Abstract
Quantal variability was determined at glutamatergic synapses in the neonatal (postnatal days 1-7) rat hippocampal slice preparation. Synaptic AMPA and NMDA quantal EPSCs were recorded from CA1 pyramidal neurones using the whole-cell, or perforated, patch-clamp technique.
Release was evoked by minimal stimulation using brief trains (10 impulses, 50 Hz), and various tests ascertained that this stimulation activated single release sites releasing single vesicles.
Both AMPA and NMDA quantal responses at a given release site varied considerably in magnitude, the coefficient of variation (CV) among the synapses averaging 0.39 and 0.30, respectively. This variability differed among the synapses, from 0.2 to 0.7, and 0.10 to 0.50, respectively, and CV values of AMPA responses co-varied with those of the NMDA responses.
Both for AMPA and NMDA, low CV values were associated with a Gaussian distribution of EPSC peak values, whereas synapses with high CV values displayed distributions skewed towards lower values.
Analysis of successive NMDA responses during a train revealed a considerable degree of non-saturation of NMDA receptors by a single vesicle.
The results are compatible with a quantal variability based, to a large extent, on non-saturated AMPA and NMDA responses fluctuating as a function of the amount of transmitter released from each vesicle.
Recent studies on glutamatergic synapses in the hippocampal CA1 region have indicated the necessity to reconsider some basic issues regarding the postsynaptic quantal response. Thus, it would now seem that glutamate released from a single vesicle is not sufficient to saturate either the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors or the N-methyl-D-aspartate (NMDA) receptors at the postsynaptic site (Tong & Jahr, 1994; Liu et al. 1999; Mainen et al. 1999; McAllister & Stevens, 2000). Variations in the amount of transmitter released from a vesicle, either due to variations in the content of transmitter, or in the manner in which transmitter is released, may then directly translate into quantal responses of various magnitudes. The extent of this quantal variability in a synapse, as well as the variation of this variability among the synapses, are thus matters of considerable importance for the understanding of quantal transmission in these synapses.
The direct analysis of evoked quantal responses at a glutamatergic synapse in the central nervous system has been difficult in that it has to be certified that only a single synapse is activated, and that this synapse only releases a single vesicle in response to a presynaptic action potential (Auger & Marty, 2000). Investigations of quantal responses in the mature hippocampus is thus complicated by the fact that innervation density is high, making single afferent activation difficult, and that many axons make more than one synapse on a given postsynaptic cell (Andersen, 1990; Sorra & Harris, 1993; Hsia et al. 1998). The problem of multiple synapses from a single axon appears to be no less in hippocampal cultures (Debanne et al. 1996). Thus, the extent of quantal variability of AMPA as well as of NMDA responses in a synapse (and their co-variation), as well as the variation of this variability among the synapses, is still largely unknown.
To examine evoked quantal responses in the slice preparation, using minimal stimulation, the neonatal hippocampal slice might provide a favourable preparation. The density of glutamatergic synapses is initially very low (Steward & Falk, 1991; Durand et al. 1996; Fiala et al. 1998; Tyzio et al. 1999), and a given axon does not appear to have more than one synapse, containing one release site, on a given postsynaptic cell (Hsia et al. 1998). Neonatal pyramidal neurones also have small dendritic trees and are electrically compact (Pokorny & Yamamoto, 1981; Spigelman et al. 1992) facilitating the discrimination of unitary quantal responses. Furthermore, by using rats of different postnatal ages (< 1 week), possible developmental trends in quantal properties can be revealed.
To procure evidence for single release site activation brief trains of presynaptic activation, rather than single volleys, were used.
METHODS
Slice preparation
Hippocampal slices were prepared from Wistar rats aged 1-7 postnatal days (P1-P7). The rats were killed by decapitation in accordance with the guidelines of the local ethical committee for animal research. The brains were removed and placed in an ice-cold solution composed of (mM): 124 NaCl, 3.0 KCl, 2 CaCl2, 6 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. Transverse hippocampal slices (300 μm) were cut using a vibrating tissue slicer (Campden Instruments), transferred to a holding chamber and stored at 28°C. For recording, slices were individually transferred to a recording chamber where they were perfused (2 ml min−1) at 30-32°C. The extracellular solution contained (mM): 124 NaCl, 3.0 KCl, 4 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 0.02 bicuculline methiodide or 0.1 picrotoxin.
Patch-clamp recordings
Whole-cell recordings from visually identified CA1 pyramidal cells (Edwards et al. 1989) were performed with an EPC-9 patch-clamp amplifier (HEKA, Lambrecht, Germany). The pipette solution contained (mM): 95 caesium gluconate, 20 TEACl, 10 NaCl, 5 QX-314, 4 Mg-ATP, 0.4 Na-GTP, 0.1 EGTA and 10 Hepes (pH 7.3, adjusted with CsOH). Some of the experiments were performed in the perforated patch-clamp mode. In those experiments amphotericin B (Sigma) was added to the pipette solution (240 μg ml−1). The tips of the pipettes were filled with solution without amphotericin B. The neurones were voltage clamped at a holding potential of either-80 mV or +50 mV. Patch pipettes (1.5 mm/0.86 mm; borosilicate, Clark Electrochemical Instruments) were pulled using a horizontal puller (Sutter Instruments Co). They had a resistance of 4-6 MΩ and were not polished or coated. The series resistance, which was continuously monitored during the experiments using a 10 mV hyperpolarizing pulse, varied in different cells between 6 and 27 MΩ. Recordings included for analysis were collected during periods of stable series resistance. Responses were filtered at 2 kHz and sampled at 10 kHz. The input resistance of the cells averaged 1.02 ± 0.50 GΩ (n = 40) and showed a negative correlation with increasing age, from P1 to P7 (r =-0.43, P < 0.01). The capacitance of the cells averaged 18 ± 4.1 pF (n = 40) and showed a positive correlation with increasing age (r = 0.48, P < 0.001). The standard deviation of the baseline noise was 0.72 ± 0.10 pA (n = 40). All data are presented as means ± standard deviation (s.d.), when not otherwise indicated. Student's t test was used to determine statistical significance. Analysis was performed using custom software written in Igor Pro (Wavemetrics, Lake Oswego, OR, USA).
