Abstract
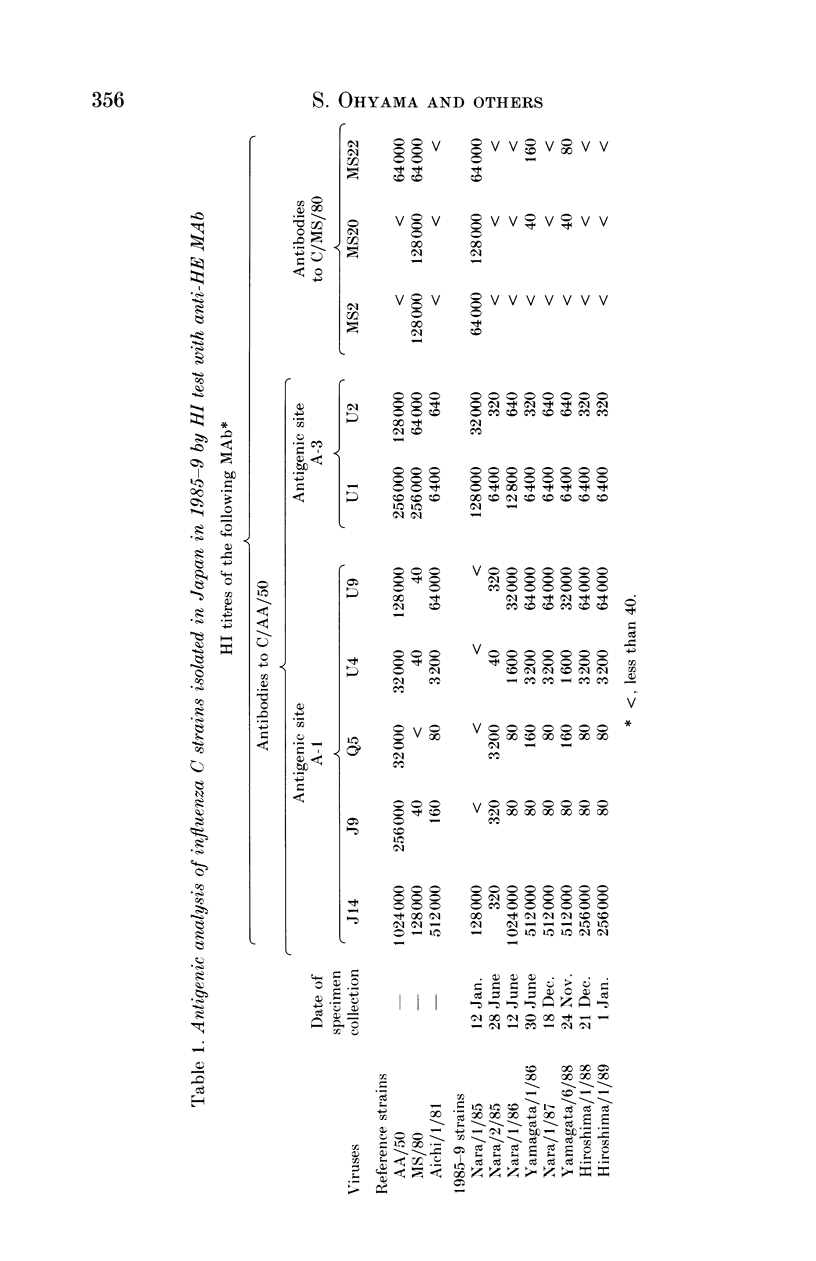
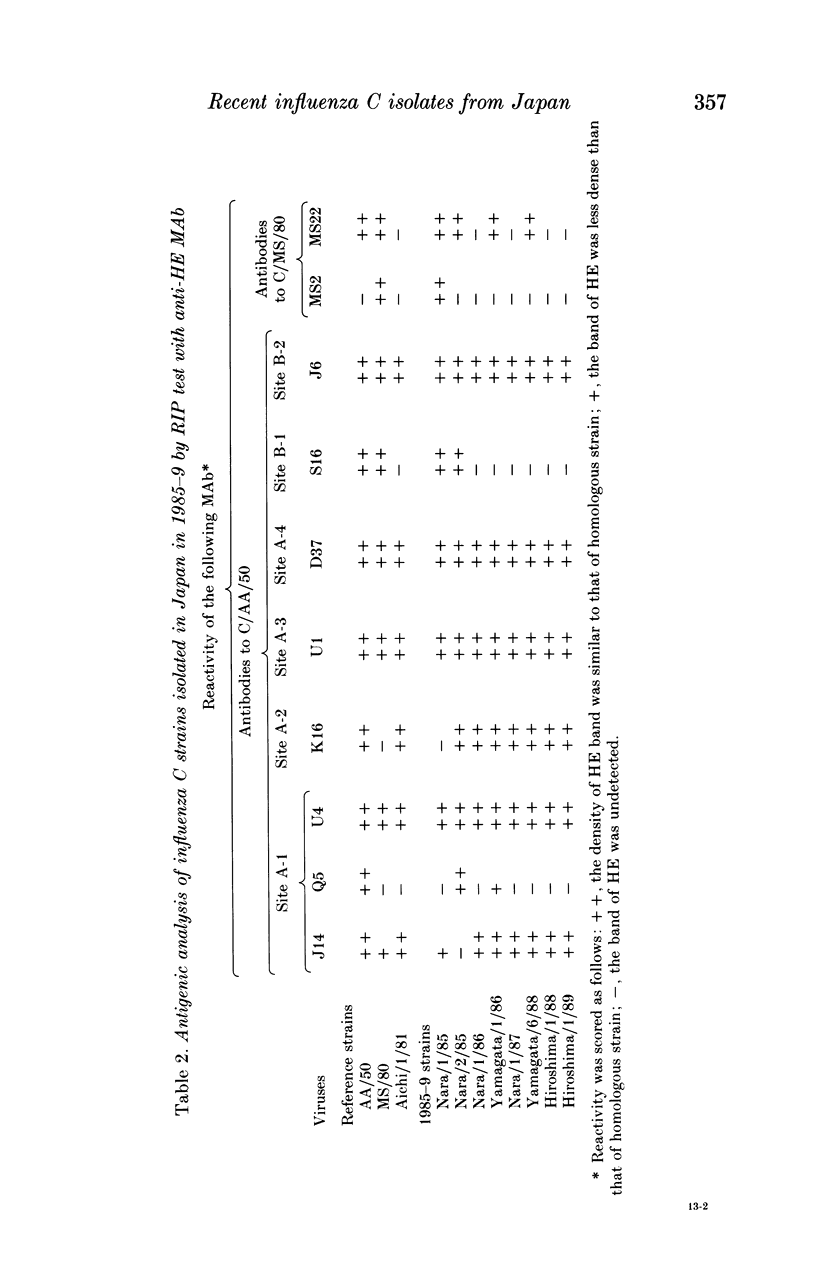
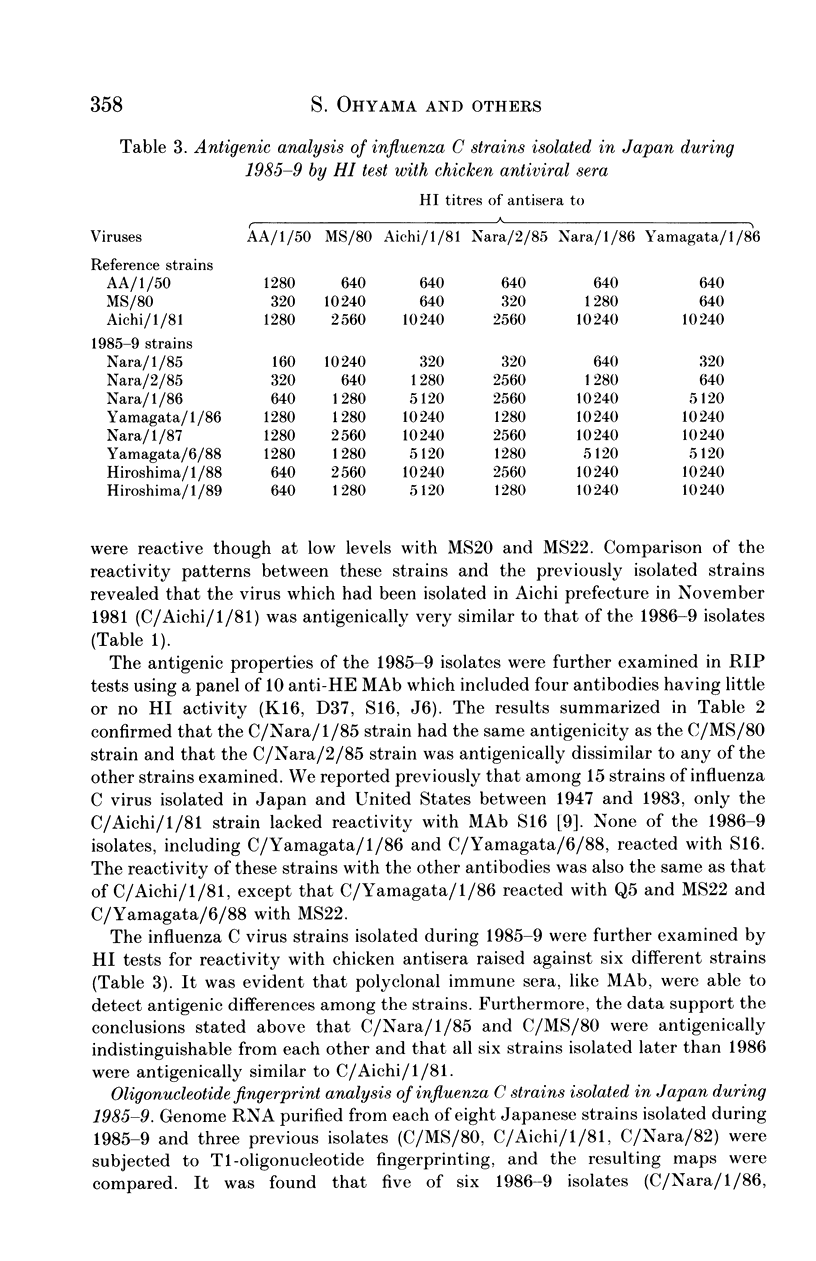
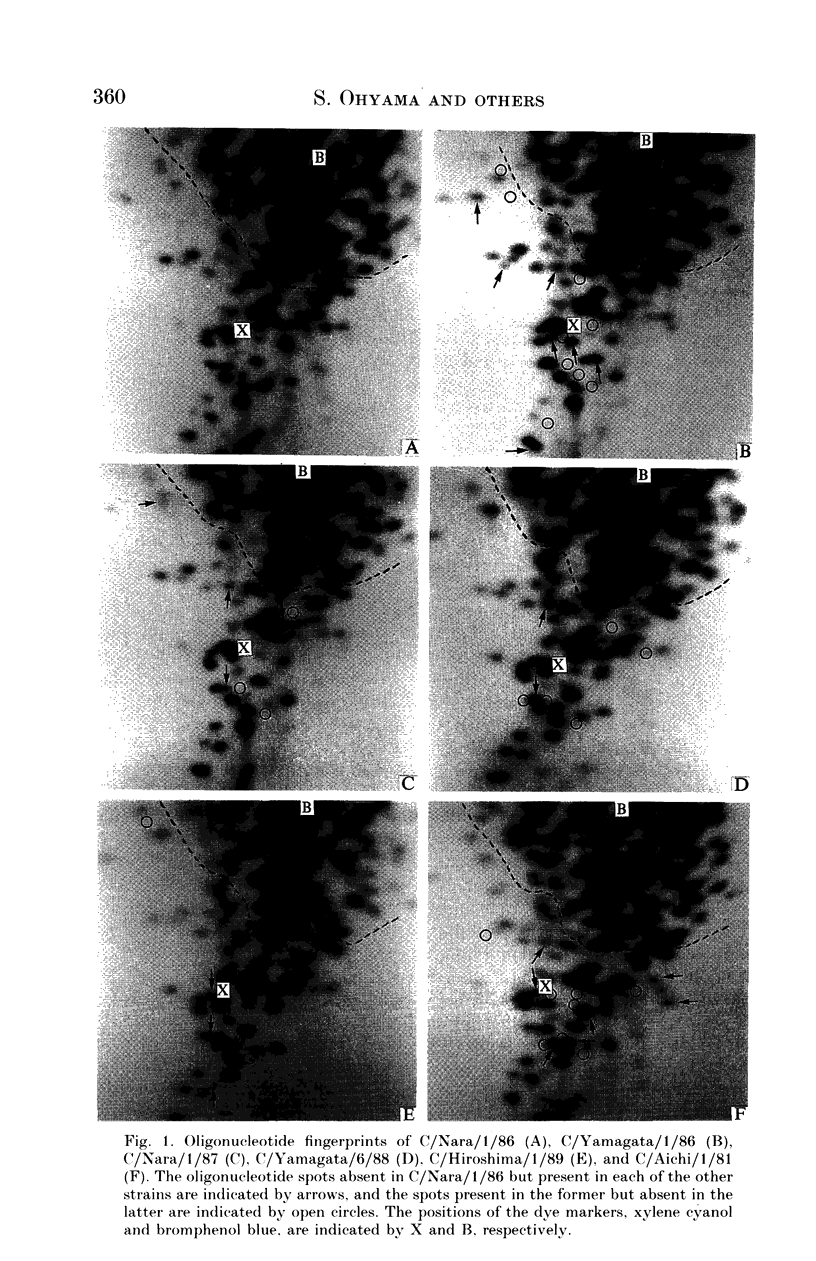
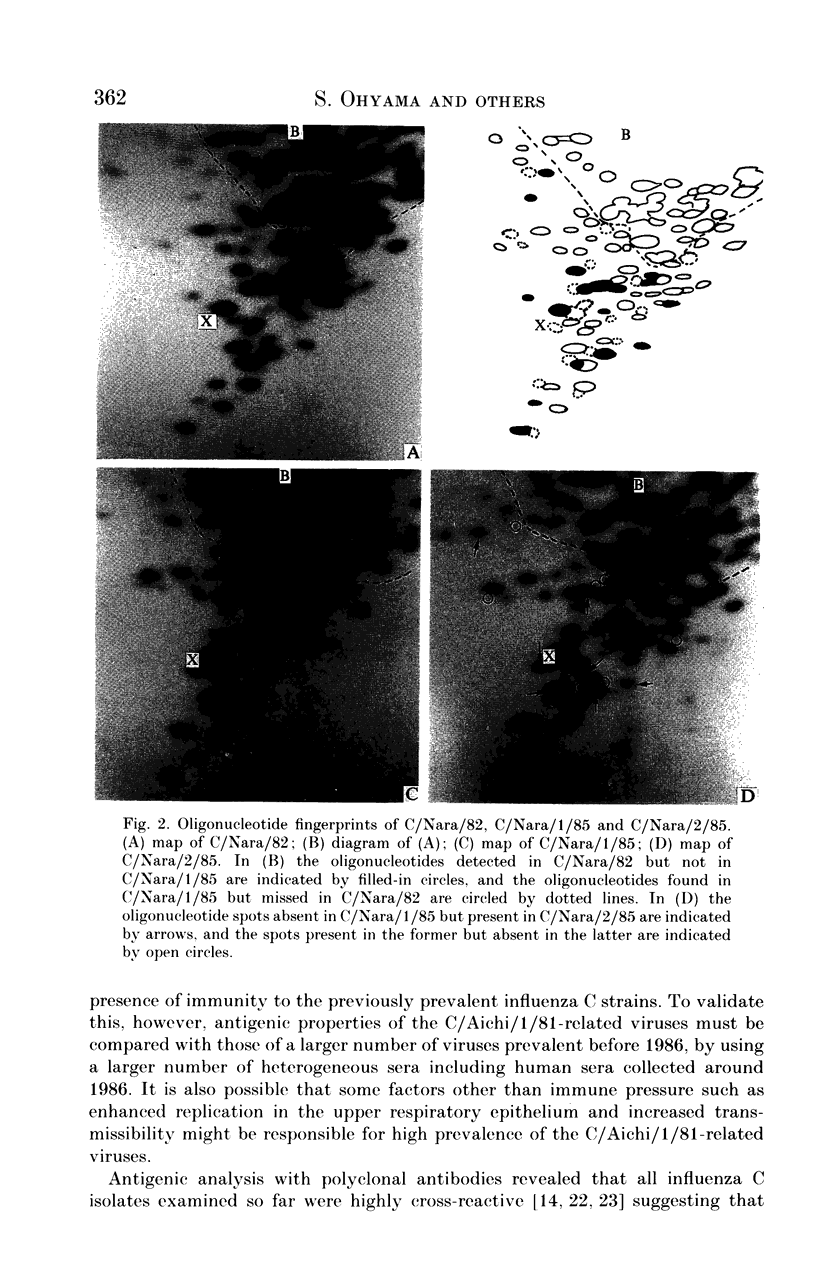
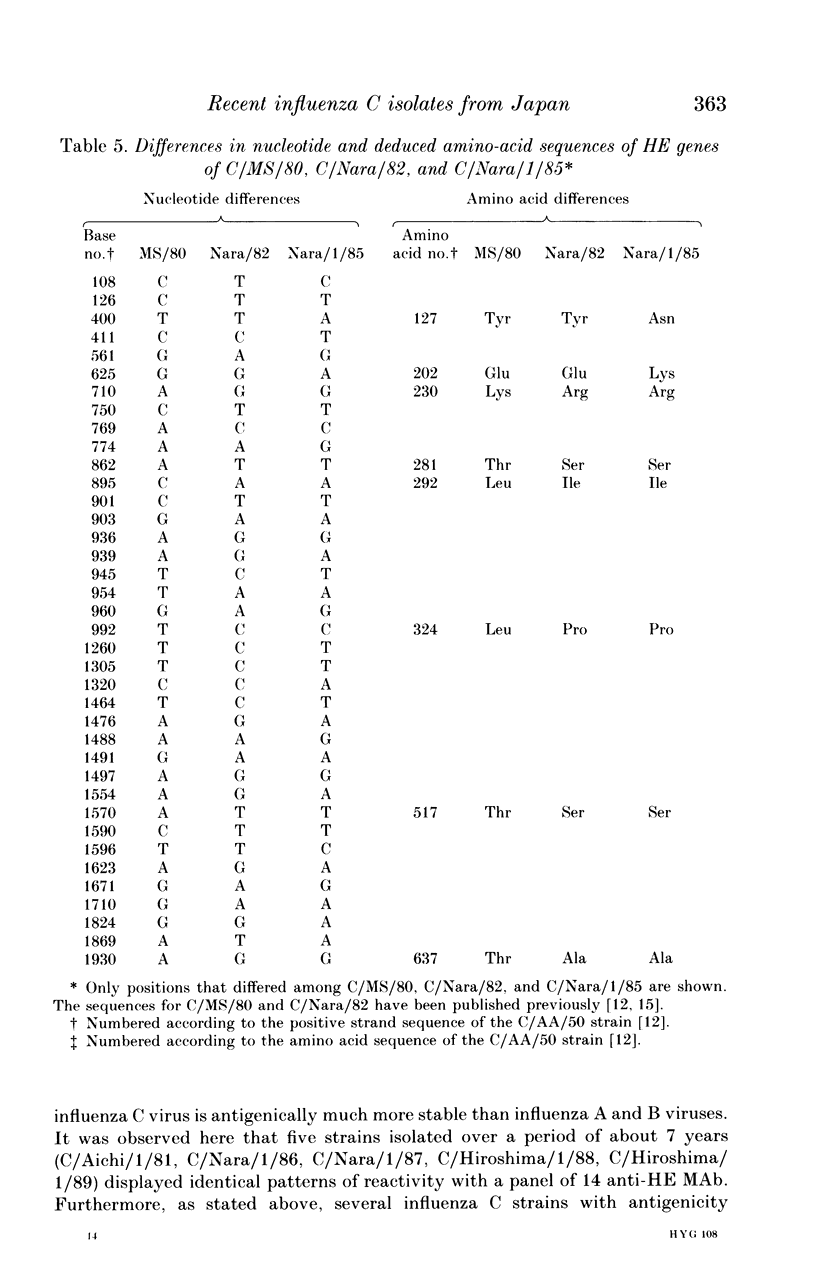
Eight strains of influenza C virus isolated in various areas of Japan between January 1985 and January 1989 were compared using monoclonal antibodies to the haemagglutinin-esterase (HE) glycoproteins and by oligonucleotide mapping of total vRNA. Five of six strains isolated during 1986-9 were closely related to one another and also resembled the virus, C/Aichi/1/81, isolated in 1981 in Aichi prefecture. This suggests that the C/Aichi/1/81-related viruses had an epidemiological advantage over any co-circulating viruses at least during that period. One of two 1985 isolates (C/Nara/1/85) was antigenically indistinguishable from the C/Mississippi/1/80 strain though their oligonucleotide patterns were markedly different from each other. This raises the possibility that C/Nara/1/85 may be a recombinant virus which receives its HE gene from the C/Mississippi/1/80-related parent.