivation except at very positive potentials (Fig. 5A and B). Voltage steps and slow voltage ramps produced very similar I-V relationships (Fig. 5C, circles and continuous lines, respectively). We termed this hyperpolarization-activated current IHA. A second current was seen upon a voltage step to −40 mV from a more negative test potential as in Fig. 5B or following the voltage ramp as in Fig. 6A. We termed this depolarization-activated current IDA. It showed rapid inactivation kinetics and thus might have been interpreted as a tail current due to the deactivation of the inwardly rectifying conductance, but further experiments showed that this was not the explanation. Both currents progressively increased in size with time after divalent cation removal (Fig. 6B, top panel). However, the time course of their increases was different. The inwardly rectifying current reached a maximum 75 s after external divalent cation removal (e.g. Fig. 6B, top panel, circles) whereas in the same cell the current seen upon the voltage step to −40 mV continued to increase in size and stabilized in different cells after about 100-200 s (e.g. Fig. 6B, top panel, triangles). Furthermore, the dependence of IHA and IDA on the external calcium concentration was different. The currents stabilized 50 s after Ca2+ readmission to the external solution. IDA was inhibited with an IC50 of 311 nm whereas for IHA the IC50 value in the same cell was 20 μm and the concentration-effect curve was less steep (Fig. 6B, bottom). These observations indicated that the currents were not related. Overall statistics for the occurrence of these currents in different cells also supported this conclusion. Thus, in experiments with Na+ or K+ as the main cation in the external solution, in only 35 % of cells studied (14 out of 40) were the two currents observed simultaneously. IHA was present in the majority of these cells whereas IDA was seen less frequently (in 35 and 16 out of 40 cells studied, respectively). In 20 cells studied using Cs+ as the main external cation, IDA was undetectable whereas IHA was seen in 19 cells. These observations implied different ion selectivity of the channels underlying these currents. To investigate this further, ion substitution experiments were performed. In the experiments illustrated in Fig. 6C and D, 130 mm Na+ in the external solution was replaced by equimolar amounts of Cs+, K+ or NMDG+. The sizes of the current in the different solutions were in the following descending order: K+ > Cs+ > Na+ for IHA (Fig. 6C) and Na+ > K+ >> Cs+ for IDA (Fig. 6D). Similar results were obtained from five cells. Substitution with the large impermeable NMDG+ cation completely abolished both IHA (Fig. 6C; note that control current was recorded in Na+ solution containing 2.5 mm Ca2+) and IDA (Fig. 6D; note the small inward current in control solution, Na+ with 2.5 mm Ca2+, compared to the NMDG+-based solution). Moreover, the reversal potential was close to 0 mV and remained almost the same: +5.5 mV with Na+, +3.3 mV with Cs+ and +2.6 mV with K+ in the external solution. In three cells expressing IHA alone, changing the pH of the external solution from 7.4 to 6.4 or 8.4 had almost no effect on the amplitude of the current or its reversal potential (data not shown).
Figure 5. Inward currents in cultured HISM cells developing in divalent cation-free external solution.
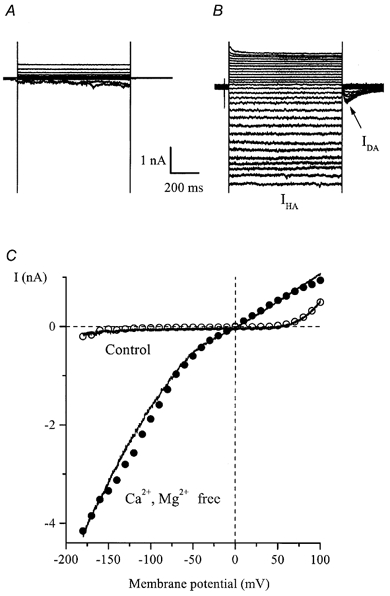
A and B, superimposed current traces elicited by 800 ms duration voltage steps from −40 mV to test potentials between +100 and −180 mV in 10 mV increments in control (130 mm Na+, 2.5 mm Ca2+, 1.2 mm Mg2+; A) and after external Ca2+ and Mg2+ removal (B, denoted as IHA). Note the instantaneous activation and deactivation of IHA as well as the noisy appearance of the current at negative potentials. The current seen upon repolarization to −40 mV is denoted as IDA. C, I-V relationships for currents measured at the end of the pulse (○ and •) and, in the same cell, by applying 1 s duration voltage ramps from +100 to −180 mV (continuous lines). The ramp protocol is illustrated in Fig. 6A.
Figure 6. Kinetics, [Ca2+]o dependence and ion permeation properties of IHA and IDA.
