Abstract
Despite extensive theoretical and empirical research into offspring food solicitation behaviour as a model for parent–offspring conflict and communication, the adaptive value of parental responsiveness to begging has never been tested experimentally. Game theory models, as well as empirical studies, suggest that begging conveys information on offspring state, which implies that parental investment can be better translated to fitness by responding to begging when allocating resources rather than by ignoring it. However, this assumption and its underlying mechanisms have received little or no attention. Here we show by experiments with hand-raised house sparrow (Passer domesticus) nestlings that a ‘responsive parent’ will do better than a hypothetical ‘non-responsive’ mutant (that provides similar food amounts, but irrespective of begging). This is neither because food-deprived nestlings convert food to mass more efficiently, however, nor because responsiveness reduces costly begging. Rather, responsiveness to begging is adaptive because it reduces two opposing risks: one is wasting time when returning too soon to feed already satiated nestlings and the other is repeatedly overlooking some nestlings as a result of the stochastic nature of a random, non-responsive strategy. This study provides the first experimental evidence for the adaptive value of parental responsiveness to offspring begging.
Keywords: parent–offspring conflict, animal communication, parental care, signalling
1. Introduction
The young of many birds and mammals solicit food from their parents using begging displays that are often extravagant and might be costly (Kilner & Johnstone 1997; Budden & Wright 2001; Kilner 2001; Wright & Leonard 2002). Parents seem to use these begging displays to decide how to allocate food within a brood (Kacelnik et al. 1995; Kilner 1995; Mondloch 1995; Leonard & Horn 2001), as well as to adjust food delivery rate to the whole brood (Burford et al. 1998; Leonard & Horn 1998; Price 1998; Kilner et al. 1999, but see Clark & Lee 1998). The evolutionary theory of begging behaviour was initially based on a ‘scramble competition’ scenario in which offspring compete by begging, and parents accept the outcome of this competition, thereby responding to begging (e.g. Macnair & Parker 1979; Parker et al. 2002). An alternative view that has developed more recently suggests that begging displays evolved (initially or subsequently) as honest signals of offspring ‘need’ (Godfray 1991, 1995; Rodríguez-Gironés et al. 1996; Godfray & Johnstone 2000; Johnstone 2004). These signalling models have elicited extensive empirical research showing that begging intensifies with need variables such as food deprivation time (Kilner & Johnstone 1997; Iacovides & Evans 1998; Leonard & Horn 1998; Kilner et al. 1999), and that parents indeed use begging intensity to adjust their food delivery (e.g. Kacelnik et al. 1995; Kilner 1995; Leonard & Horn 2001). These findings are consistent with the predictions made by both honest signalling models and scramble competition models (Royle et al. 2002). According to both analyses, offspring begging should reflect, at least to some degree, the offspring's potential fitness gain from receiving extra resources (offspring need; see Godfray & Johnstone 2000; Parker et al. 2002). Thus, whether it is an honest signal of need, or an outcome of sibling competition, parents should respond to begging (even if they merely succumb to the strongest stimulus, as in scramble competition) simply because it directs them to feed offspring that can better translate food into fitness.
The notion that parental responsiveness to offspring begging is adaptive goes back to Parker and Macnair's seminal paper (Parker & Macnair 1979), where a cost sustained by non-responsive parents was suggested to emerge ‘because ‘real’ needs are ignored’ (pp. 1221; see also Mock & Parker 1997, pp. 161). In the more recent signalling models (Godfray 1991, 1995; Rodríguez-Gironés et al. 1996; Godfray & Johnstone 2000; Johnstone 2004), parental responsiveness is an obtained evolutionarily stable strategy, implying that at signalling equilibrium responsive parents gain more fitness than would non-responsive parents. It is quite surprising that such a widespread theoretical notion has never been tested directly, but perhaps this is due to the obvious difficulties in manipulating parental response strategies. Moreover, the possible mechanisms underlying the adaptive value of parental responsiveness to begging have received little or no attention (but see Karasov & Wright (2002) for a discussion of digestive efficiency and begging). Thus, while current models assume certain relationships among offspring fitness, parental provisioning and offspring state, the biological factors, or mechanisms, that generate these fitness functions are virtually unknown (Cotton et al. 1999). In fact, some of these biological factors may result from the dynamic nature of feeding sequences that is admittedly not captured by current modelling (Godfray & Johnstone 2000).
There are at least four, not mutually exclusive, mechanisms that may underlie the adaptive value of parental responsiveness to begging: (i) Owing to some physiological constraints, a given amount of food can be converted to more mass when the offspring's digestive system is emptier (e.g. as a result of increased digestive efficiency). A version of this mechanism, assumed by Price et al. (2002), may have underpinned the fixation of parental responsiveness in their computer simulation and is somewhat supported by the fact that nestlings fed with excessive daily amounts showed a lower digestive efficiency (Lepczyk et al. 1998), or did not grow faster (Bengtsson & Rydén 1983; Stamps et al. 1985; Mock et al. 2005). (ii) Considering that the digestive system is limited in size, feeding an ‘empty’ offspring may be faster and easier for the parents than feeding a ‘full’ offspring that might even refuse to eat. Responsive parents can therefore save time, which would have been wasted by non-responsive parents when trying to feed recently fed offspring. Alternatively, such feeding refusals may cause a non-responsive parent to eat the food, and eventually to shift from its intended balance between present and future parental investment. (iii) In species where discrete units of food are allocated, in each visit, to only one or two of a group of offspring (as in many bird nestlings), a non-responsive parent faces the risk of repeatedly overlooking a particular offspring simply by chance (to the extent of causing it harm). Such a possibility was suggested by Davis et al.' s computer simulation (1999), where a random feeding rule may have caused some of the nestlings to die because they were ‘randomly skipped for too many feedings’ (pp. 1793). Responding to begging reduces this risk because food-deprived offspring, which beg intensely, will be fed promptly and will cease to be neglected. (iv) Finally, responsive parents may also benefit from minimizing costly begging by their offspring (after begging has evolved), if satiated offspring beg less or less intensely.