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Kitame F., Sugawara K., Nishimura H., Nakamura K. Antigenic and genetic characterization of three influenza C strains isolated in the Kinki district of Japan in 1982-1983. Virology. 1989 Sep;172(1):125–133. doi: 10.1016/0042-6822(89)90114-1. [DOI] [PubMed] [Google Scholar]
- Both G. W., Sleigh M. J., Cox N. J., Kendal A. P. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J Virol. 1983 Oct;48(1):52–60. doi: 10.1128/jvi.48.1.52-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Desselberger U., Krystal M., Palese P. Noncumulative sequence changes in the hemagglutinin genes of influenza C virus isolates. Virology. 1985 Oct 30;146(2):221–232. doi: 10.1016/0042-6822(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Fitch W. M., Palese P. Epidemiology of influenza C virus in man: multiple evolutionary lineages and low rate of change. Virology. 1986 Aug;153(1):12–21. doi: 10.1016/0042-6822(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Czekalowski J. W., Prasad A. K. Studies on influenza virus. I. Antigenic variation in influenza virus type C. Arch Gesamte Virusforsch. 1973;42(3):215–227. doi: 10.1007/BF01265646. [DOI] [PubMed] [Google Scholar]
- Homma M., Ohyama S., Katagiri S. Age distribution of the antibody to type C influenza virus. Microbiol Immunol. 1982;26(7):639–642. doi: 10.1111/mim.1982.26.7.639. [DOI] [PubMed] [Google Scholar]
- Hongo S., Sugawara K., Homma M., Nakamura K. The functions of oligosaccharide chains associated with influenza C viral glycoproteins. I. The formation of influenza C virus particles in the absence of glycosylation. Arch Virol. 1986;89(1-4):171–187. doi: 10.1007/BF01309887. [DOI] [PubMed] [Google Scholar]
- Hongo S., Sugawara K., Homma M., Nakamura K. The functions of oligosaccharide chains associated with influenza C viral glycoproteins. II. The role of carbohydrates in the antigenic properties of influenza C viral glycoproteins. Arch Virol. 