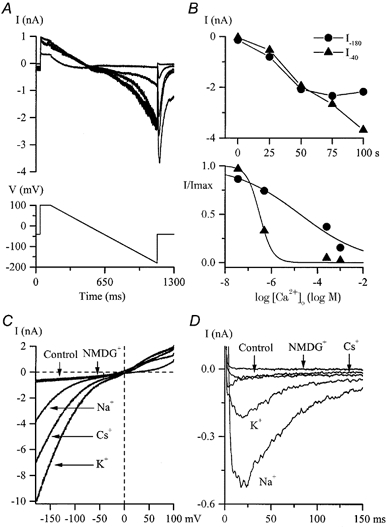
A, typical currents during a slow voltage ramp from +100 to −180 mV and a voltage step to −40 mV slowly developing after Ca2+ and Mg2+ removal from the external solution. Superimposed current traces were recorded at 25 s intervals. B, top, maximal amplitudes of inward currents at −180 mV (•, IHA) and at −40 mV after the ramp (8, IDA) plotted against time where time zero represents the moment of Ca2+ and Mg2+ removal from the external solution. Bottom, dependence of IHA (•) and IDA (8) in the same cell on the external free Ca2+ concentration. Relative currents were fitted by logistic functions with IC50 values of 20 μm for IHA and 311 nm for IDA. C and D, external cation substitution experiments were performed on two different cells after IHA(C) and IDA(D) had been stabilized in a divalent cation-free external solution. The control I-V relationship for IHA (voltage protocol as in A) and control current response for IDA (voltage step from −100 to −40 mV) were obtained in high-Na+, 2.5 mm CaCl2-containing external solution. Traces labelled ‘Na+’ were obtained after Ca2+ removal whereas all other traces were obtained in solutions with Na+ replacement as described in the Methods. High-Cs+ pipette solution was used.
The above results clearly indicate that IHA and IDA are carried via distinct cation channels which differ in their relative permeabilities to Na+, K+ and Cs+ and in their voltage and extracellular Ca2+ dependence.
Pharmacological properties of IHA and IDA cationic currents
It is now well established that a variety of cationic channels are commonly expressed in various smooth muscles. The gating events which open these channels are also diverse and include membrane stretch, Ca2+ release and/or a rise in [Ca2+]i, receptor and G-protein activation, a change in membrane polarization, protein kinase C activation, etc. Our observation that IHA and IDA were activated by Ca2+ removal from the external solution indicated a crucial role of external Ca2+ in the channel gating. A wide variety of cell types respond to external Ca2+ removal by generating cationic currents or non-selective currents carried by both cations and anions. The known examples include epithelium (Van Driessche & Zeiske, 1985; Desmedt et al. 1993), skeletal muscle (Almers et al. 1984), chick embryo ectoderm (Li et al. 1994), cardiac muscle (Mubagwa et al. 1997) and Xenopus oocytes (Zhang et al. 1998).
Another possibility was that in Ca2+-free external solution the intracellular stores could be passively depleted and this could account for the slow development of IHA and IDA due to the opening of calcium release-activated channels (CRACs). Thus, we performed experiments to see whether Ca2+ store depletion could induce such currents per se.
In these cultured intestinal smooth muscle cells both ryanodine- and InsP3-sensitive Ca2+ stores have been identified and the cholinergic agonist carbachol has been shown to release Ca2+ from both pools (Oh et al. 1997; Bielefeldt et al. 1997). Thus, we tested further the effects of carbachol. In the presence of Ca2+, carbachol application induced no or a very small inward current. It is also interesting to note here that the delayed rectifier K+ current was strongly inhibited by carbachol (by 60 % at 50 μm); the inhibition was voltage independent between 0 and +80 mV. Such an effect has been previously demonstrated in freshly isolated gastric myocytes (Lammel et al. 1991) as well as for the cloned smooth muscle-derived delayed rectifier potassium channels (Vogalis et al. 1995).
However, after IHA had developed in Ca2+-free solution, it was rapidly increased by carbachol application in a dose-dependent manner; carbachol at 1 μm was nearly maximally effective (Fig. 7A). The amplitude of IHA in the presence of saturating agonist concentrations (10- 100 μm) relative to that before carbachol application was 2.77 ± 0.50 (n = 5). The effect of carbachol was practically abolished in the presence of 1 μm atropine (ratio of amplitudes, 1.09 ± 0.05 (n = 4) with and without atropine plus 10 μm carbachol) or in cells where GDPβS (2 mm) was allowed to diffuse into the cell from the pipette solution for about 5 min to inhibit G-proteins prior to carbachol application (there was little increase in IHA; the ratio of amplitudes in these cells was 1.06 ± 0.08 (n = 4) with 10 μm carbachol). Thus, both effects were statistically significant (P < 0.03; two-tailed unpaired t test) indicating the involvement of muscarinic receptors/G-proteins in the signal transduction pathways. As mentioned above, IDA occurred very infrequently but in two cells where this current was present the same carbachol concentrations (10-100 μm) strongly inhibited and finally abolished IDA (one example is shown in Fig. 7B).
Figure 7. Effects of carbachol on IHA and IDA.