Minimal stimulation
Minimal stimulation (Konnerth et al. 1990; Allen & Stevens, 1994; Stevens & Wang, 1995; Raastad, 1992, Raastad, 1995; Isaac et al. 1996; Dobrunz & Stevens, 1997) was used to activate single axons in the stratum radiatum. A 10 impulse, 50 Hz stimulation was used for test stimulation, and constant current pulses (100 μs) were passed through pipettes (0.5 MΩ), filled with extracellular solution. The inter-trial frequency was 0.2 Hz. To search for a synaptic input stimulus intensity was increased (up to 50 μA) and the area of the burst response was measured. Often, particularly in slices from the youngest animals, no synaptic response could be detected despite high stimulation intensities. The position of the stimulation pipette was then moved to a new position in the stratum radiatum and the procedure was repeated. When EPSC(s) appeared in response to the burst, stimulation intensity was increased in steps of about 10 % of the threshold intensity to check for constancy of the burst response. If a further increase of the burst response was detected within the first two to three stimulation increments a new position of the stimulation pipette was tried. Minimal stimulation was deemed successful either when there was no further increase, or when there was an increase of the burst response only after more than three stimulation increments. In these cases stimulation intensity was set at a value of 110-120 % of the threshold intensity and was kept constant throughout the rest of the experiment. As a test for possible increasing, or decreasing, excitation of the axon during the burst activation, the pattern of release was quantified by calculating the point in the burst where 50 % of the release had occurred. This point was not found to change when the stimulus intensity was varied within the range of stimulus intensities that produced the constant minimal burst response. As a test of whether the evoked action potentials faithfully invade the terminal we adopted the method described by Stevens & Wang (1995) (see also Allen & Stevens, 1994). If a failure in stimulus position 1 is due to an invasion failure (or an excitation failure), then any action potential-dependent changes in release probability in stimulus position 2 should be absent. However, we found that release probability in position 2 was independent of whether release occurred in position 1, or not. The average ratio between the release probability in stimulus position 2, when release had occurred in stimulus position 1, and release probability in stimulus position 2, when there was no release in stimulus position 1, was 0.98 ± 0.07 (mean ± standard error of the mean (s.e.m.), n = 29).
Detection of EPSCs
After subtraction of the stimulus artefact (using clear failures selected by eye), the sweeps were smoothed using a binomial smoothing operation (20 iterations), and preliminary EPSCs were sampled automatically based on a threshold amplitude of 2 pA (about 3 × noise s.d.). The rise time and time to peak of the average preliminary EPSC was then used to delimit the ‘time window’ to search for EPSC peak amplitudes to time to peak ± rise time. After scaling the peak amplitude to 2 pA, the area of the average preliminary EPSC during the ‘time window’ was calculated. The procedure was then reapplied and the EPSCs that were deemed successful, now based on both amplitude and area criteria during the ‘time window’, were also inspected by eye to exclude spurious events that passed the criteria. The amplitude of each detected EPSC was calculated from the smoothed trace (from which the artefact was subtracted) by subtracting the average baseline value during the 3 ms preceding stimulation from the peak value. In the absence of an EPSC, a measurement was taken at a time point corresponding to that of the EPSC peak value to obtain noise variance. For the NMDA EPSC the same procedure was used, but the ‘time window’ was set between 15 and 20 ms after stimulation. The amplitude of the NMDA EPSC was calculated by subtracting the average baseline amplitude during the 3 ms preceding stimulation from the average amplitude between 15 and 20 ms after the stimulation. When, for the same synaptic input, NMDA EPSCs were compared with AMPA EPSCs, NMDA responses were evoked at a holding potential of +50 mV for 2-3 min (24-36 burst responses) and the corresponding AMPA responses were collected during a 2-3 min period before and after the collection of the NMDA responses.
Data analysis
Coefficient of variation (CV) was calculated as:
(s.d.EPSCs2–s.d.failures2)0.5/mean EPSC amplitude (excluding failures).
Rise time was calculated between points corresponding to 20 and 80 % of peak amplitude. Onset latency was calculated from the intercept between a line obtained by linear regression throughout the 20-80 % rising phase and the horizontal projection of the baseline measured during the 3 ms before the artefact. Before further analysis, mean EPSC amplitude (excluding failures), CV, and probability of release (binned into 1 min groups) were plotted against time and linear fits were applied to the data. Only experiments, or part of experiments, in which these linear regressions did not significantly (at the 0.05 level) deviate from zero slope were selected for further analysis. There was no significant (P > 0.05) correlation between EPSC rise time and the series resistance (r = 0.18, n = 52), or between EPSC rise time and the mean EPSC amplitude (r = 0.07, n = 52).
RESULTS
Minimal stimulation activates a single synapse
As noted in the Introduction, slices from the neonatal (< 1 week old) rat are favourable for the examination of release from single synaptic terminals. Nevertheless, it has to be ascertained that only a single axon and a single synapse are actually activated. Moreover, it has to be demonstrated whether or not this synapse only contains a single release site when activated. To examine this, the stimulation consisted of 10 impulse, 50 Hz trains (at 0.2 Hz) (Fig. 1a).
Figure 1. Uniform EPSC characteristics during minimal burst stimulation.
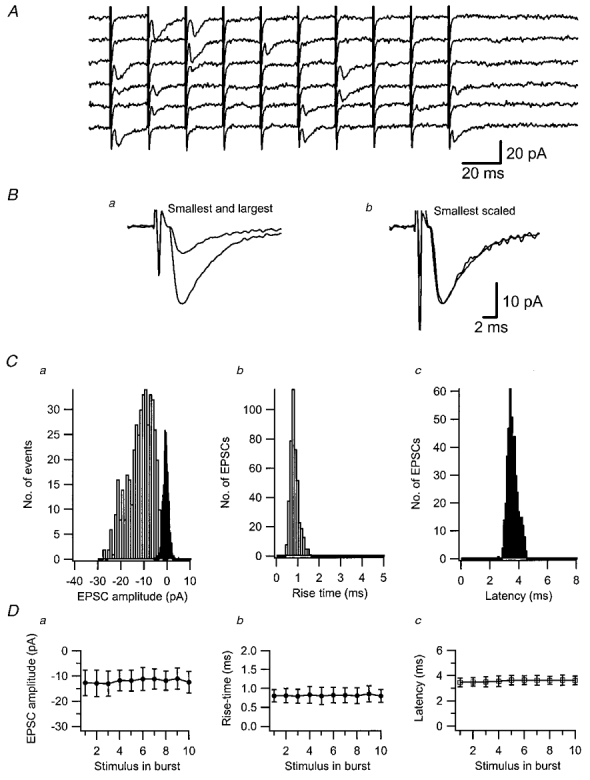
A, six consecutive traces (0.2 Hz) in response to burst stimulation (10 impulses, 50 Hz). B, average traces of the 25 % smallest and the 25 % largest EPSCs taken from all stimulus positions in the burst. To compare kinetics, the smaller average EPSC is scaled to the same peak amplitude as the larger average EPSC in Ba. C, distributions of EPSC amplitudes (Ca), rise times (Cb), and latencies (Cc). The CVs for these distributions are 44 %, 22 % and 8 %, respectively. In Ca the distribution of amplitudes when there was no response (failures) is included (filled columns). D, EPSC amplitudes (excluding failures) (Da), rise times (Db), and latencies (Dc) as a function of stimulus position in the burst, given as means ±s.d. All graphs in this figure refer to values from one specific synaptic input.