To test whether parental response to begging is potentially adaptive and which of the above mechanisms may underlie its adaptive value, we hand-raised pairs of house sparrow nestlings while simulating either a ‘responsive parent’ (responsive treatment, RT) or a hypothetical ‘non-responsive’ mutant (non-responsive treatment, NRT). For RT pairs, we allocated all or most of the food in each feeding visit to the nestling begging most intensely, and then used the pair's joint effort to determine the subsequent visit rate. NRT pairs received exactly the same number of feeding visits as RT pairs had received (with equal meal sizes), but the distribution of food within the brood, as well as the distribution of visits along the day, were random and irrespective of begging intensity. To determine whether the RT resulted in a better outcome than the NRT (and why), we compared the nestlings' mass gain over the course of the experiment (nestling growth rate has been shown to correlate with post-fledging fitness, e.g. Gebhardt-Henrich & Richner 1998), as well as their digestive efficiency, feeding chronology and begging intensity.
2. Material and methods
(a) Subjects and general methods
The 40 pairs of nestlings used for the experiment were taken from different broods in captive (10 pairs in each treatment) and free-living (10 pairs in each treatment) house sparrow colonies in the I. Meier Segals Garden for Zoological Research of Tel-Aviv University during the spring of 2005. In the population from which these colonies are derived, a positive relationship has been found between the begging posture and the probability of being fed, and food-deprived nestlings have been shown to use more erect postures (Yedvab 1999; Kedar 2003). Each pair of siblings (chosen as the two nestlings closest in mass) was taken from the nest between 06.40 and 07.00 hours on day 4 post hatching (hatching=day 0), an age at which house sparrow nestlings are in the middle of the linear phase of their growth curve (Lepczyk & Karasov 2000). Nestlings were then marked with non-toxic acrylic paint to enable individual recognition and were kept together for 48 hours in a custom-made incubator (D.M.P. Engineering Ltd. based on the Lory10 model) set to 37°C at 50–70% RH. The light in the incubator, kept only bright enough to allow video recording, was on from 07.30 to 19.30 hours. Throughout this period, the two experiment days, we simulated a large number of parental feeding visits. At the onset of each visit, we stimulated the nestlings to beg by turning the incubator light switch off and on (thereby also causing a sound stimulus), and then let them beg for 4 s with no intervention. After this period, each nestling was fed either a ‘small meal’ (0.1 ml of chopped fly larvae through a syringe), a ‘large meal’ (two small meals one after the other) or not fed at all. This, as well as the number of feeding visits throughout the day and the length of the inter-visit intervals, was determined according to the different treatment protocols (see below). In both treatment groups, when a nestling was to be fed but its beak was closed, another darkening stimulus was given, and then if needed also one light touch to the beak with the syringe. If the beak still remained closed, the meal (or half-meal, if the nestling had already been fed the first half of a large meal) was not given and was noted as ‘untaken food’. At the end of the experiment, nestlings were fed to satiation and returned either to their original nest (n=34 pairs) or, if impossible, to another nest of similar age (n=6 pairs). No mortality or apparent stress was recorded during the experiments.
(b) Simulating the responsive parent
In the responsive parent treatment group (RT) begging intensity affected food delivery in two ways, simulating the response of natural parents to begging. First, all or most of the food in each visit was allocated to the nestling using the most erect begging posture (for the effect of begging intensity on within-brood food allocation see Kacelnik et al. 1995; Kilner 1995; Mondloch 1995; Leonard & Horn 2001); during the 4 s of uninterrupted begging, the experimenter determined which of the nestlings was using a more erect begging posture, and that nestling was subsequently fed a large meal (0.2 ml). If the experimenter could not decide (this happened in 19.6±8.8% of feeding visits), the large meal was given to one of the nestlings at random (according to a chart prepared in advance). To mimic the situation at the nest (Dor et al. 2007), in half of the feeding visits (randomly selected in advance) a small meal (0.1 ml) was given to the remaining nestling after its sibling was first offered a large meal (based on its higher begging). The second way by which begging intensity affected food delivery was by determining the food delivery rate to the whole ‘brood’ (the pair of nestlings in this case; for the effect of begging intensity on food delivery to the brood see Burford et al. 1998; Leonard & Horn 1998; Price 1998; Kilner et al. 1999); after each feeding visit was over, the experimenter used the video recording of the 4 s of begging to score the begging posture of each nestling from 0 to 3 (see ‘measuring begging intensity’ below). The sum of scores for the two nestlings (0–6) was then used to determine the time interval until the next visit: more erect begging postures led to a shorter time interval until the next visit. Begging score sums of 0, 1, 2, 3, 4, 5 and 6 led to inter-visit intervals of 35, 30, 25, 20, 15, 10 and 5 min, respectively. These intervals (together with meal sizes, see above) ensured that not begging at all would have led to not receiving enough food, while maximal begging (throughout the day) would have led to at least twice the food amount needed for normal growth of house sparrow nestlings of this age (according to the amounts fed to control nestlings in Lepczyk et al. (1998)).
