Abstract
A fundamental property of animal cells is the ability to regulate their own cell volume. Even under hypotonic stress imposed by either decreased extracellular or increased intracellular osmolarity, the cells can re-adjust their volume after transient osmotic swelling by a mechanism known as regulatory volume decrease (RVD). In most cell types, RVD is accomplished mainly by KCl efflux induced by parallel activation of K+ and Cl− channels. We have studied the molecular mechanism of RVD in a human epithelial cell line (Intestine 407). Osmotic swelling results in a significant increase in the cytosolic Ca2+ concentration and thereby activates intermediate-conductance Ca2+-dependent K+ (IK) channels. Osmotic swelling also induces ATP release from the cells to the extracellular compartment. Released ATP stimulates purinergic ATP (P2Y2) receptors, thereby inducing phospholipase C-mediated Ca2+ mobilization. Thus, RVD is facilitated by stimulation of P2Y2 receptors due to augmentation of IK channels. In contrast, stimulation of another G protein-coupled Ca2+-sensing receptor (CaR) enhances the activity of volume-sensitive outwardly rectifying Cl− channels, thereby facilitating RVD. Therefore, it is possible that Ca2+ efflux stimulated by swelling-induced and P2Y2 receptor-mediated intracellular Ca2+ mobilization activates the CaR, thereby secondarily upregulating the volume-regulatory Cl− conductance. On the other hand, the initial process towards apoptotic cell death is coupled to normotonic cell shrinkage, called apoptotic volume decrease (AVD). Stimulation of death receptors, such as TNFα receptor and Fas, induces AVD and thereafter biochemical apoptotic events in human lymphoid (U937), human epithelial (HeLa), mouse neuroblastoma × rat glioma hybrid (NG108-15) and rat phaeochromocytoma (PC12) cells. In those cells exhibiting AVD, facilitation of RVD is always observed. Both AVD induction and RVD facilitation as well as succeeding apoptotic events can be abolished by prior treatment with a blocker of volume-regulatory K+ or Cl− channels, suggesting that AVD is caused by normotonic activation of ion channels that are normally involved in RVD under hypotonic conditions. Therefore, it is likely that G protein-coupled receptors involved in RVD regulation and death receptors triggering AVD may share common downstream signals which should give us key clues to the detailed mechanisms of volume regulation and survival of animal cells. In this Topical Review, we look at the physiological ionic mechanisms of cell volume regulation and cell death-associated volume changes from the facet of receptor-mediated cellular processes.
Cell volume regulation and cell death
The regulation of cell volume is an essential function coupled to a variety of physiological processes, such as cell proliferation, differentiation and migration (see Lang et al. 1998a; Okada, 1998a). Living cells need to regulate their volume even at constant extracellular osmolarity, because the presence of membrane-impermeable polyvalent anion macromolecules within the cytosol physico-chemically produces colloid-osmotic pressure due to cationic leak influx with a driving force towards Donnan equilibrium. Animal cells can exhibit steady-state volume regulation by a Na+ pump-mediated mechanism (Fig. 1, centre), called the ‘pump-leak balance mechanism’ (Leaf, 1959; Tosteson & Hoffman, 1960; see Macknight & Leaf, 1977).
Figure 1. Schematic illustration of the ionic mechanisms for RVD and RVI under physiological conditions as well as for AVD and NVI under pathophysiological conditions.

The pump-leak balance mechanism is depicted in the centre cell. Three different mechanisms for RVI and RVD are depicted in the upper left and upper right cells, respectively. The AVD- and NVI-inducing mechanisms are given in the lower left and lower right cells, respectively. Apoptotic and necrotic cell death may be triggered by persistent cell shrinkage and swelling, as depicted in the left and right cells, respectively. (See text for details.)
Cells respond with swelling or shrinkage to osmotic perturbation, but swollen and shrunken cells thereafter exhibit volume regulation under physiological conditions. However, cells undergoing programmed cell death (apoptosis) exhibit persistent cell shrinkage even under physiological normotonic conditions. Under pathophysiological conditions, cells often undergo a persistent swelling or shrinkage without showing volume regulation. Such impaired volume regulation is coupled to the initial steps of necrotic and apoptotic cell death.
Regulatory volume decrease (RVD) and regulatory volume increase (RVI)
Cell activities themselves cause fluctuations in intracellular osmolarity due to changes in osmolyte transport and metabolism. In the face of anisotonic conditions, cells can regulate, in a non-steady-state manner, their volume after transient osmotic shrinkage or swelling, and RVI and RVD are attained by water flux driven mainly by NaCl influx and KCl efflux, respectively. Most cell types have been shown to possess the ability to undergo RVD (see Hoffmann & Simonsen, 1989; Lang, 1998; Okada, 1998a). Hippocampal neurons have, in contrast, been reported to lack volume regulation under anisotonic conditions (Andrew et al. 1997; Aitken et al. 1998), whereas RVD capacity has been observed in cerebellar neurons (Pasantes-Morales et al. 1993; Patel et al. 1998) and sympathetic neurons (Horie et al. 1989; Quinn & Pierce, 1992; Leaney et al. 1997) as well as in neuroblastoma cells (Lippmann et al. 1995; Basavappa & Ellory, 1996; Mackert et al. 1996; Basavappa et al. 1998; Rouzaire-Dubois et al. 1999). Since cerebral cortical neurons were found to exhibit RVD only in the presence of an NMDA receptor antagonist (Churchwell et al. 1996), it is possible that stimulation of NMDA receptors overrides or inhibits the RVD mechanism.