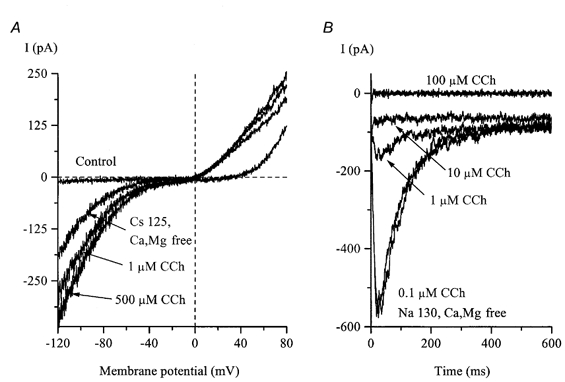
A, the control I-V relationship was measured as described for Fig. 6C. After IHA had developed in divalent cation-free, high-Cs+ external solution, it was rapidly potentiated by carbachol (CCh) application. IDA was abolished in Cs+-containing external solution and was inactivated at −40 mV. B, in a different cell, IDA measured at −40 mV in divalent cation-free, high-Na+ external solution was strongly inhibited by carbachol.
Cyclopiazonic acid (CPA), an inhibitor of the sarco-(endo)plasmic reticulum Ca2+ pump (SERCA), is widely used as a pharmacological tool to deplete intracellular Ca2+ stores. In four cells tested, application of CPA at 30 μm for 5-8 min failed to induce inward cationic currents when Ca2+ was present in the external solution but both currents could be readily induced in the same cells in Ca2+-free solution (Fig. 8A). Thus, it appeared that Ca2+ store depletion alone, if this was an underlying mechanism, was insufficient for IHA or IDA activation as the channels are primarily controlled (suppressed) by external calcium ions. It was also notable in all cells tested that CPA did induce an outward current. Its amplitude at very positive potentials was the same as that of IHA during subsequent Ca2+-free solution application (Fig. 8A).
Figure 8. Pharmacological properties of IHA and IDA.
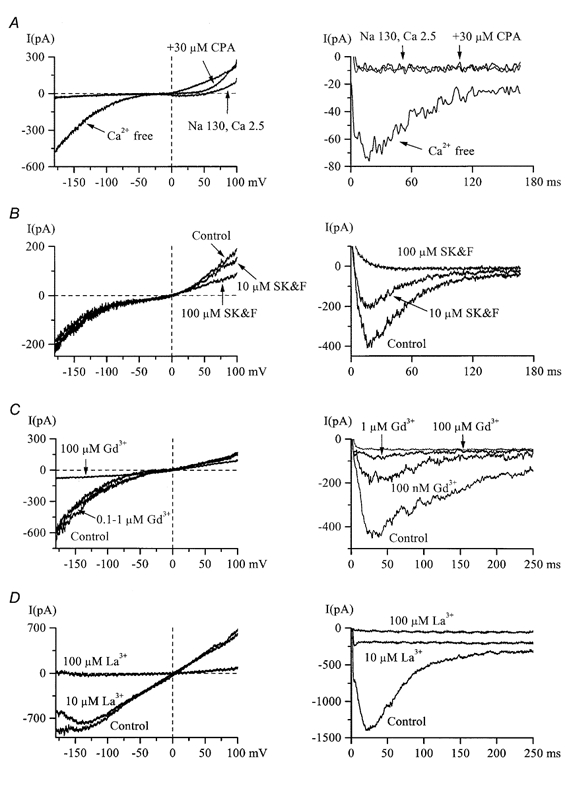
A, 5 min treatment with 30 μm CPA failed to induce an inward IHA (left) or IDA (right) in 2.5 mm Ca2+, high-Na+ external solution (n = 5), but both currents were readily activated following Ca2+ removal. Note that at positive potentials current could be activated by CPA. B, SK&F 96365 at concentrations of up to 100 μm did not inhibit inward IHA (left), but completely abolished IDA (right) in the same cell (n = 7). Current at positive potentials at which it could be activated by CPA (left panel in A) was also inhibited by SK&F 96365. C and D, IDA (right) was more sensitive to the inhibitory action of Gd3+ or La3+ compared to IHA (left) but both currents were abolished by 100 μm of either blocker. In all panels IHA and IDA were measured in the same cells.
SK&F 96365, a blocker of store-operated Ca2+ channels (Clementi & Meldolesi, 1996), suppressed IDA with a half-maximal inhibitory concentration of 9 μm but was ineffective in blocking inward IHA at concentrations up to 100 μm (Fig. 8B). Interestingly, an outward current at the same potentials at which it could be induced by CPA was inhibited by SK&F 96365. Also, at 100 μm SK&F 96365 almost completely blocked the TTX-sensitive Na+ current; the inhibition was reversible (τon = 7.5 s; τoff = 103 s).
Gadolinium and lanthanum are useful pharmacological tools as antagonists of mechano-sensitive (e.g. stretch- or swelling-activated) cationic channels (Hamill & McBride, 1996), as well as external Ca2+-blockable cationic channels (Van Driessche et al. 1988; Mubagwa et al. 1997; Zhang et al. 1998). In 13 HISM cells tested, IDA was found to be more sensitive to the blocking action of Gd3+ and La3+ compared to IHA, the latter being completely inhibited only at 100 μm (Fig. 8D).