To activate an axon minimal stimulation was used (for criteria, see Methods). Such stimulation resulted in only a few EPSCs per train, ranging from mean values of 0.7 to 4.1 among the inputs examined (accompanying paper, Hanse & Gustafsson, 2001). The release pattern varied considerably among the inputs. Thus, there was a spectrum ranging from inputs demonstrating strong facilitation in release probability during the train stimulation to those displaying a large depression. Due to this large variability in release behaviour one may expect that synaptic events evoked at various positions in the train may, on average, vary, if this release should come from more than one axon, or synapse. As exemplified from one experiment in Fig. 1, rise times, and latencies of evoked EPSCs (sampled for all positions) displayed uni-modal distributions (Fig. 1C), and when plotted against position in the train, no trends were observed (Fig. 1D). None of the inputs (n = 51) showed any significant (P > 0.05) deviation from the zero slope (using linear regression), latency and rise time changing only on average 2.5 % and 4.2 %, respectively, from the first to the last stimulus position. This small intra-experimental variation should be compared with the large inter-experimental variation, average latencies and rise times among the inputs ranging from 2.2 to 7.2 ms, and from 0.6 to 2.2 ms, respectively.
EPSC peak amplitude distribution in the illustrated case was also uni-modal, but markedly skewed, and peak amplitudes varied over a large range, from a few picoamps up to 30 pA (Fig. 1Ca). Nonetheless, EPSCs of various amplitudes had shapes that were superimposable. This is illustrated in Fig. 1B where averages of the 25 % smallest and the 25 % largest EPSCs are shown at their original amplitudes (Ba) and after scaling (Bb). In contrast to latency and rise time, EPSC peak amplitude (excluding failures) did tend to decrease somewhat with position in the train (Fig. 1Da), a feature that was observed for many of the inputs. The slope of regression lines were on average-1.89 ± 0.4 (n = 51) indicating a 19 % decrease in EPSC amplitude from the first to last position in the train. This significant decrease is, however, due to an interaction between successive EPSCs from one synapse, rather than indicating the superposition of release from two synapses (see below). Thus, taken together, latency, rise time and peak amplitude data are consistent with activation of a single axon and a single synapse. However, interactions between successive EPSCs will only allow for use of the first EPSC to occur in the train for analysis of quantal variance.
Minimal stimulation activates a single release site
How then to ascertain that the single synapse only contains a single release site? In quite a number of the synapses release probability changed considerably during the train, being more than 0.5 initially, thereafter decreasing to about 0.1. If such synapses release more than one vesicle per stimulus due to the presence of several release sites, the likelihood of such multiple release would change considerably during the train (Stevens & Wang, 1995). The average EPSC amplitude (excluding failures) would then differ initially and later during the train (see below). Figure 2A and B shows from one experiment that initial release with a probability of 0.61 resulted in an average EPSC (excluding failures) of the same amplitude (and time course) as that evoked later with a probability of 0.11. The same result was observed when averaged for 19 synapses with such release characteristics (Fig. 2C).
Figure 2. EPSC amplitude is independent of release probability.
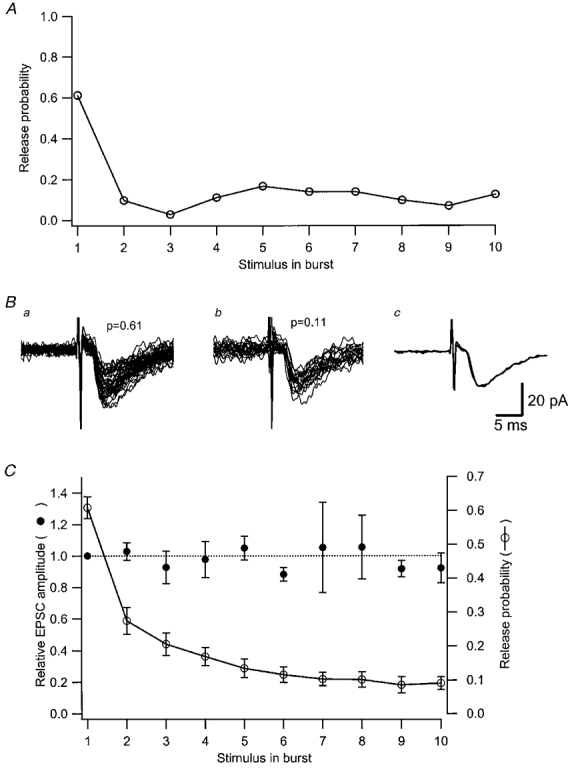
A, release probability, for one synaptic input, is plotted against position in the stimulus train. Ba, all EPSCs (n = 44) from the first position of the stimulus train where the release probability was 0.61. Bb, all EPSCs (n = 22) from the second to the tenth position of the stimulus train where the average release probability was 0.11. Only EPSCs that were not preceded by other EPSCs in the burst are included. Bc, average of the EPSCs in Ba and Bb superimposed. C, summary graph (n = 19 synaptic inputs) showing both the relative amplitude of the EPSC (○) and the average release probability (○) as a function of position in the stimulus train. Only synaptic inputs showing a release probability >= 0.5 in the first stimulus position are included. The EPSC amplitude is expressed as the fraction (±s.e.m.) of EPSC amplitude in the first stimulus position in all experiments before averaging. The number of synapses contributing to the average value in each stimulus position in this plot decreases from 19 in the first two positions to 6 in the last three positions.
If the release illustrated in Fig. 2A were to be attributed to release from two different release sites with the same release characteristics, what then would the discrepancy in EPSC magnitude (excluding failures) be? The decrease in the number of simultaneous releases from these two sites, when the ‘combined’ release probability of these synapses drops from 0.61 to 0.11 can be calculated using the binomial theorem. For a combined release probability of 0.61 and 0.11, respectively, the individual probabilities will be 0.38 and 0.06. The probabilities for double releases would then change during the train from 0.14 (0.382) to 0.003 (0.062), i.e. in 23 % of the trials (0.14/0.61) the high probability EPSCs will contain double releases whereas this will be true for only about 3 % (0.003/0.11) of those with low probability. The low probability EPSCs would then be about 80 % of those of high probability. As can be seen in Fig. 2C, this result was not observed. We thus conclude that our minimal stimulation protocol activates single synapses containing a single release site, and thus that the EPSCs observed can be considered as quantal responses.
Interaction between successive EPSCs
As shown above, the average EPSC (excluding failures) showed a small, but significant, decrease during the train. To analyse this behaviour we computed the ratio between the second and first EPSCs to occur in the train, regardless of their position, against the interval between these EPSCs. This ratio averaged 0.90 ± 0.04 (n = 42) for all intervals, being 0.86 at intervals between 20 and 80 ms and 1.01 at longer intervals (100-140 ms). There was a significant correlation (r = 0.57; n = 42; P < 0.001) between the decrease in EPSC magnitude during the train and the reduction in this second/first EPSC ratio. We then computed the EPSC magnitude as a function of position using only the first EPSC to appear during the train for all inputs. In this case EPSC magnitude did not deviate at any position from that in position 1, and the slope of a regression line through the average values at each position indicated an increase of 1.9 % of the EPSC from the first to the last position in the train. Thus, EPSCs following the first EPSC in the train seem subjected to a possible desensitizing influence from the first one.