(c) Simulating the non-responsive parent
In the ‘non-responsive’ parent treatment group (NRT), begging intensity did not affect food delivery. In each feeding visit, the nestling that received the large meal was randomly chosen according to a chart prepared in advance (the other nestling received a small meal in half of the feeding visits, as in the RT group). In addition, the time intervals between the visits (and consequently the total number of visits) were not related to begging intensity, as they had been for RT pairs. Instead, each NRT pair was yoked (matched) to a specific RT pair of similar initial mass (as far as possible) that came from a similar colony (captive or free-living). The daily protocols that had been created according to that specific RT pair's begging intensity in the first and second days of the experiment (see §2b above) were used for the first and second days of the yoked NRT pair, after the sequence of inter-visit intervals had been randomly mixed. The NRT pair thus received the same number of daily visits as had the RT pair it was yoked to, with the same time intervals, but in a random sequence (unrelated to its begging). Note that this procedure means that the non-responsive mutant parent we have simulated is assumed to be able to estimate the daily amount of resources appropriate for its brood. It is also important to note that although the experimental design makes it unavoidable that each NRT experiment was conducted (soon) after its respective RT experiment, both RT and NRT experiments were conducted throughout the entire breeding season.
(d) Measuring begging intensity
All feeding visits were recorded using a digital Sony video camera (DCR-TRV355E). After the breeding season was over, the begging postures used by each of the nestlings at each visit were analysed (blind to treatment group) on a computer screen using Adobe Premier v. 6.5. Each nestling was given a begging posture score for one frame (1/25 s) from each of the 4 s of uninterrupted begging in each visit, and a mean visit score was calculated per chick. The graphic scale used for scoring begging postures was 0, no begging; and 1–3 representing increasingly erect body positions while gaping (with 3 given for fully erect body postures, i.e. standing up; for similar methodology see Kacelnik et al. 1995; Kilner 1995; Lotem 1998; Kedar et al. 2000; Dor et al. 2007). This 0–3 scale was also the one used during the RT experiments to determine the time interval between visits (see §2b above).
(e) Chick growth and digestive efficiency data
To compare the two treatment groups, we weighed the nestlings on the first and last mornings (to the nearest 0.1 g) and calculated their mass gain over the course of the experiment. Wing length measurements were also taken at these times (to the nearest 0.1 mm). To assess each pair's digestive efficiency, food samples were kept (one sample from each batch), and all the faeces excreted by the pair were collected each morning and throughout the experimental days (see Guglielmo & Karasov 1993; Afik & Karasov 1995; Kilner 2001 for using nestling faeces to assess digestive efficiency). Only the second experimental day's data were used, in order to reduce the effect of food present in the digestive tract prior to the experiment. Excreta and food samples were frozen at −20°C, weighed to the nearest 0.0001 g (using Sartorius BP121S electronic scales), dried at 60°C and weighed again. These data, together with the exact food amounts consumed by each pair on the second day, were used to calculate the apparent assimilation mass coefficient (AMC*, calculated as (dry mass consumed−dry mass excreted)/dry mass consumed; see Guglielmo & Karasov 1993; Afik & Karasov 1995). (Two pairs were not included in this analysis because faecal sacs were not collected). The energy content of food samples and second day excreta of each pair was measured on a ballistic bomb calorimeter (Gallenkamp cb-370), with a benzoic acid standard. In addition to the two pairs for which faeces were not collected, one pair with insufficient faecal dry mass to measure energy content was excluded from this analysis. These data were then used to calculate another index of digestive efficiency (or utilization efficiency), the apparent metabolizable energy coefficient (MEC* calculated as (energy consumed−energy excreted)/energy consumed; see Guglielmo & Karasov 1993; Afik & Karasov 1995).
(f) Statistics
Brood averages were calculated for all variables and used as the independent data point for all analyses and figures, unless otherwise specified (including the mean±s.d. values given in the text). Distributions of all variables used for parametric tests were first found not to differ significantly from normal using the Kolmogorov–Smirnov test. For the three dependent variables that were based on a ratio or on a conventional index that includes a ratio (mass gain per food consumed, apparent AMC and apparent MEC), we also analysed the data using the SAS system for mixed models (v. 8.2) to exclude possible artefacts caused from using ratios. These Proc Mixed models, with the numerator of each ratio as the dependent variable, treatment as a fixed effect, RT–NRT yoked pairs as a random effect, and with the denominator as a covariate, all produced similar results (non-significant, all p values>0.29) to those of the tests performed on the calculated indices (see §3).