Voltage-dependent properties of IDA
Since IDA showed steady-state inactivation properties very similar to those for INa in these cells (see Fig. 10C) we also considered the possibility that modulation of Na+ channel gating in Ca2+-free solution could somehow be involved (Armstrong, 1999; Armstrong & Cota, 1999). However, TTX at 1 μm had no effect on IDA. This current was also distinct from INa in its kinetics and voltage dependence of activation. Figure 9 shows current traces recorded in high-Na+, divalent cation-free external solution upon stepping from the holding potential of −100 mV to various levels indicated beside each current trace. Activation of IDA occurred at potentials more negative than activation of INa (e.g. −60 mV). Since IDA activation was much slower than that of INa these currents produced two distinct peaks (e.g. between −45 and −35 mV). The rate of IDA inactivation was also considerably slower. At more positive test potentials, IDA was masked by a generally much larger INa. Thus, to study the voltage dependence of its activation and inactivation we employed the protocols shown schematically in Fig. 10A and B, respectively. These voltage-dependent parameters for IDA were assessed by measuring tail current amplitude at −100 mV after voltage steps to different test potentials (activation; Fig. 10A) or after a voltage step to +10 mV applied from different holding potentials (inactivation; Fig. 10B). The amplitude of the tail current was measured by fitting a single exponential function with extrapolation to the beginning of repolarization. Normalized values are shown in Fig. 10C by triangles, with open symbols for inactivation and filled symbols for activation. For comparison, steady-state activation and inactivation curves for INa in the same cell are also shown; these were obtained as described above (e.g. Fig. 3). Steady-state inactivation for the two currents was surprisingly similar both in the slope and in the position on the voltage axis (only 5 mV difference in the V1/2 values). However, the activation curve for IDA was steeper and positioned about 35 mV more negative than that for INa. Thus, a small steady IDA (‘window current’) can be generated in the voltage range −60 to −30 mV under conditions when Ca2+ stores are depleted (compare to Fig. 9).
Figure 10. Comparison of the voltage dependence of activation and inactivation of INa and IDA.
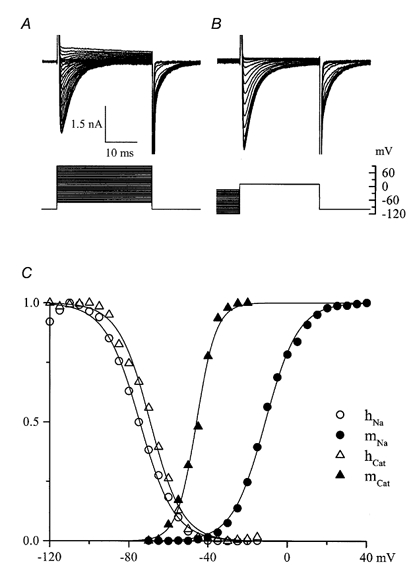
A and B, families of superimposed current traces and voltage protocols used to study the voltage dependence of activation (A) and inactivation (B). Measurements were made of INa at its peak and activation of IDA 30 ms after stepping to various potentials from a holding potential of −100 mV; at 30 ms INa is largely inactivated (cf. Fig. 3) but IDA is close to its peak value. In B the test potential was +10 mV, close to the reversal potential (Vrev) for IDA, thus minimizing its contribution during measurements of INa inactivation. Again, a 30 ms pulse allows INa to inactivate leaving IDA close to its peak value. C, open and filled circles show, respectively, normalized INa amplitude from B (hNa) and relative Na+ conductance calculated from INa amplitudes in A (mNa) as described for Fig. 3C, respectively. Filled and open triangles show relative tail current amplitudes from A (mCat) and B (hCat), respectively, measured by fitting single exponential functions and extrapolating to time zero at the end of each test pulse. Data points were fitted by Boltzmann functions with the following best fit parameters: INa activation: V1/2 = −11.1 mV; k = −8.3 mV; INa inactivation: V1/2 = −74.5 mV, k = 8.8 mV; IDA activation: V1/2 = −45.8 mV, k = −5.0 mV; IDA inactivation: V1/2 = −69.2 mV, k = 8.1 mV. This cell was in 130 mm Na+, divalent cation-free solution; IHA was small (-145 and +155 pA at −100 and +90 mV, respectively).
Figure 9. Amplitude and kinetic relationships between INa and IDA.