Quantal size and variability of AMPA EPSCs
Due to the interaction between successive EPSCs, mean value and variability (CV, coefficient of variation) of the EPSC peak amplitude for a release site was estimated using only the first EPSC (quantal response) to appear during the train. Both these parameters were found to vary considerably among the synapses. The mean quantal size among the synapses averaged 15.8 ± 7.6 pA (n = 52), but ranged between 5 and 30 pA (Fig. 3Aa). Similarly, the average CV was 39 ± 11 % (n = 52), but could be as low as 20-30 %, and as high as 60-70 % (Fig. 3Ab). There was no significant correlation between the variability and the mean size of the quanta of a synapse (Fig. 3B). Neither mean quantal size, nor variability was found to be correlated with the average initial release probability of the synapse (Fig. 3C) or with postnatal age (Fig. 3D). The lack of correlation with initial release probability would seem to imply that quantal size does not depend on the amount of transmitter release. However, initial release probability does not predict the amount of release during burst activation (Hanse & Gustafsson, 2001). Plotting the quantal size against the total release during the train did not, however, produce any significant correlation (not shown).
Figure 3. Mean quantal size and mean quantal variability of AMPA EPSCs.
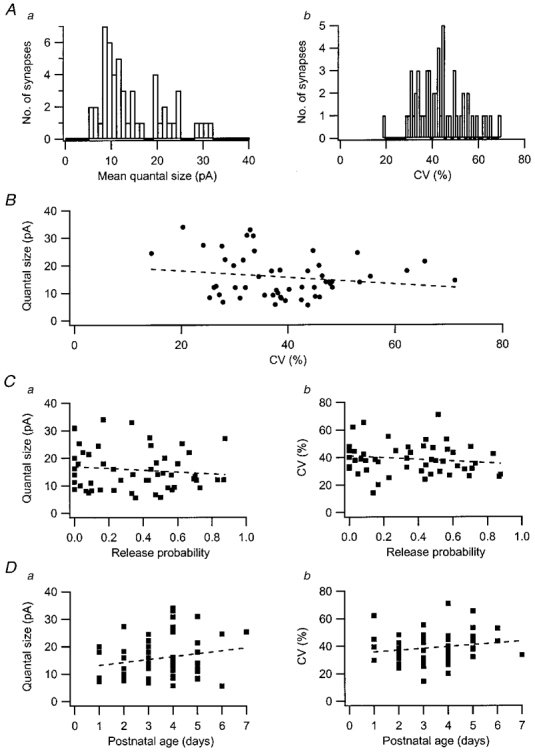
Aa, distribution of values for the mean quantal size (n = 52 synapses). Ab, distribution of values for the coefficient of variation (CV, n = 52 synapses). B, relation between mean quantal size and CV (r =-0.18; P > 0.05). Ca, relation between mean quantal size and release probability in the first stimulus position of the burst (r =-0.12; P > 0.05). Cb, relation between CV and release probability in the first stimulus position of the burst (r =-0.16; P > 0.05). Da, relation between mean quantal size and postnatal age (r = 0.20; P > 0.05). Db, relation between CV and postnatal age (r = 0.17; P > 0.05). Dashed lines in the graphs are linear regression lines.
In the example illustrated in Fig. 1 (Ca), the distribution of EPSC peak amplitudes was clearly skewed towards lower values. When pooling distributions from synapses with low variability (CVs within the lower one-fifth of the range) after normalization to common peak amplitude (equal to the average mean quantal size), the peak amplitude distribution was well described by a Gaussian distribution (Fig. 4a). However, in synapses demonstrating larger quantal variability, distributions were more skewed, as illustrated in Fig. 4B for synapses exhibiting CVs within the higher one-fifth of the CV range. A plot of the skew value against that of CV indicated a positive correlation (r = 0.53).
Figure 4. AMPA EPSC amplitude distributions for synapses with low and high quantal variability.
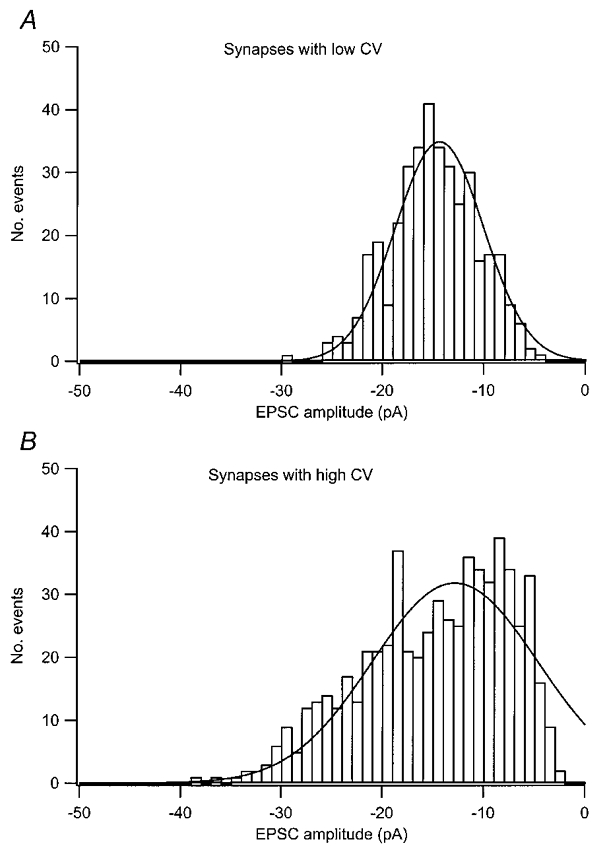
A, distribution of normalized AMPA EPSC amplitudes from the ten synapses with the lowest CV (skew =-0.15). B, distribution of normalized AMPA EPSC amplitudes from the ten synapses with the highest CV (skew =-0.65). Before pooling, EPSCs were normalized with respect to the average mean quantal size among the synapses (15 pA).
Baseline noise could possibly influence the shape of EPSC peak amplitude distributions towards a more Gaussian appearance. However, no correlation was found between the degree of skewness and the s.d. of baseline noise (r = 0.17, P > 0.05). Moreover, the skew was significantly (P < 0.01) more evident in synapses with small quantal size (-0.60 ± 0.12, 8.9 ± 0.35 pA, n = 26) than in those with a larger size (-0.20 ± 0.10, 19.9 ± 1.15 pA, n = 26), contrary to what one would expect if noise had been the explanation for the Gaussian distributions presently observed.
Quantal size and variability of NMDA EPSCs
In a subset of synapses (n = 23), train stimulation was also applied when the cell was held at +50 mV, to examine the quantal size and variability of NMDA EPSCs (Fig. 5a). When compared to the AMPA EPSCs at the same synapse, the NMDA EPSCs occurred with the same release probability, number of responses per burst and response pattern (Fig. 5B), indicating that both resulted from activation of the same release site. As for AMPA EPSCs, the mean quantal size of the NMDA EPSCs, selecting only the first to appear during each train, varied considerably among the synapses, from 10 up to 60 pA (Fig. 6Aa). Moreover, in any given synapse the NMDA EPSC fluctuated considerably from trial to trial, with CV values for peak amplitude distributions ranging from about 10 to 50 % (Fig. 6Ab). No significant relation was observed between the variability of the quantal NMDA EPSC and its mean quantal size (Fig. 6B).
Figure 5. AMPA and NMDA EPSCs from the same synapse exhibit the same release characteristics.