3. Results
(a) Verifying treatment integrity
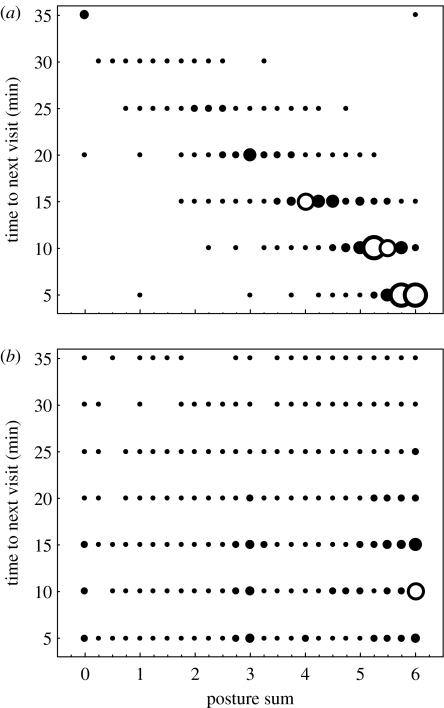
Because the simulation of responsive parents in the RT group was based on the experimenter's assessment of begging postures at each visit (see §2b), it was important to verify that this assessment was sufficiently accurate to create the intended differences between the two treatment groups. The video analysis of begging postures that was carried out after the breeding season allowed us to verify treatment integrity. First, as intended for food allocation within the brood, in feeding visits where begging postures differed between the two nestlings (79±11% of visits), the large meal was given to the nestling that used the more erect body posture in 87.5±5% of cases in the RT pairs, and only in 49.9±4.4% of the cases in the NRT pairs (as expected for a random food allocation). Moreover, the effect of experimenter errors in the RT group (i.e. the cases not included in the above-mentioned 87.5% correct decisions) was not severe as they occurred mainly when differences in begging score were small (only 0.48±0.37, compared with 1.19±0.81 when correct decisions were made). Second, as intended for the feeding schedule, only in RT pairs was there a clear negative correlation between the pair's begging posture sum at each visit (see §2b) and the time interval until the next visit (figure 1a). Data for each pair separately show significant negative correlation with p<0.0001 for all 20 RT pairs and with an average rs value of −0.87±0.06. In contrast, no such correlation was found in the NRT group (figure 1b). Data for each pair separately show significant correlation in only one of the 20 NRT pairs (with an rs value of only −0.23), and the average rs value for the 20 NRT pairs was −0.005±0.11. Thus, we can conclude that the assessment of begging postures by the experimenter was sufficiently accurate to create the intended differences between the treatments.
Figure 1.
Time interval to the next feeding visit as a function of the pair's ‘begging posture sum’ (sum of the visit posture score for both nestlings, see §2). (a) All the visits in the RT group. (b) All the visits in the NRT group. Six increasing circle sizes represent <20, 20–40, 40–60, 60–80, 80–100 and 100<overlapping data points. Correlation values for the separate pairs' data are given in the text.
(b) Analysing treatment effect
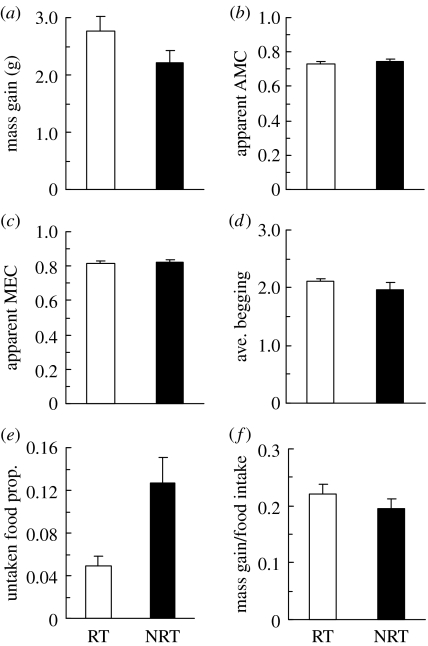
The daily numbers of feeding visits for both treatments (resulting from responding to the begging intensity of RT pairs) were 47.8±7.58 and 55.8±8.48 visits for the first and second day of the experiment, respectively (corresponding to 5.97±0.97 and 6.98±1.04 ml of chopped fly larvae per nestling, respectively). During the course of the experiment, RT nestlings gained more mass than did NRT nestlings (2.77±1.13 and 2.21±0.95 g, respectively; paired t19=2.49, p=0.0219; figure 2a), while wing growth did not differ between the treatment groups (8.16±1.69 and 7.99±1.89 mm for RT and NRT nestlings, respectively; paired t19=0.43, p=0.67). In contrast to the difference in mass gain, there was no difference in the two indices of digestive efficiency: AMC* (0.73±0.06 and 0.74±0.07 for RT and NRT pairs, respectively; paired t17=0.58, p=0.57; figure 2b) and MEC* (0.81±0.06 and 0.82±0.07 for RT and NRT pairs, respectively; paired t16=0.47, p=0.64; figure 2c). There was also no difference in the average begging posture score of the two treatment groups (2.10±0.26 and 1.97±0.54 for RT and NRT pairs, respectively; paired t19=0.95, p=0.36; figure 2d), implying that the NRT nestlings had not learnt the irrelevance of their begging intensity to their being fed (which is consistent with additional recent experiments with house sparrow nestlings, U. Grodzinski et al. 2004–2005, unpublished data). In light of the above results, the lower mass gain of nestlings of the NRT group (figure 2a) is explained neither by a lower digestive efficiency nor by the possible cost of additional begging.
Figure 2.
Comparison of the RT and NRT nestlings in respect to: (a) mass gain; (b) apparent AMC; (c) apparent MEC; (d) average begging posture; (e) the proportion of untaken food (due to feeding refusals) and (f) mass gain per food consumed (g ml−1). Means with standard error bars are shown.