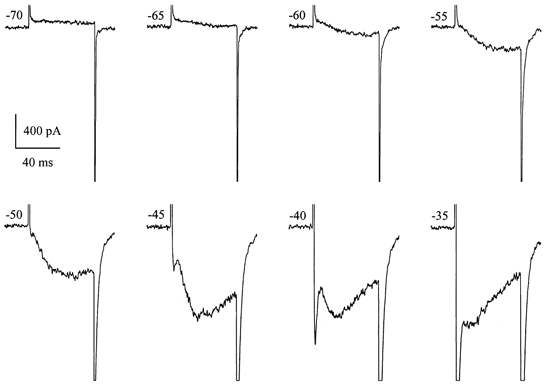
Current traces were recorded in a high-Na+, divalent cation-free solution upon voltage steps from −100 mV to various test potentials indicated beside each trace. IHA was 160, 122 and 81 pA at −100, −70 and −35 mV, respectively.
The position of the activation curve for IDA on the voltage axis is very similar to that for the muscarinic cationic conductance in guinea-pig ileal smooth muscle cells (compare with Inoue & Isenberg, 1990; Zholos & Bolton, 1994). Given that both currents are cation currents, IDA might be expected to show a similar U-shaped I-V relationship. In the experiment illustrated in Fig. 11A, IDA was measured by applying voltage steps from −100 mV to various test potentials. The instantaneous I-V relationship for this current was also evaluated in the same cell by stepping to 0 mV (maximal activation) followed by voltage steps to between −70 and +60 mV (Fig. 11B). Figure 11C compares I-V relationships obtained using these protocols. The instantaneous I-V relationship (○) is nearly linear implying an almost linear dependence of single channel current amplitude on the membrane potential (constant single channel conductance), whereas peak IDA showed a bell-shaped voltage dependence (•) declining almost to the zero level at very negative potentials (compare with Fig. 5 of Inoue & Isenberg, 1990). The reversal potential in the two cases was nearly the same.
Figure 11. I-V relationships for IDA.
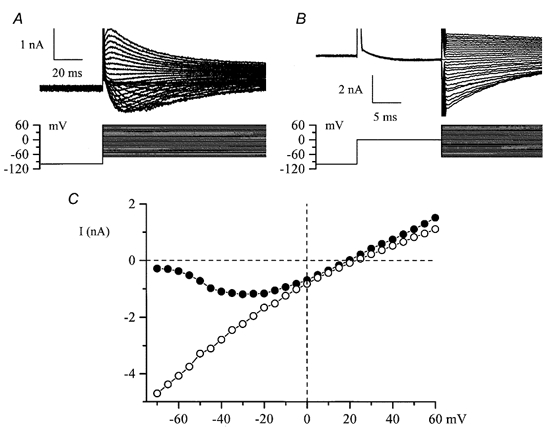
A and B, voltage protocols and superimposed current traces recorded in the same cultured HISM cell using high-Cs+ pipette and high-K+, divalent cation-free external solution in which INa is abolished and IDA can be measured. IHA was relatively small in this cell: measured by applying voltage ramps from −40 mV to inactivate IDA, IHA amplitude was −427 pA at −70 mV and 315 pA at +60 mV. In B steps were in the range −70 to +60mV. Tail current was flat at +20 mV. C, I-V relationships for the peak IDA in A (•) and instantaneous tail current amplitude in B (○), measured as described for Fig. 10B. Tail current reversed at +23 mV which was 3 mV more positive than the peak IDA reversal potential. This discrepancy could arise as a result of some contamination of the peak IDA by IHA, as explained above, for which Vrev 0 mV.
DISCUSSION
Smooth muscle cell cultures offer a valuable approach to study the mechanisms and potential regulatory pathways controlling cell differentiation (Owens, 1995). However, substantial modification of cell properties means that careful studies are required in each particular case and the results obtained may not represent in vivo properties of fully differentiated cells. In the present patch-clamp study we investigated the electrophysiological behaviour of cultured HISM cells, which until now were poorly characterized.
The lack of voltage-gated Ca2+ channels (Fig. 2A and B) in these cells is clearly the result of altered properties of cultured HISM cells as compared with their native counterparts. The L-type channel proteins are ubiquitously expressed in all freshly isolated gastrointestinal smooth muscle cells studied, including human jejunal and colonic myocytes (Farrugia et al. 1995; Xiong et al. 1995). It must be noted that in a previous study the inward current in cultured HISM cells was erroneously identified as a voltage-gated Ca2+ current via L-type Ca2+ channels despite its atypical rapid inactivation for such a current. No ion substitution tests were performed and this conclusion was drawn from the partial inhibition by nifedipine (20 μm) and verapamil (Bielefeldt et al. 1996). In our experiments this fast inward current persisted in a Ca2+-free external solution (e.g. Fig. 9 and 10) and was abolished in Na+-free solutions (replacement by Cs+, K+ or NMDG+; e.g. Figs 2A and 11A). Moreover, the current was TTX sensitive (IC50 ≈ 100 nm). Many different smooth muscle cells normally express both Ca2+ channels and voltage-gated, TTX-sensitive Na+ channels (e.g. rat ileum, Smirnov et al. 1992; for review see Kuriyama et al. 1998). Na+ channels may also be expressed in cultured smooth muscle cells, usually in a relatively minor population of cells, even though they are not observed in their native counterparts (e.g. Snetkov et al. 1996). TTX-sensitive Na+ channels were present in all HISM cells tested though INa greatly varied in amplitude with no obvious relation to cell size. It is interesting to note that arresting cell growth by using serum-free conditions for up to 19 days did not abolish INa but substantially accelerated its inactivation kinetics; for example at +10 mV the decay time constant decreased from 5.2 to 0.9 ms (data not shown).