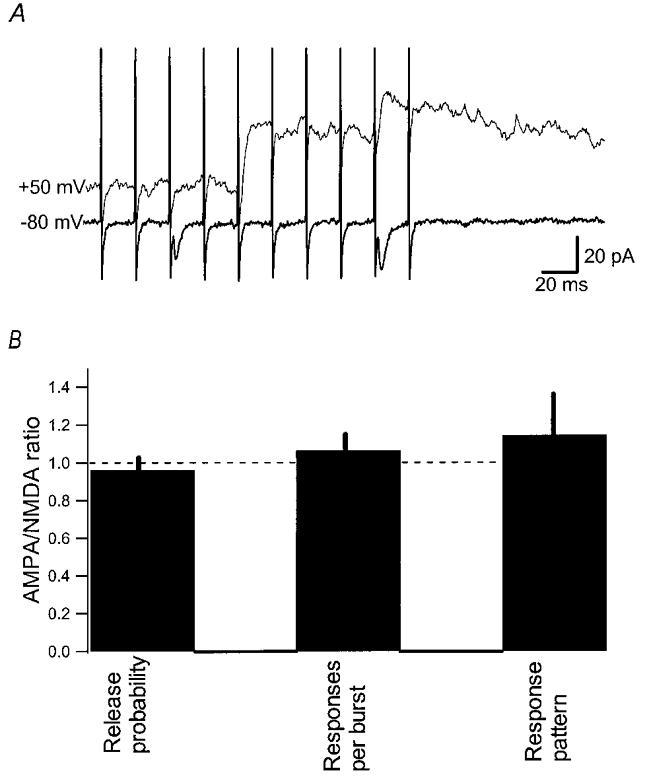
A, example of burst responses recorded at-80 mV and +50 mV. B, AMPA/NMDA ratio for release probability in the first stimulus position of the burst, the mean number of responses during a burst and the response pattern during a burst. The data are based on 18 synapses and the bars are the mean ratio ±s.e.m. Response pattern during the burst was quantified by calculating the position in the burst where 50 % of the release had occurred.
Figure 6. Mean quantal size and mean quantal variability of NMDA EPSCs.
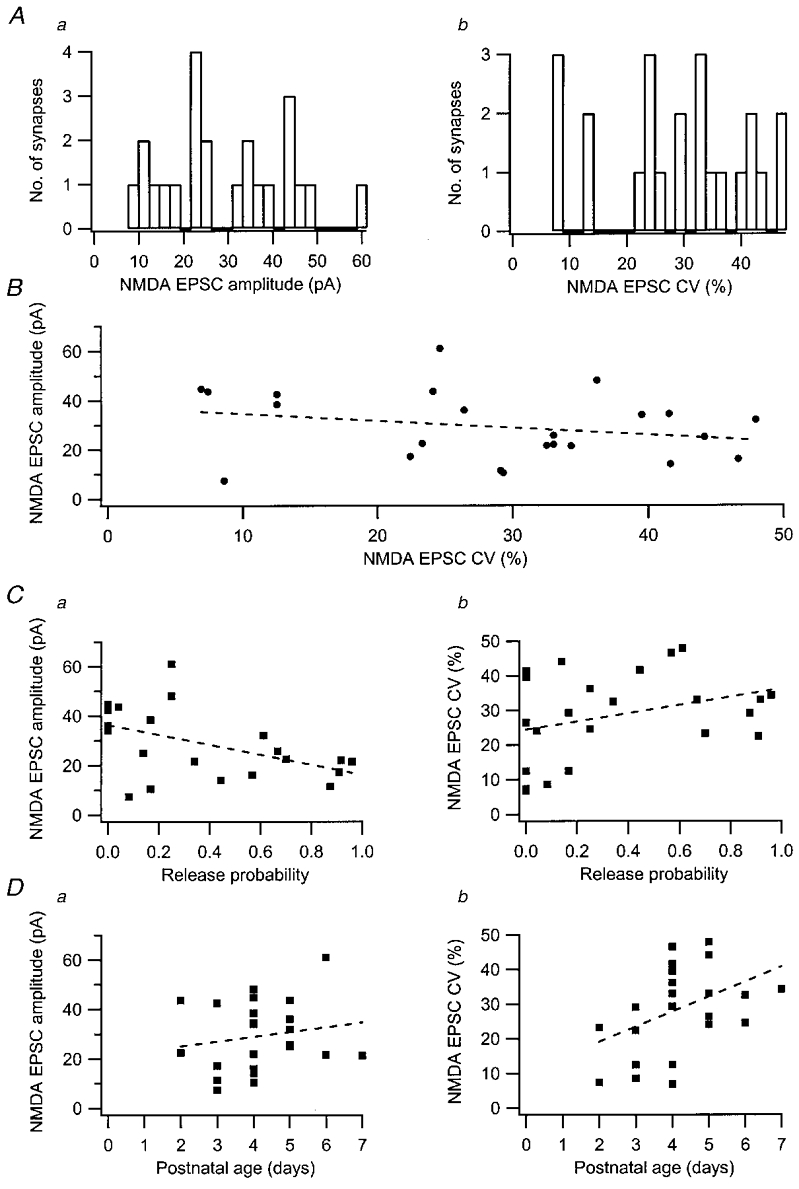
Aa, distribution of values for the mean quantal size (n = 23 synapses). Ab, distribution of values for the coefficient of variation (CV, n = 23 synapses). B, relation between mean quantal size and CV (r = 0.26; P > 0.05). Ca, relation between mean quantal size and release probability in the first stimulus position in the burst (r =-0.50; P < 0.05). Cb, relation between CV and release probability in the first stimulus position of the burst (r = 0.33; P > 0.05). Da, relation between mean quantal size and postnatal age (r = 0.17; P > 0.05). Db, relation between CV and postnatal age (r = 0.42; P < 0.05). Dashed lines in the graphs are linear regression lines.
NMDA EPSC quantal size, but not its variability, did tend to decrease in size with increased initial release probability of the presynaptic terminal (P < 0.05) (Fig. 6C), unlike AMPA EPSCs where no such tendency was observed. Increased postnatal age had no influence on NMDA quantal size (Fig. 6Da), but was associated with an increase in variability (Fig. 6Db, P < 0.01). Thus, our results do not seem to indicate that quantal variability, either with respect to AMPA or to NMDA, decreases with age, but rather the opposite.
The normalized NMDA EPSC amplitude distribution from synapses with CVs from the lower half of the CV range was well described by a Gaussian distribution (Fig. 7a), whereas that from the synapses with the highest CVs displayed a broad peak and a skew towards lower values (Fig. 7B).
Figure 7. NMDA EPSC amplitude distributions for synapses with low and high quantal variability.