Rather, the main source for the differences in mass gain appears to be the proportion of food untaken by the nestlings (of the total amount offered to them, see §2a), which was significantly lower for RT chicks (0.05±0.04 versus 0.13±0.11 for RT and NRT chicks, respectively; Wilcoxon matched pair test Z=2.58, N=20, p=0.01; figure 2e). The lower rate of such food refusals by RT nestlings resulted in a higher overall food intake throughout the experiment (12.35±2.07 versus 11.25±1.94 ml for RT and NRT nestlings, respectively; paired t19=3.34, p=0.0034). Therefore, there was no difference in the mass gain relative to the total amount of food consumed (mass gain/food consumed; 0.22±0.07 and 0.19±0.08 g ml−1 for RT and NRT nestlings, respectively; paired t19=1.45, p=0.1634; figure 2f). Finally, because the difference in mass gain between RT and NRT nestlings can be explained by the difference in untaken food, there is no evidence that NRT nestlings have also suffered a greater risk of being overlooked.
(c) The reasons for food refusal in the NRT
In theory, the reason for feeding refusal by nestlings can either be because non-responsive parents sometimes bring too much food to the nest, or because although they bring the correct daily amount of food, their arbitrary feeding schedule causes them to occasionally try to feed satiated nestlings. Our experimental set-up allowed us to explore the relative contribution of these aspects. Despite our attempt to match each NRT pair to an RT pair by initial mass, there was always some difference between the average initial mass of each NRT pair and its RT pair counterpart (with absolute values of 0.54±0.43 g). These differences were not biased towards a certain treatment, so the average initial mass was not different between treatments (paired t19=0.79, p=0.44). However, the differences did create a situation in which for each NRT pair, the daily food amount (determined by the begging of its RT pair counterpart, see §2c) could be slightly above or below the daily amount suitable for its mass. It was therefore interesting to examine whether these deviations had any effect on the proportion of untaken food.
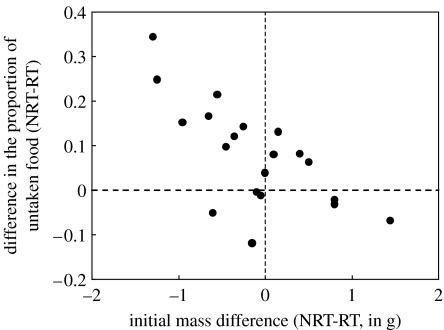
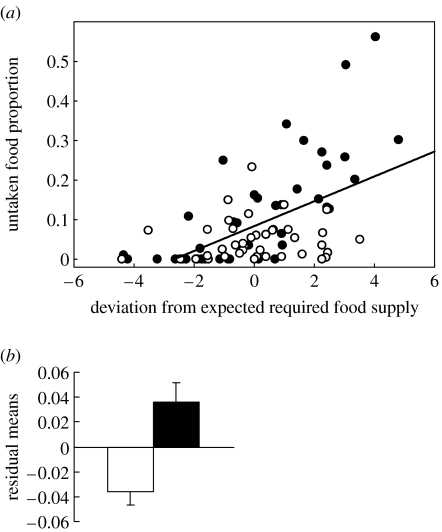
Figure 3 shows the difference in the proportion of untaken food between each NRT pair and its respective RT pair plotted against the initial difference in mass between the pairs, revealing that these variables are indeed related (rs=−0.66, p=0.0016). This finding is a clear indication that, at least to some extent, feeding refusals by our NRT nestlings occurred when their daily food requirements were overestimated, or in other words, when we tried to feed them too much (figure 3, left side). However, subsequent analysis revealed that this overestimation of food requirements could not fully account for the differences in untaken food (figure 2e). We statistically controlled for this ‘overestimation effect’ by calculating the amount of food that should have been offered to each nestling, based on its initial mass (using a regression line of the total amount of food offered to RT nestlings over their initial mass; food amount=1.20×(initial mass)+3.67, , p<0.0001), and then calculating the extent to which the actual amount offered to each nestling deviated from the required amount (i.e. the residuals from this regression). We then plotted the proportion of untaken food for each nestling against this deviation (figure 4a). The residuals taken from this new regression line (figure 4b) were still significantly lower for RT than for NRT nestlings (Wilcoxon matched pair test Z=2.61, N=20, p=0.009). Thus, food refusal was not only a consequence of occasionally offering excessive daily food amounts to NRT nestlings, but also a result of the random, non-responsive distribution of the daily amount throughout the day and among the nestlings.
Figure 3.
The difference in the proportion of untaken food between each NRT pair and the RT pair it was matched to, plotted against the initial difference in mass between the pairs. Dashed lines indicate no difference between NRT pairs and their respective RT pairs.
Figure 4.
Controlling for the effect of errors in estimating daily food requirements on the proportion of untaken food. (a) The proportion of untaken food plotted against the deviation of the actual amount of food offered to each nestling from the food amount required according to its initial mass (see §3c for details), for RT (open circles) and NRT (filled circles) nestlings. The regression line for both treatment groups together is shown. (b) Mean±s.e. residuals from the regression line shown in (a) for RT (open bar) and NRT (filled bar) nestlings. Data for all nestlings are shown here, but we conservatively used the mean residual for each pair to compare statistically between treatment groups.