The overall properties of INa in cultured HISM cells appear very similar to those in freshly isolated human colonic myocytes except for an approximately 7-fold higher IC50 value for TTX (Xiong et al. 1993). In both cases the currents peaked at about 0 mV and inactivated within about 10 ms. The availability curves had similar slopes and V1/2 values but the activation curve in HISM was positioned 19 mV more positive in HISM cells compared to native cells without any significant change in the voltage dependence (slopes, 6.7 vs. 7.6 mV, respectively). There was also a clear indication of the overexpression of Na+ channels in cultured cells as the current density on average was 20 times higher compared to that of freshly isolated human cells (Xiong et al. 1993).
BKCa channels are ubiquitously expressed in visceral smooth muscles giving rise to STOC discharge or large outward currents in response to intracellular Ca2+ release (Bolton et al. 1999). In cultured HISM cells both ryanodine- and InsP3-sensitive Ca2+ stores are functional (Oh et al. 1997), but under conditions of low intracellular Ca2+ buffering we observed neither STOCs nor outward currents in response to caffeine or carbachol. This suggests that BKCa channels are not expressed in these cells, which is further confirmed by the lack of block by IbTX (Fig. 1). However, it is unlikely that these channels are lost in culture since in cells freshly dispersed from human jejunum, Ca2+-dependent K+ channels are also lacking. The major difference was in the activation range for the TEA+-sensitive K+ current, which was about 60 mV more positive in cultured cells compared to freshly isolated jejunal cells (Farrugia et al. 1993).
Upon carbachol application, even at high concentrations, muscarinic receptor cationic current, ICAT, with a characteristic U-shaped voltage dependence was not observed in HISM cells. Since in guinea-pig ileal cells ICAT is strongly inhibited by external divalent cations (Zholos & Bolton, 1995), Ca2+ and Mg2+ were removed in an attempt to unmask this current. Though ICAT was not revealed under these conditions we found two other cationic currents previously not seen in freshly dissociated smooth muscle cells. Under divalent cation-free conditions they developed very slowly and initially almost in parallel (Fig. 6A and B). At later times the current termed IHA stabilized whereas the other current, termed IDA, continued to increase. This was the first indication that different ion channels mediate these two currents. IDA turned out to be much more sensitive to [Ca2+]o (Fig. 6B). The channels also had different ion-selectivity profiles and different voltage-dependent and pharmacological properties.
The slow appearance of the currents in divalent cation-free medium could indicate the involvement of passive Ca2+ store depletion in their generation. Thus, the channels could be potentially related to CRACs through which capacitative refilling of Ca2+ stores is believed to occur. This has been found to be a dominant Ca2+ entry pathway in a large variety of cells, particularly in non-excitable cells (reviewed by Parekh & Penner, 1997). Store-operated Ca2+ influx has also been reported in cultured smooth muscle cells such as A7r5 vascular cells (Blatter, 1995; Byron & Taylor, 1995) and the DDT1MF-2 cell line (Ufret-Vincenty et al. 1995).
ICRAC is difficult to measure directly in smooth muscle cells. However, recent studies on other cell types have established that monovalent cation outward currents can be generated by CRACs even when Ca2+ is present in the external solution (Hoth, 1996). When the external free Ca2+ concentration was reduced to micromolar levels in the absence of Mg2+, ICRAC in Jurkat T-cells was significantly increased (Lepple-Wienhues & Cahalan, 1996) and single CRAC conductance for Na+ was much higher compared to the Ca2+ current through CRACs (Kerschbaum & Cahalan, 1999). Using CPA, a sarco-endoplasmic reticulum Ca2+ pump (SERCA) inhibitor, to deplete the store we found that an outward current developed and the same component of the current was inhibited by SK&F 96365, a blocker of store-operated Ca2+ channels (Fig. 8A and B). However, beyond this there were more differences than similarities between IHA and IDA in HISM cells and ICRAC in other cells. To summarize, it appears that some properties of a CRAC current are shared with IHA (e.g. voltage dependence, kinetics, inward rectification, the lack of inactivation in Ca2+-free solution, similar IC50 values for inhibition by external Ca2+, effects of carbachol) whereas other properties are shared with IDA (e.g. the blocking action of Gd3+, La3+ and SK&F 96365, and the sequence of conductance Na+ > K+ >> Cs+ is identical to that in Jurkat T cells). Thus, an amplified CRAC component may be present in divalent cation-free solution but the properties of neither IHA nor IDA are the same as those described for ICRAC in other cell types.