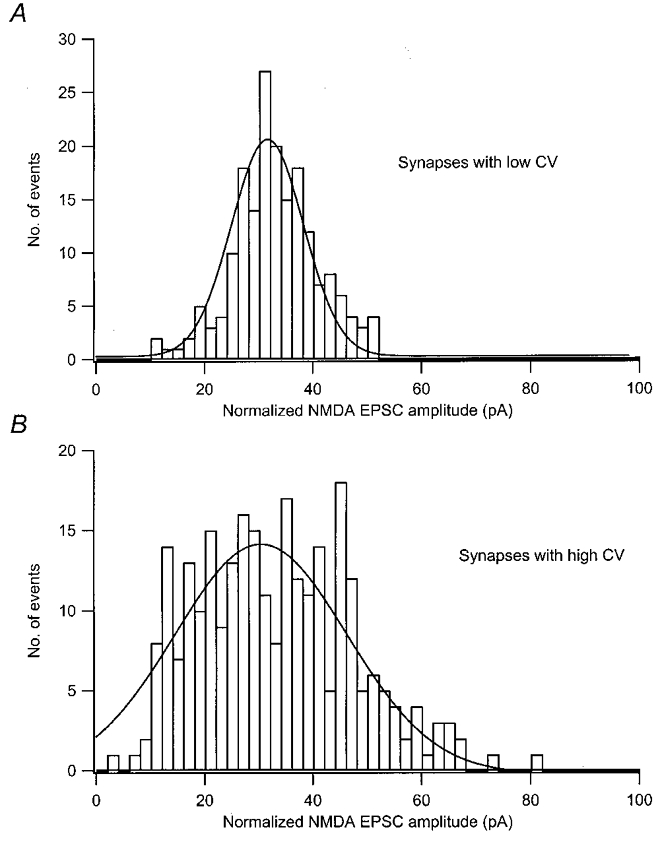
A, distribution of normalized NMDA EPSC amplitudes from the synapses with the lowest CV (n = 11 synapses, skew =-0.009). B, distribution of normalized NMDA EPSC amplitudes from synapses with highest CV (n = 12 synapses, skew =+0.42). Before pooling, NMDA EPSCs were normalized with respect to the average mean quantal size among the synapses (33 pA).
The quantal variability that was found regarding NMDA EPSCs was not restricted to dual NMDA and AMPA signalling synapses, but was also found in those only signalling via NMDA (Fig. 8Db), so called ‘deaf’ synapses (Malgaroli, 1999). Such a ‘deaf’ synapse showed no response at-80 mV (Fig. 8B), and a quite variable NMDA response at +50 mV (Fig. 8a). The CV of the NMDA EPSC at this synapse was 36 %. It can be noted that even burst stimulation at-80 mV did not reveal any AMPA EPSCs (Fig. 8C), indicating that the absence of such an EPSC is not merely due to a reduced release probability at negative holding potentials (Gasparini et al. 2000). On average, the ‘deaf’ synapses tended to have higher release probabilities (Fig. 8Dc) and the quantal EPSCs were smaller (Fig. 8Da) and displayed a greater variability (Fig. 8Db) compared to the dual-signalling synapses. However, none of these differences reached statistical significance.
Figure 8. Postsynaptically silent (‘deaf’) synapses exhibit similar properties to dual synapses.
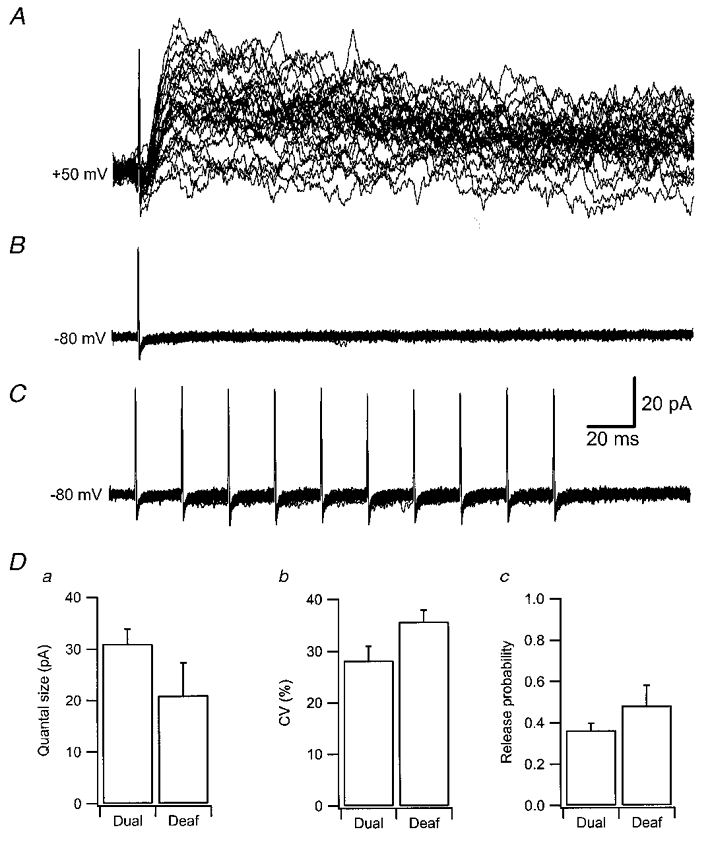
A, 30 consecutive sweeps evoked by single volley stimulation recorded at a holding potential of +50 mV. B, 30 consecutive sweeps evoked by single volley stimulation recorded at a holding potential of-80 mV. C, 30 consecutive sweeps evoked by burst stimulation (10 impulses, 50 Hz) recorded at a holding potential of-80 mV. D, mean quantal size (Da), CV (Db) and release probability (Dc) ±s.e.m. for 18 dual and 5 deaf synapses. None of the differences reached statistical significance.
Comparison of AMPA and NMDA EPSCs with respect to quantal size and variability
As shown above, AMPA and NMDA EPSCs varied considerably among the synapses both with respect to their mean quantal sizes and to their variability. With respect to mean quantal amplitudes, AMPA and NMDA EPSCs were only weakly correlated (Fig. 9A), indicating separate control in a given synapse for these two EPSCs. With respect to quantal variability NMDA EPSCs showed, on average, a lower CV value (28 ± 13 %) than that of AMPA EPSCs (39 ± 11 %). Nevertheless, the CVs for the two types of EPSCs were correlated, indicating a common underlying mechanism (Fig. 9B). Postnatal age neither changed the amplitude ratio between the two EPSCs nor the ratio between their CVs (not illustrated).
Figure 9. Relation between corresponding AMPA and NMDA EPSCs.
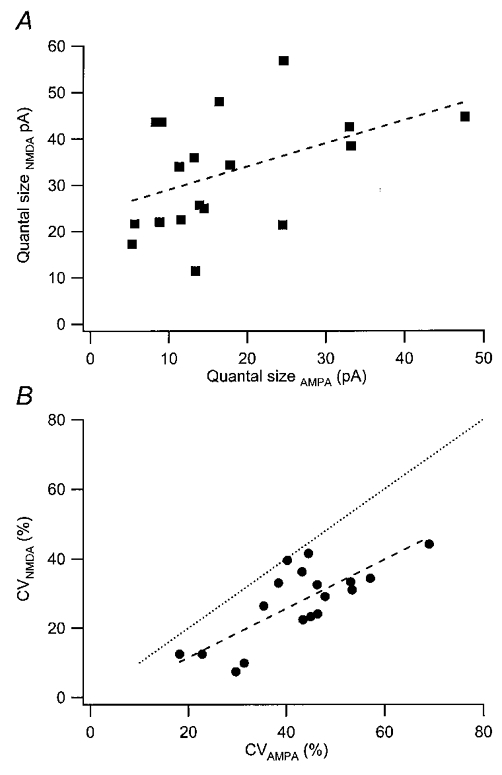
A, relation between mean quantal size for NMDA and AMPA EPSCs (n = 18 synapses). The correlation is borderline significant (r = 0.45; P < 0.05). B, relation between CV for NMDA and AMPA EPSCs (n = 18 synapses). The correlation is highly significant (r = 0.77; P < 0.001). Dashed lines in the graphs are linear regression lines and the dotted line in B indicates equality.