4. Discussion
This study was a first attempt to examine whether responding to nestling begging is in any way better than providing the same amount of food resources in a random, non-responsive manner. While nestlings that were subjected to a treatment responsive to their begging intensity gained more mass than those subjected to a non-responsive treatment (figure 2a), this was neither due to better digestive efficiency, which could have led to better food-to-mass transformation (figure 2b,c,f), nor was it because responsiveness reduced begging (figure 2d). Rather, the proportion of food that was not given due to nestling refusal was lower in the RT (figure 2e), leading to higher food intake and the consequent higher mass gain. In other words, mass gain was related only to the total amount of food consumed by nestlings, and not to whether or not nestlings consumed more of the food when begging intensely. The results therefore do not support the first proposed mechanism, according to which responsiveness is adaptive because food is converted to more mass when the offspring's digestive system is emptier (see §1). On the contrary, it seems that as long as the nestlings are willing to take the food, their digestive system is sufficiently robust to use it equally well. The rejection of this ‘efficiency-based’ mechanism by our data is important because, although no mechanisms are explicitly specified in analytical models of parent–offspring interaction (e.g. Godfray 1991, 1995; Godfray & Johnstone 2000; Parker et al. 2002; Johnstone 2004), this was the mechanism assumed in the few previous attempts to simulate this dynamic interaction (Price et al. 2002; Biran 2004) and is held as a common interpretation of current theory by people in the field.
(a) Parental responsiveness and the cost of feeding refusals
Our results do support the second mechanism proposed in the introduction, according to which responsive parents may simply save time that would have been wasted trying to feed satiated nestlings that refuse to eat. In our experiment, feeding refusals ended with our taking the food away, which resulted in a smaller mass gain by nestlings of the NRT group. Under natural conditions, there might be several possible outcomes of such events, but all are likely to be costly for the parent. First, the parent itself may eat the food (e.g. Canestrari et al. 2004), but then it would have wasted the time and effort of flying from where the food was found, and may eventually shift from its intended balance between present and future parental investment. Another option is to persist for longer, perhaps pecking the nestling gently until it opens its beak and is willing to take the food. This behaviour is commonly observed when feeding young nestlings (Stamps et al. 1985), but is clearly time-consuming and may come at the expense of future parental foraging and feeding trips to the nest. Alternatively, after one nestling refuses feeding, the parent may switch to feeding another. However, this would also take some time (needed for testing the first nestling) and can help only when other nestlings are willing to eat (i.e. it can correct for random errors in food allocation within the nest, but not for a temporal excess in food supply to the entire brood). Thus, there seems to be no simple way to escape the cost entailed by feeding refusals. On the other hand, our results show that responsiveness to begging can minimize such refusals in the first place. Moreover, as illustrated by our analysis (figures 3 and 4), parental responsiveness to begging can minimize feeding refusals originating from two sources: those that result from overestimating the daily amount of food and those that result from distributing an appropriate amount of food during the day without considering the nestlings' willingness to eat.
(b) Parental responsiveness and the risk of neglecting a nestling by chance
While the cost of feeding refusals may explain why parental responsiveness to begging is adaptive, it may not be sufficient to explain why begging has evolved as a graded signal of food deprivation (e.g. Iacovides & Evans 1998; Leonard & Horn 1998; Kilner et al. 1999). In other words, if all that parents need to know in advance is whether offspring will take the food or not, then an ‘all or none’ signal (or cue) of food receptivity should do the job well enough. In fact, our results suggest that as long as the nestlings can take the food, the parents should feed them, irrespective of how long ago they received their last meal. The solution to this problem may come when considering also the third mechanism proposed in the introduction: namely, that a non-responsive parent faces the risk of repeatedly neglecting a particular offspring simply by chance. This cost was unlikely to be expressed in our experiment because we used a brood size of only two nestlings and provided secondary feedings (of half the size of primary feedings) in half of the visits. Under such conditions, the risk of repeatedly overlooking one of the two nestlings by chance was negligible, and even less likely to result in poor growth of this nestling. However, under natural conditions, random food allocation in larger broods inevitably increases this risk, which under poor conditions can reduce the survival probability of such neglected chicks. In this case, the evolution of begging as a graded signal of starvation risk (Clark 2002) makes adaptive sense: the longer a nestling is food deprived, the greater the risk for its survival, which can then justify higher begging (even at a greater begging cost). Seen in this light, begging conveys two main messages to the parents: the first is that of feeding receptivity (an ‘all or none’ signal, or primitive cue) and the second is the risk of suffering from starvation (a graded signal).
(c) The fuel gauge analogy for parental responsiveness
The results of this study offer a model to explain the adaptive value of parental responsiveness to begging that is somewhat analogous to people's response to the fuel gauge in cars. We offer this analogy because it represents a clear case in which the amount of fuel already present in the system does not change the efficiency by which added fuel will be used. What does matter is not to waste time by returning to the gas station too often and, on the other hand, not to forget to refuel before it is too late. The fuel gauge signals the proximity to each of these opposing risks. While this analogy may not be accurate or appropriate for all parent–offspring communication systems, it offers a clear contrast with efficiency-based models, which assume that food-deprived offspring convert food to mass more efficiently than satiated ones. In future theoretical and experimental works, these contrasting models might be helpful in making explicit assumptions about the mechanisms involved in parent–offspring communication. The results of our study with house sparrow nestlings are consistent with the fuel gauge analogy. They suggest that parental response to begging is adaptive because it reduces two opposing risks: the risk of wasting time when returning too soon to feed satiated nestlings, and the risk of repeatedly overlooking some nestlings as a result of the stochastic nature of a random, non-responsive strategy. Future work is still needed to clarify the extent to which these results are general and robust, or whether different mechanisms may underlie the adaptive value of parental responsiveness to offspring begging in different species.