Since no stimulation of the cells was employed (e.g. mechanical stimuli or agonists) to evoke these currents, the remaining possibility is that IHA and IDA belong to the family of Ca2+-blockable cationic currents which appear to be ubiquitous in various cell types but so far have not been described in smooth muscle cells. The opening of such channels is believed to produce the membrane depolarization commonly occurring in Ca2+-free solutions and two mechanisms have been proposed to explain this phenomenon.
One possibility is that the removal of external Ca2+ alters the selectivity and/or gating properties of Ca2+ or Na+ channels (e.g. Almers et al. 1984; Armstrong, 1999; Armstrong & Cota, 1999). In HISM cells this seems unlikely since Ca2+ channels are absent and TTX did not affect the cationic currents. The voltage-dependent availability curves for INa and IDA were strikingly similar (Fig. 10C), but other properties were different and it is an important observation that all three currents could be observed in the same cell. Moreover, INa was abolished in K+ solutions but both cationic currents remained.
The other possibility is that Ca2+ removal unmasks cationic channels (e.g. Van Driessche & Zeiske, 1985; Mubagwa et al. 1997). Activation of hemi-gap-junctional channels has recently been implicated in Xenopus oocytes (Zhang et al. 1998), but these channels are permeable to anions and even to large organic monovalent cations such as NMDG+, which is clearly not the case in HISM cells (e.g. Fig. 6C and D). Also, the voltage dependence and channel kinetic properties of HISM cells and oocytes are different.
In ventricular myocytes, Mubagwa et al. (1997) described a novel Ca2+-blockable cationic current with properties remarkably similar to those of IHA in HISM cells. The current was blocked by Ca2+ with an IC50 of 60 μm (compare to 20 μm for IHA). Thus, the authors postulated the existence of a high-affinity binding site for Ca2+ at or near the extracellular site of the cationic channel, so that the slow off-rate could explain the slow development (> 5 min) of the current, which is also the case for IHA. IDA developed even more slowly, consistent with an even lower apparent dissociation constant for Ca2+ of about 0.3 μm. IHA and cationic current in cardiac cells both show inward rectification, and have a very similar noisy appearance, amplitude and instantaneous activation/ deactivation during voltage jumps (compare our Fig. 5B with Fig. 2A in Mubagwa et al. 1997). Both currents could be carried by monovalent cations in the same sequence K+ > Cs+ > Na+ >> NMDG+, but not by Cl−, and were abolished in the presence of 100 μm Gd3+.
The physiological relevance of such a conductance in cells in vivo exposed to [Ca2+]o in the millimolar range remains largely unclear. However, in cardiac cells there is a difference in the [Ca2+]o sensitivity between intact tissue and single cells. Also, Mubagwa et al. (1997) raised the possibility that these channels can be physiologically regulated by agonists (though they did not observe any response to β-adrenergic or muscarinic receptor stimulation) or change their calcium sensitivity under pathophysiological conditions such as ischaemia or ‘Ca2+ paradox’. Our findings that carbachol application can strongly potentiate IHA via a muscarinic receptor/ G-protein pathway support this hypothesis. Our first demonstration of IHA in smooth muscle cells also shows that such a current is not tissue specific. Moreover, IDA in HISM cells is a novel Ca2+-blockable cationic current with no resemblance to previously described currents. Thus, even in the same cell distinct channels can mediate cationic fluxes in divalent cation-free solutions but their physiological function remains to be defined. HISM cells may provide a useful experimental model for future studies since these channels, like Na+ channels, are apparently overexpressed in these cultured cells, explaining why such currents were not previously found in freshly isolated smooth muscle cells.
Acknowledgments
This work was supported by grant number 051162/Z from The Wellcome Trust.
