Abstract
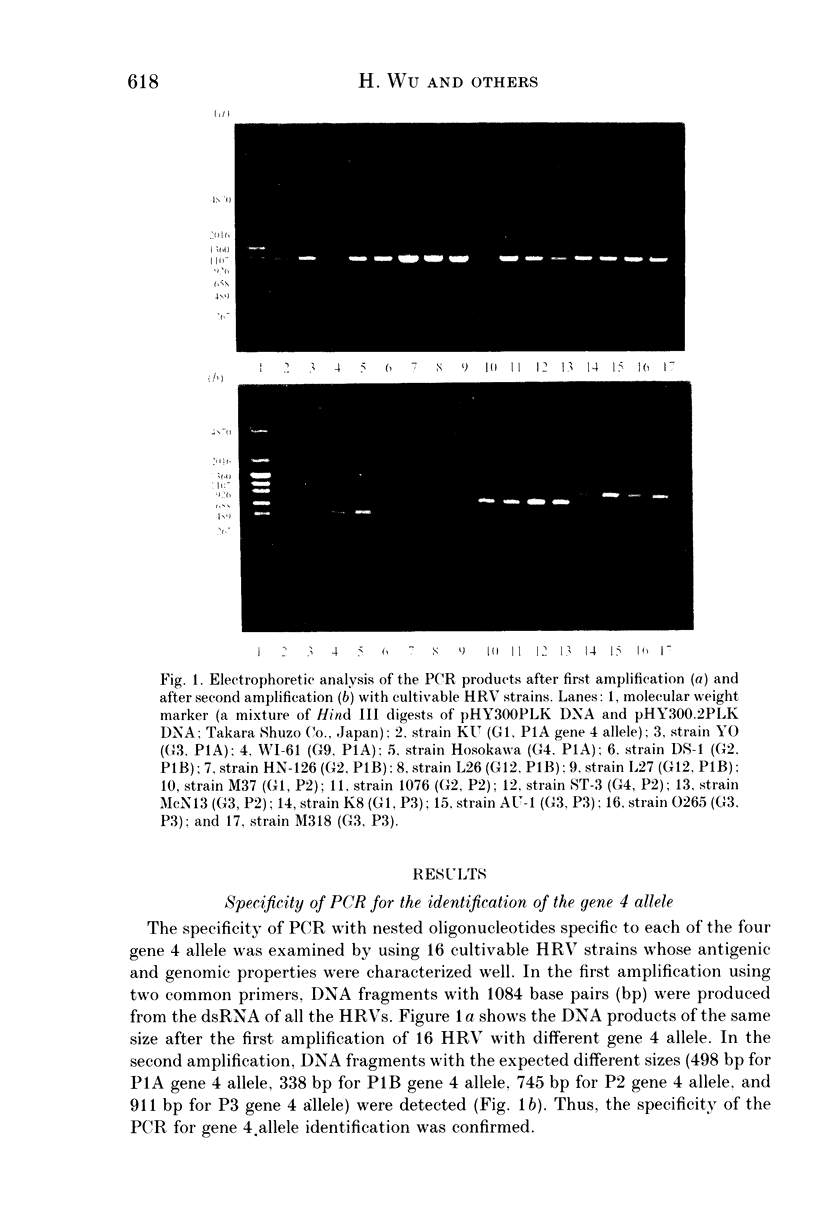
The presence of six gene 4 alleles (or VP4 genotypes) in human rotaviruses has been recognized. Using 16 representative cultivable human rotavirus strains, we confirmed the specificity of VP4 genotyping by polymerase chain reaction (PCR) using the nested oligonucleotides specific to each of the four representative gene 4 alleles. Using the PCR, we surveyed the gene 4 alleles of 199 human rotaviruses in stools collected in Japan and Thailand. Strains with the gene 4 allele, corresponding to P1A serotype, were shown to be the most prevalent, but two strains with P2 gene 4 allele and one strain with P3 gene 4 allele were detected in Thailand and in Japan, respectively.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beards G., Xu L., Ballard A., Desselberger U., McCrae M. A. A serotype 10 human rotavirus. J Clin Microbiol. 1992 Jun;30(6):1432–1435. doi: 10.1128/jcm.30.6.1432-1435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Dunn S. J., Woode G. N., Greenberg H. B., Rao C. D. Both surface proteins (VP4 and VP7) of an asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology. 1993 May;194(1):374–379. doi: 10.1006/viro.1993.1271. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Das B. K., Jiang B., Bhan M. K., Glass R. I. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology. 1993 May;194(1):424–430. doi: 10.1006/viro.1993.1280. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Glass R. I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B. K., Bhan M. K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992 Jun;30(6):1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Green K., Nishikawa K., Taniguchi K., Jones R., Kapikian A. Z., Chanock R. M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988 Aug;62(8):2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A. Z., Chanock R. M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isegawa Y., Nakagomi O., Nakagomi T., Ueda S. A VP4 sequence highly conserved in human rotavirus strain AU-1 and feline rotavirus strain FRV-1. J Gen Virol. 1992 Aug;73(Pt 8):1939–1946. doi: 10.1099/0022-1317-73-8-1939. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Larralde G., Li B. G., Kapikian A. Z., Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991 Jun;65(6):3213–3218. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Clark H. F., Gouvea V. Nucleotide sequence of the VP4-encoding gene of an unusual human rotavirus (HCR3). Virology. 1993 Oct;196(2):825–830. doi: 10.1006/viro.1993.1540. [DOI] [PubMed] [Google Scholar]