Acknowledgments
This study was carried out under an animal care permit from the Tel-Aviv University Animal Care Committee (no. L-02-09).
We thank the staff of the I. Meier Segals Garden for Zoological Research of Tel-Aviv University for their help and facilities; I. Choshniak, N. Kronfeld-Schor and A. Hefetz for use of laboratory and equipment; I. Choshniak, N. Kronfeld-Schor and W. H. Karasov for their advice concerning digestive efficiency estimation; and I. Erev, W. H. Karasov, R. M. Kilner, M. A. Rodríguez-Gironés, N. J. Royle, H. Withers, J. Wright, R. Ydenberg and two anonymous reviewers for their comments. This study was funded by the Israel Science Foundation grant 353/03-17.2.
References
- Afik D, Karasov W.H. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology. 1995;76:2247–2257. doi:10.2307/1941699 [Google Scholar]
- Bengtsson H, Rydén O. Parental feeding rate in relation to begging behavior in asynchronously hatched broods of the great tit Parus major—an experimental-study. Behav. Ecol. Sociobiol. 1983;12:243–251. doi:10.1007/BF00290777 [Google Scholar]
- Biran, I. 2004 Nestling begging strategies and learning rules in virtual nests: a computer simulation study. MSc thesis, Tel-Aviv University, Israel.
- Budden A.E, Wright J. In: Begging in nestling birds. Current ornithology. Nolan V, Ketterson E, editors. vol. 16. Kluwer Academic/Plenum; New York, NY: 2001. pp. 83–118. [Google Scholar]
- Burford J.E, Friedrich T.J, Yasukawa K. Response to playback of nestling begging in the red-winged blackbird Agelaius phoeniceus. Anim. Behav. 1998;56:555–561. doi: 10.1006/anbe.1998.0830. doi:10.1006/anbe.1998.0830 [DOI] [PubMed] [Google Scholar]
- Canestrari D, Marcos J.M, Baglione V. False feedings at the nests of carrion crows Corvus corone corone. Behav. Ecol. Sociobiol. 2004;55:477–483. doi:10.1007/s00265-003-0719-8 [Google Scholar]
- Clark A.B. Appetite and the subjectivity of nestling hunger. In: Wright J, Leonard M.L, editors. The evolution of begging: competition, cooperation and communication. Kluwer; Dordrecht, The Netherlands: 2002. pp. 173–198. [Google Scholar]
- Clark A.B, Lee W.H. Red-winged blackbird females fail to increase feeding in response to begging call playbacks. Anim. Behav. 1998;56:563–570. doi: 10.1006/anbe.1998.0831. doi:10.1006/anbe.1998.0831 [DOI] [PubMed] [Google Scholar]
- Cotton P.A, Wright J, Kacelnik A. Chick begging strategies in relation to brood hierarchies and hatching asynchrony. Am. Nat. 1999;153:412–420. doi: 10.1086/303178. doi:10.1086/303178 [DOI] [PubMed] [Google Scholar]
- Davis J.N, Todd P.M, Bullock S. Environment quality predicts parental provisioning decisions. Proc. R. Soc. B. 1999;266:1791–1797. doi:10.1098/rspb.1999.0848 [Google Scholar]
- Dor R, Kedar H, Winkler D.W, Lotem A. Begging in the absence of parents: a “quick on the trigger” strategy to minimize costly misses. Behav. Ecol. 2007;18:97–102. doi:10.1093/beheco/arl056 [Google Scholar]
- Gebhardt-Henrich S, Richner H. Causes of growth variation and its consequences for fitness. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press; New York, NY: 1998. pp. 324–339. [Google Scholar]
- Godfray H.C.J. Signaling of need by offspring to their parents. Nature. 1991;352:328–330. doi:10.1038/352328a0 [Google Scholar]
- Godfray H.C.J. Evolutionary-theory of parent–offspring conflict. Nature. 1995;376:133–138. doi: 10.1038/376133a0. doi:10.1038/376133a0 [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J, Johnstone R.A. Begging and bleating: the evolution of parent–offspring signalling. Phil. Trans. R. Soc. B. 2000;355:1581–1591. doi: 10.1098/rstb.2000.0719. doi:10.1098/rstb.2000.0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmo C.G, Karasov W.H. Endogenous mass and energy-losses in ruffed grouse. Auk. 1993;110:386–390. [Google Scholar]
- Iacovides S, Evans R.M. Begging as graded signals of need for food in young ring-billed gulls. Anim. Behav. 1998;56:79–85. doi: 10.1006/anbe.1998.0742. doi:10.1006/anbe.1998.0742 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. Begging and sibling competition: how should offspring respond to their rivals? Am. Nat. 2004;163:388–406. doi: 10.1086/375541. doi:10.1086/375541 [DOI] [PubMed] [Google Scholar]
- Kacelnik A, Cotton P.A, Stirling L, Wright J. Food allocation among nestling starlings—sibling competition and the scope of parental choice. Proc. R. Soc. B. 1995;259:259–263. doi:10.1098/rspb.1995.0038 [Google Scholar]
- Karasov W.H, Wright J. Nestling digestive physiology and begging. In: Wright J, Leonard M.L, editors. The evolution of begging: competition, cooperation and communication. Kluwer; Dordrecht, The Netherlands: 2002. pp. 199–219. [Google Scholar]
- Kedar, H. 2003 The role of learning in parent–offspring communication in the house sparrow (Passer domesticus). PhD dissertation, Tel-Aviv University, Israel.