Non-saturation of NMDA EPSCs by single releases
When a second NMDA EPSC appeared during the train stimulus it produced levels of peak current that were, on average, 155 % (n = 19 synapses) of that of the first EPSC. This would suggest that the release of a single vesicle does not saturate the NMDA receptors. If now such non-saturation underlies quantal variability of NMDA EPSCs one may expect that synapses in which the above ratio is smaller, i.e. indicates a larger saturation by the first release, would also show less variability. This was found to be the case (Fig. 10), the regression line crossing the y-axis at a CV value of about 20 %. This result would then imply that at full saturation by a single vesicle there would remain a quantal variability of about 20 %.
Figure 10. The degree of additivity between consecutive NMDA EPSCs is correlated with the CV of the first NMDA EPSC.
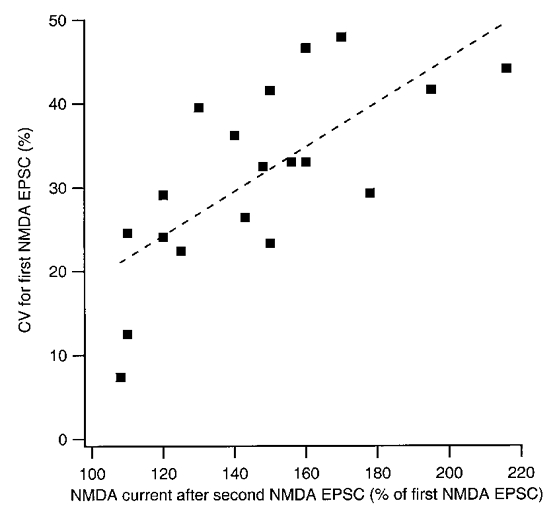
CV of the first NMDA EPSC in the burst is plotted against the total outward current obtained after a second NMDA EPSC in the burst (expressed as percentage of the amplitude of the first NMDA EPSC). The plot is based on the mean values from 19 synapses that exhibited more than one NMDA EPSC per 10 impulse burst. The dashed line is the linear regression line and the correlation is statistically significant (r = 0.71; P < 0.01).
DISCUSSION
The main findings of the present study can be summarized as follows. Both AMPA and NMDA receptor-mediated quantal responses from a single release site show considerable inter-trial variability. The amount of this variability varies considerably among the synapses and in such a manner that quantal variability of the AMPA response co-varies with that of the NMDA response. It was also observed that transmitter released by a single vesicle did not saturate the postsynaptic NMDA receptors. These results are compatible with a quantal variability based to a large extent on non-saturated AMPA and NMDA responses fluctuating as a function of the amount of transmitter released from each vesicle.
Release of a single vesicle at a single release site
How certain can we be that the minimal stimulation only activates a single afferent with a single release site releasing a single vesicle upon activation, i.e. that we are studying quantal responses? Our best evidence is the constancy of the response size observed in synapses in which an initial high release probability during the train stimulation was followed by a later much lower release probability. If more than a single vesicle is released by a stimulus then such multiple-release should occur more often when the release probability is high, thus producing larger responses in the initial part of the train stimulation. Such larger responses were not observed in any of the 19 synapses displaying the above release behaviour. This result excludes the possibility that the EPSCs originate from more than one synapse, either from more than one axon or from one axon, or from more than one independent release site in the same synapse.
Since the AMPA receptors are unlikely to be saturated by the release from a single vesicle (Liu et al. 1999; Umemiya et al. 1999; McAllister & Stevens, 2000; present study), the above result should, in principle, also exclude multiple release from a single release site. However, since the effect of a double release will be moderated by possible saturation, the existence of such multiple release from a single release site may escape notice using the above test. However, such possible multiple releases would be expected (except larger amplitudes) to be associated with shorter latencies, longer rise times, and longer durations of the EPSCs (Auger & Marty, 2000). In our analysis of latency and rise time of the EPSCs for each synapse, these parameters displayed overall sharp Gaussian distributions of latency and rise time, and the average values of these parameters did not change during the train stimulation. Moreover, EPSCs averaged from the initial period of high initial release probability were superimposable with those averaged later in the train, and the same was true for small and large EPSCs in the same synapse.
Quantal amplitude
From the above we conclude that the observed responses were produced by a single vesicle released from a single release site. This conclusion thus supports the notion that release in these synapses is uni-quantal (Raastad et al. 1992; Allen & Stevens, 1994; Forti et al. 1997; Hsia et al. 1998; Liu et al. 1999). The mean quantal amplitude thus estimated varied considerably among the synapses, from about 5 to 30 pA, averaging 15 pA. These values are considerably greater than the values of a few picoamps commonly attributed to the quantal response. This discrepancy could be explained by a reduction in receptor number/channel conductance with development. However, reported values of quantal amplitude are likely to be much underestimated due to the filtering effects of dendrites and recording electrode, effects that may lower the actual currents by one order of magnitude (Spruston et al. 1994). Recordings close to the synapse, using loose patch recording, have also indicated large quantal amplitudes (Forti et al. 1997). It is thus not implausible that the values presently observed, due to the more compact electrotonic structure in these neonatal pyramidal neurones (Pokorny & Yamamoto, 1981; Spigelman et al. 1992), are compatible with those attributed to the quantal response in older animals.
Quantal variability
The quantal amplitude was found to vary considerably not only among the synapses, but also for a given synapse. Thus, the present study substantiates earlier reports (Bekkers et al. 1990; Raastad et al. 1992; Forti et al. 1997; Liu et al. 1999; Mainen et al. 1999; McAllister & Stevens, 2000; Murthy et al. 2000) that uni-quantal release can be associated with a large quantal variability (CV) of both AMPA and NMDA receptor-mediated responses. These CVs averaged about 39 % and 30 %, respectively, and did not tend to decrease with age of the animal (P1-P7). To the extent that synapses in older animals should have lower quantal variability, this must result from some maturation process that is initiated later than the first neonatal week.