- Li B., Larralde G., Gorziglia M. Human rotavirus K8 strain represents a new VP4 serotype. J Virol. 1993 Jan;67(1):617–620. doi: 10.1128/jvi.67.1.617-620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Offit P. A., Vo P. T., Mackow E. R., Benfield D. A., Shaw R. D., Padilla-Noriega L., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989 Apr;27(4):780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Green K. Y. Human rotavirus strain 69M has a unique VP4 as determined by amino acid sequence analysis. Virology. 1991 May;182(1):407–412. doi: 10.1016/0042-6822(91)90691-4. [DOI] [PubMed] [Google Scholar]
- Steele A. D., Garcia D., Sears J., Gerna G., Nakagomi O., Flores J. Distribution of VP4 gene alleles in human rotaviruses by using probes to the hyperdivergent region of the VP4 gene. J Clin Microbiol. 1993 Jul;31(7):1735–1740. doi: 10.1128/jcm.31.7.1735-1740.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Nishikawa K., Urasawa T., Urasawa S., Midthun K., Kapikian A. Z., Gorziglia M. Complete nucleotide sequence of the gene encoding VP4 of a human rotavirus (strain K8) which has unique VP4 neutralization epitopes. J Virol. 1989 Sep;63(9):4101–4106. doi: 10.1128/jvi.63.9.4101-4106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Independent segregation of the VP4 and the VP7 genes in bovine rotaviruses as confirmed by VP4 sequence analysis of G8 and G10 bovine rotavirus strains. J Gen Virol. 1993 Jun;74(Pt 6):1215–1221. doi: 10.1099/0022-1317-74-6-1215. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Hasegawa A., Urasawa T., Taniguchi K., Wakasugi F., Suzuki H., Inouye S., Pongprot B., Supawadee J., Suprasert S. Antigenic and genetic analyses of human rotaviruses in Chiang Mai, Thailand: evidence for a close relationship between human and animal rotaviruses. J Infect Dis. 1992 Aug;166(2):227–234. doi: 10.1093/infdis/166.2.227. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Wakasugi F., Kobayashi N., Chiba S., Sakurada N., Morita M., Morita O., Tokieda M. Survey of human rotavirus serotypes in different locales in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989 Jul;160(1):44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Wakasugi F., Kobayashi N., Taniguchi K., Lintag I. C., Saniel M. C., Goto H. Presumptive seventh serotype of human rotavirus. Arch Virol. 1990;113(3-4):279–282. doi: 10.1007/BF01316680. [DOI] [PubMed] [Google Scholar]
- Urasawa T., Taniguchi K., Kobayashi N., Wakasugi F., Oishi I., Minekawa Y., Oseto M., Ahmed M. U., Urasawa S. Antigenic and genetic analyses of human rotavirus with dual subgroup specificity. J Clin Microbiol. 1990 Dec;28(12):2837–2841. doi: 10.1128/jcm.28.12.2837-2841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods P. A., Gentsch J., Gouvea V., Mata L., Santosham M., Bai Z. S., Urasawa S., Glass R. I. Distribution of serotypes of human rotavirus in different populations. J Clin Microbiol. 1992 Apr;30(4):781–785. doi: 10.1128/jcm.30.4.781-785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]