1986;89(1-4):189–201. doi: 10.1007/BF01309888. [DOI] [PubMed] [Google Scholar]
- Katagiri S., Ohizumi A., Homma M. An outbreak of type C influenza in a children's home. J Infect Dis. 1983 Jul;148(1):51–56. doi: 10.1093/infdis/148.1.51. [DOI] [PubMed] [Google Scholar]
- Katagiri S., Ohizumi A., Ohyama S., Homma M. Follow-up study of type C influenza outbreak in a children's home. Microbiol Immunol. 1987;31(4):337–343. doi: 10.1111/j.1348-0421.1987.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Kawamura H., Tashiro M., Kitame F., Homma M., Nakamura K. Genetic variation among human strains of influenza C virus isolated in Japan. Virus Res. 1986 May;4(3):275–288. doi: 10.1016/0168-1702(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert H., Petri T., Bishop D. H. Oligonucleotide fingerprint analyses of influenza C virion RNA recovered from five different isolates. Arch Virol. 1981;67(2):141–147. doi: 10.1007/BF01318597. [DOI] [PubMed] [Google Scholar]
- Nakada S., Creager R. S., Krystal M., Aaronson R. P., Palese P. Influenza C virus hemagglutinin: comparison with influenza A and B virus hemagglutinins. J Virol. 1984 Apr;50(1):118–124. doi: 10.1128/jvi.50.1.118-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Sugawara K., Kitame F., Nakamura K., Sasaki H. Prevalence of the antibody to influenza C virus in a northern Luzon Highland Village, Philippines. Microbiol Immunol. 1987;31(11):1137–1143. doi: 10.1111/j.1348-0421.1987.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. B., Compans R. W. Structure of the influenza C glycoprotein gene as determined from cloned DNA. Virus Res. 1984;1(4):281–296. doi: 10.1016/0168-1702(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Palese P. Isolation of influenza C virus recombinants. J Virol. 1979 Dec;32(3):1006–1014. doi: 10.1128/jvi.32.3.1006-1014.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond F. L., Caton A. J., Cox N. J., Kendal A. P., Brownlee G. G. The antigenicity and evolution of influenza H1 haemagglutinin, from 1950-1957 and 1977-1983: two pathways from one gene. Virology. 1986 Jan 30;148(2):275–287. doi: 10.1016/0042-6822(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Kitame F., Nishimura H., Nakamura K. Operational and topological analyses of antigenic sites on influenza C virus glycoprotein and their dependence on glycosylation. J Gen Virol. 1988 Mar;69(Pt 3):537–547. doi: 10.1099/0022-1317-69-3-537. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Nishimura H., Kitame F., Nakamura K. Antigenic variation among human strains of influenza C virus detected with monoclonal antibodies to gp88 glycoprotein. Virus Res. 1986 Oct;6(1):27–32. doi: 10.1016/0168-1702(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Yuanji G., Desselberger U. Genome analysis of influenza C viruses isolated in 1981/82 from pigs in China. J Gen Virol. 1984 Nov;65(Pt 11):1857–1872. doi: 10.1099/0022-1317-65-11-1857. [DOI] [PubMed] [Google Scholar]