- Kedar H, Rodríguez-Gironés M.A, Yedvab S, Winkler D.W, Lotem A. Experimental evidence for offspring learning in parent–offspring communication. Proc. R. Soc. B. 2000;267:1723–1727. doi: 10.1098/rspb.2000.1201. doi:10.1098/rspb.2000.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R. When do canary parents respond to nestling signals of need. Proc. R. Soc. B. 1995;260:343–348. doi:10.1098/rspb.1995.0102 [Google Scholar]
- Kilner R.M. A growth cost of begging in captive canary chicks. Proc. Natl Acad. Sci. USA. 2001;98:11 394–11 398. doi: 10.1073/pnas.191221798. doi:10.1073/pnas.191221798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R, Johnstone R.A. Begging the question: are offspring solicitation behaviours signals of needs. Trends Ecol. Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. doi:10.1016/S0169-5347(96)10061-6 [DOI] [PubMed] [Google Scholar]
- Kilner R.M, Noble D.G, Davies N.B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature. 1999;397:667–672. doi:10.1038/17746 [Google Scholar]
- Leonard M.L, Horn A.G. Need and nestmates affect begging in tree swallows. Behav. Ecol. Sociobiol. 1998;42:431–436. doi:10.1007/s002650050457 [Google Scholar]
- Leonard M.L, Horn A.G. Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor) Behav. Ecol. Sociobiol. 2001;49:170–175. doi:10.1007/s002650000290 [Google Scholar]
- Lepczyk C.A, Karasov W.H. Effect of ephemeral food restriction on growth of house sparrows. Auk. 2000;117:164–174. doi:10.1642/0004-8038(2000)117[0164:EOEFRO]2.0.CO;2 [Google Scholar]
- Lepczyk C.A, Caviedes-Vidal E, Karasov W.H. Digestive responses during food restriction and realimentation in nestling house sparrows (Passer domesticus) Physiol. Zool. 1998;71:561–573. doi: 10.1086/515965. [DOI] [PubMed] [Google Scholar]
- Lotem A. Differences in begging behaviour between barn swallow Hirundo rustica, nestlings. Anim. Behav. 1998;55:809–818. doi: 10.1006/anbe.1997.0675. doi:10.1006/anbe.1997.0675 [DOI] [PubMed] [Google Scholar]
- Macnair M.R, Parker G.A. Models of parent–offspring conflict. 3. Intra-brood conflict. Anim. Behav. 1979;27:1202–1209. doi:10.1016/0003-3472(79)90067-8 [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Mock D.W, Schwagmeyer P.L, Parker G.A. Male house sparrows deliver more food to experimentally subsidized offspring. Anim. Behav. 2005;70:225–236. doi:10.1016/j.anbehav.2004.10.020 [Google Scholar]
- Mondloch C.J. Chick hunger and begging affect parental allocation of feedings in pigeons. Anim. Behav. 1995;49:601–613. [Google Scholar]
- Parker G.A, Macnair M.R. Models of parent–offspring conflict. 4. Suppression—evolutionary retaliation by the parent. Anim. Behav. 1979;27:1210–1235. doi:10.1016/0003-3472(79)90068-X [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol. Lett. 2002;5:206–215. doi:10.1046/j.1461-0248.2002.00301.x [Google Scholar]
- Price K. Benefits of begging for yellow-headed blackbird nestlings. Anim. Behav. 1998;56:571–577. doi: 10.1006/anbe.1998.0832. doi:10.1006/anbe.1998.0832 [DOI] [PubMed] [Google Scholar]
- Price K, Ydenberg R, Daust D. State-dependent begging with asymmetries and costs: a genetic algorithm approach. In: Wright J, Leonard M.L, editors. The evolution of begging: competition, cooperation and communication. Kluwer; Dordrecht, The Netherlands: 2002. pp. 21–42. [Google Scholar]
- Rodríguez-Gironés M.A, Cotton P.A, Kacelnik A. The evolution of begging: signaling and sibling competition. Proc. Natl Acad. Sci. USA. 1996;93:14 637–14 641. doi: 10.1073/pnas.93.25.14637. doi:10.1073/pnas.93.25.14637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 2002;17:434–440. doi:10.1016/S0169-5347(02)02565-X [Google Scholar]
- Stamps J, Clark A, Arrowood P, Kus B. Parent–offspring conflict in budgerigars. Behaviour. 1985;94:1–40. [Google Scholar]
- Wright J, Leonard M.L. Kluwer; Dordrecht, The Netherlands: 2002. The evolution of begging: competition, cooperation and communication. [Google Scholar]
- Yedvab, S. 1999 Begging strategies of house sparrow nestlings. MSc thesis, Tel-Aviv University, Israel.