References
- Almers W, Mc Cleskey EW, Palade PT. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. Journal of Physiology. 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM. Distinguishing surface effects of calcium ion from pore-occupancy effects in Na+ channels. Proceedings of the National Academy of Sciences of the USA. 1999;96:4158–4163. doi: 10.1073/pnas.96.7.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Cota G. Calcium block of Na+ channels and its effect on closing rate. Proceedings of the National Academy of Sciences of the USA. 1999;96:4154–4157. doi: 10.1073/pnas.96.7.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Waite L, Abboud FM, Conklin JL. Nongenomic effects of progesterone on human intestinal smooth muscle cells. American Journal of Physiology. 1996;271:G370–376. doi: 10.1152/ajpgi.1996.271.2.G370. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Whiteis CA, Sharma RV, Abboud FM, Conklin JL. Reactive oxygen species and calcium homeostasis in cultured human intestinal smooth muscle cells. American Journal of Physiology. 1997;272:G1439–1450. doi: 10.1152/ajpgi.1997.272.6.G1439. [DOI] [PubMed] [Google Scholar]
- Blatter LA. Depletion and filling of intracellular calcium stores in vascular smooth muscle. American Journal of Physiology. 1995;268:C503–512. doi: 10.1152/ajpcell.1995.268.2.C503. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annual Review of Physiology. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Brittingham J, Phiel C, Trzyna WC, Gabbeta V, McHugh KM. Identification of distinct molecular phenotypes in cultured gastrointestinal smooth muscle cells. Gastroenterology. 1998;115:605–617. doi: 10.1016/s0016-5085(98)70140-4. [DOI] [PubMed] [Google Scholar]
- Byron KL, Taylor CW. Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. Journal of Physiology. 1995;485:455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiological Reviews. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Clementi E, Meldolesi J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- Desmedt L, Simaels J, Van Driessche W. Ca2+-blockable, poorly selective cation channels in the apical membrane of amphibian epithelia. Tetracaine blocks the UO22+-insensitive pathway. Journal of General Physiology. 1993;101:103–116. doi: 10.1085/jgp.101.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G, Rae JL, Sarr MG, Szurszewski JH. Potassium current in circular smooth muscle of human jejunum activated by fenamates. American Journal of Physiology. 1993;265:G873–879. doi: 10.1152/ajpgi.1993.265.5.G873. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Rich A, Rae JL, Sarr MG, Szurszewski JH. Calcium currents in human and canine jejunal circular smooth muscle cells. Gastroenterology. 1995;109:707–717. doi: 10.1016/0016-5085(95)90377-1. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacological Reviews. 1996;48:231–252. [PubMed] [Google Scholar]
- Hoth M. Depletion of intracellular calcium stores activates an outward potassium current in mast and RBL-1 cells that is correlated with CRAC channel activation. FEBS Letters. 1996;390:285–288. doi: 10.1016/0014-5793(96)00673-4. [DOI] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. Journal of Physiology. 1990;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiological Reviews. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- Lammel E, Deitmer P, Noack T. Suppression of steady membrane currents by acetylcholine in single smooth muscle cells of the guinea-pig gastric fundus. Journal of Physiology. 1991;432:259–282. doi: 10.1113/jphysiol.1991.sp018384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Cahalan MD. Conductance and permeation of monovalent cations through depletion-activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophysical Journal. 1996;71:787–794. doi: 10.1016/S0006-3495(96)79278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JQ, Prodhom B, Kucera P. Cation channel blocked by extracellular Ca2+ in the apical membrane of the chick embryonic ectoderm. Pflügers Archiv. 1994;429:183–192. doi: 10.1007/BF00374311. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Stengl M, Flameng W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. Journal of Physiology. 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ST, Yedidag E, Conklin JL, Martin M, Bielefeldt K. Calcium release from intracellular stores and excitation-contraction coupling in intestinal smooth muscle. Journal of Surgical Research. 1997;71:79–86. doi: 10.1006/jsre.1997.5134. [DOI] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiological Reviews. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Zholos AV, Shuba MF. Potential-dependent inward currents in single isolated smooth muscle cells of the rat ileum. Journal of Physiology. 1992;454:549–571. doi: 10.1113/jphysiol.1992.sp019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snetkov VA, Hirst SJ, Ward JPT. Ion channels in freshly isolated and cultured human bronchial smooth muscle cells. Experimental Physiology. 1996;81:791–804. doi: 10.1113/expphysiol.1996.sp003977. [DOI] [PubMed] [Google Scholar]
- Ufret-Vincenty CA, Short AD, Alfonso A, Gill DL. A novel Ca2+ entry mechanism is turned on during growth arrest induced by Ca2+ pool depletion. Journal of Biological Chemistry. 1995;270:26790–26793. doi: 10.1074/jbc.270.45.26790. [DOI] [PubMed] [Google Scholar]
- Van Driessche W, Simaels J, Aelvoet I, Erlij D. Cation-selective channels in amphibian epithelia: electrophysiological properties and activation. Comparative Biochemistry and Physiology. 1988;90:693–699. doi: 10.1016/0300-9629(88)90686-x. [DOI] [PubMed] [Google Scholar]
- Van Driessche W, Zeiske W. Ca2+-sensitive, spontaneously fluctuating, cationic channels in the apical membrane of the adult frog skin epithelium. Pflügers Archiv. 1985;405:250–259. doi: 10.1007/BF00582569. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Ward M, Horowitz B. Suppression of two cloned smooth muscle-derived delayed rectifier potassium channels by cholinergic agonists and phorbol esters. Molecular Pharmacology. 1995;48:1015–1023. [PubMed] [Google Scholar]
- Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Fast Na+ current in circular smooth muscle cells of the large intestine. Pflügers Archiv. 1993;423:485–491. doi: 10.1007/BF00374945. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Sperelakis N, Noffsinger A, Fenoglio-Preiser C. Ca2+ currents in human colonic smooth muscle cells. American Journal of Physiology. 1995;269:G378–385. doi: 10.1152/ajpgi.1995.269.3.G378. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McBride DW, Jr, Hamill OP. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. Journal of Physiology. 1998;508:763–776. doi: 10.1111/j.1469-7793.1998.763bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. Journal of Physiology. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. Journal of Physiology. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]