Quantal variability may arise as a result of factors such as the stochastic properties of transmitter-receptor interactions, or spatial variations in release zone-postsynaptic receptor alignment. However, a plausible explanation is variability in the amount of transmitter released. Vesicles in a terminal vary in size, and thus probably in the amount of transmitter released (Bekkers et al. 1990; Harris & Sultan, 1995). Paired with non-saturation of postsynaptic receptors, this variation will result in quantal variability (Liu et al. 1999). Considering the fact that a twofold variation in vesicle diameter may allow for an 8-fold variation in amount of releasable transmitter, and that even the largest NMDA responses did not reach saturation, a variation in vesicle transmitter content can potentially explain much of the quantal variability (Kullmann, 1999). A variable degree of emptying of the vesicle may also contribute to variations in transmitter output (Klingauf et al. 1998). The present analysis adds evidence in favour of an explanation based on variability in transmitter release. Thus, CV values for AMPA and NMDA quantal responses were found to co-vary, and the most reasonable common link is transmitter output. Moreover, the shapes of the size distributions of AMPA and NMDA quantal responses displayed similar features in the present study. Thus, in both cases, synapses within the lower range of CV values exhibited distributions well fitted by a Gaussian curve. And, in synapses displaying a higher degree of quantal variability, both distributions had broader peaks and were skewed towards lower values.
This conclusion of a presynaptic basis for quantal variability was also reached in recent studies on spontaneous synaptic responses in cultures of cortical (Umemiya et al. 1999) and hippocampal neurones (McAllister & Stevens, 2000). However, postsynaptic factors may also contribute to the quantal variability, at least of NMDA EPSCs, since even in synapses indicating full saturation of these receptors after a single response there remained some variability (about 20 %, cf. Fig. 10).
Variability in quantal variability
The synapses did not only show considerable quantal variability in AMPA and NMDA responses, but also a large variation among them in the magnitude of the variability, the CV values ranging from about 20 to 70 % (AMPA) and 10 to 50 % (NMDA). This variability in CV was not related to variations in mean quantal amplitude indicating that stochastic properties of transmitter-receptor interactions are not the major explanation (Faber et al. 1992). As noted above, the variations in CV of AMPA and NMDA EPSCs co-varied among the synapses, not only in magnitude but also in the shape of the amplitude distributions. That is, from Gaussian distributions at low CVs to more skewed ones at high CVs. All this may be explained by various concentrations of transmitter operating on non-saturated receptors. One may then imagine two scenarios to explain variability in CV among the synapses. Either that differently sized variations in vesicle volume among the synapses are operating against the same level of receptor non-saturation. Or that similarly sized variations in vesicle volume are operating against different levels of receptor non-saturation. As judged from the interaction of successive NMDA EPSCs, higher degrees of NMDA saturation were associated with smaller CV values, favouring the latter notion.
In the mossy fibre-granule cell synapse in the cerebellum, a broad skew quantal distribution at an early age (11-15 days) is later replaced by a narrow Gaussian distribution (Wall & Usowicz, 1998). Such behaviour seems compatible with the present observation that ‘deaf’ synapses, that may be considered more immature than AMPA-signalling ones, tended to have smaller quantal peaks and larger CVs. However, this tendency was not statistically significant. Moreover, AMPA-signalling synapses that exhibited narrow Gaussian quantal distributions were found to coexist with synapses exhibiting broad skewed distributions at the same, early, developmental stage. These findings suggest that a small quantal variability is not simply a passive consequence of development, but that it may instead be subject to an active regulation. The basis for such an active regulation remains to be understood, but it may represent a form of synaptic plasticity that controls the dynamics of trial-to-trial signalling without affecting the average signalling strength.
Acknowledgments
This project was supported by the Swedish Medical Research Council (project number 12600 and 05180), the Swedish Society of Medicine, Harald Jeanson's foundation, The Royal Society of Arts and Sciences in Göteborg, Magnus Bergvall's Foundation and Adlerbertska Research Foundation.
References
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proceedings of the National Academy of Sciences of the USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P. Synaptic integration in hippocampal CA1 pyramids. Progress in Brain Research. 1990;83:215–222. doi: 10.1016/s0079-6123(08)61251-0. [DOI] [PubMed] [Google Scholar]
- Auger C, Marty A. Quantal currents at single-site central synapses. The Journal of Physiology. 2000;526:3–11. doi: 10.1111/j.1469-7793.2000.t01-3-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Richerson GB, Stevens CF. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proceedings of the National Academy of Sciences of the USA. 1990;87:5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: Quantal fluctuation affects subsequent release. The Journal of Physiology. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Edwards F, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Faber DS, Young WS, Legendre P, Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992;258:1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. Journal of Neuroscience. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti L, Bossi M, Bergamaschi A, Villa A, Malgaroli A. Loose-patch recordings of single quanta at individual hippocampal synapses. Nature. 1997;388:874–878. doi: 10.1038/42251. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proceedings of the National Academy of Sciences of the USA. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Vesicle release probability and pre-primed pool at glutamatergic synapses in area CA1 of the rat neonatal hippocampus. The Journal of Physiology. 2001;531:481–493. doi: 10.1111/j.1469-7793.2001.0481i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Sultan P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology. 1995;34:1387–1395. doi: 10.1016/0028-3908(95)00142-s. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. Journal of Neurophysiology. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Hjelmstad GO, Nicoll RA, Malenka RC. Long-term potentiation at single fiber inputs to hippocampal CA1 pyramidal cells. Proceedings of the National Academy of Sciences of the USA. 1996;93:8710–8715. doi: 10.1073/pnas.93.16.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Keller BU, Lev Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflügers Archiv. 1990;417:285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Excitatory synapses. Neither too loud nor too quiet. Nature. 1999;399:111–112. doi: 10.1038/20089. [DOI] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proceedings of the National Academy of Sciences of the USA. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- Malgaroli A. Silent synapses: I can't hear you! Could you please speak aloud? Nature Neuroscience. 1999;2:3–5. doi: 10.1038/4503. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Dynamics of dendritic calcium transients evoked by quantal release at excitatory hippocampal synapses. Proceedings of the National Academy of Sciences of the USA. 2000;97:901–906. doi: 10.1073/pnas.97.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Research Bulletin. 1981;7:113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Raastad M. Extracellular activation of unitary excitatory synapses between hippocampal CA3 and CA1 pyramidal cells. European Journal of Neuroscience. 1995;7:1882–1888. doi: 10.1111/j.1460-9568.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Raastad M, Storm JF, Andersen P. Putative single quantum and single fibre excitatory postsynaptic currents show similar range and variability in rat hippocampal slices. European Journal of Neuroscience. 1992;4:113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. Journal of Neuroscience. 1993;13:3736–3748. doi: 10.1523/JNEUROSCI.13-09-03736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Zhang L, Carlen PL. Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: Membrane excitability and K+ currents. Journal of Neurophysiology. 1992;68:55–69. doi: 10.1152/jn.1992.68.1.55. [DOI] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends in Neurosciences. 1994;17:161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Steward O, Falk PM. Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. Journal of Comparative Neurology. 1991;314:545–557. doi: 10.1002/cne.903140311. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. Journal of Neuroscience. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Senda M, Murphy TH. Behaviour of NMDA and AMPA receptor-mediated miniature EPSCs at rat cortical neuron synapses identified by calcium imaging. The Journal of Physiology. 1999;521:113–122. doi: 10.1111/j.1469-7793.1999.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of the quantal properties of evoked and spontaneous synaptic currents at a brain synapse. Nature Neuroscience. 1998;1:675–682. doi: 10.1038/3677. [DOI] [PubMed] [Google Scholar]