Upon osmotic cell shrinkage, volume-regulatory NaCl influx is produced by three different cotransporter-mediated mechanisms depending on the cell type (see Hoffmann & Simonsen, 1989; Okada, 1997; Baumgarten & Feher, 1998); that is, by parallel operation of Na+-H+ and Cl−-HCO3− antiporters or by operation of either the Na+-K+-2Cl− or Na+-Cl− symporter, as depicted in Fig. 1 (upper left). Upon osmotic cell swelling, as summarized in Fig. 1 (upper right), a volume-regulatory KCl efflux is induced as a result of the activation of K+ and Cl− channels in most cell types or as a result of electroneutral operation of cotransporters, such as the K+-Cl− symporter and K+-H+plus Cl−-HCO3− antiporters, in several cell types (see Hoffmann & Simonsen, 1989; Okada 1997; Baumgarten & Feher, 1998). Similar ionic mechanisms operate during volume regulation after isotonic shrinkage induced by active solute secretion in enterocytes (O'Brien et al. 1993; Diener, 1994) as well as after isotonic swelling coupled to active uptake of organic solutes by enterocytes (MacLeod & Hamilton, 1991), proximal tubule cells (Mounfield & Robson, 1998; Robson & Hunter, 1999) and hepatocytes (Lidofsky & Roman, 1997).
In the face of a swelling emergency, most cells extrude K+ by operation of the available K+ channels (see Hoffmann & Pedersen, 1998). In order to lead to an osmoeffective efflux, net anion efflux should be accompanied by K+ efflux in order to maintain electroneutrality. Osmotic swelling is associated with the activation of a variety of anion channels (see Okada, 1997). Among these swelling-activated anion channels, the most ubiquitous and specific is the volume-sensitive outwardly rectifying (VSOR) anion channel (Okada, 1997), also called the volume-regulated anion channel (VRAC) (Nilius et al. 1997) or the volume-sensitive organic osmolyte and anion channel (VSOAC) (Strange et al. 1996). VSOR channel activity is largely dependent on non-hydrolytic binding of intracellular ATP, is sensitive to cytosolic free Mg2+ (Oiki et al. 1995; Okada, 1997) and is known to be downregulated when cells switch from proliferation to differentiation (Voets et al. 1997) or are arrested in G0/G1 (Shen et al. 2000). However, the molecular identity of this channel is not firmly established (see Okada, 1997; Eggermont et al. 1998; Okada et al. 1998; Strange, 1998).
Necrotic volume increase (NVI)
Necrotic cell death is paralleled by cell swelling (Duvall & Wyllie, 1986; Kroemer et al. 1998), termed necrotic volume increase (NVI). Accidental cell damage or injury induces Na+ uptake and ATP release due to membrane leakage as well as dissipation of ATP by constrained overworking of the Na+ pump. The resulting ATP depletion, in turn, leads to a reduction of Na+ pump activity, thereby resulting in an impairment of the pump-leak balance mechanism. Ischaemia or hypoxia often produces cell swelling by this mechanism (see Macknight & Leaf, 1977; Hoffmann & Simonsen, 1989; Lipton, 1999), but sometimes triggers cell shrinkage by activation of apoptotic reactions (Lipton, 1999; Banasiak et al. 2000). Cell swelling induced by ATP depletion due to inhibition of oxidative reactions is aggravated by enhanced glycolytic reactions which lead to accumulation of glycolysis metabolites such as lactate and to intracellular acidification. Cell acidosis results in NaCl accumulation within the cells, because net uptake of Na+ is produced by stimulation of Na+-H+ antiporters and that of Cl− by operation of Cl−-HCO3− antiporters and by reduction of the negative charge valency of intracellular polyvalent anion macromolecules (see Okada, 1998b). Under acidosis with lactate accumulation, called lactacidosis, lactate entry via H+-lactate− symporters (monocarboxylate transporters) may also partake in cell swelling (Lomneth et al. 1990). In the ischaemic brain, glutamate is released from glial cells, and thereby induces neuronal swelling by Na+ entry via stimulated NMDA receptors (Choi & Rothman, 1990; Kimelberg, 1991; Rossi et al. 2000). Reactive oxygen species (ROS), including superoxide anion, hydroxyl radical and hydrogen peroxide, may also bring about Na+ influx by activating a special class of non-selective cation channels (Koliwad et al. 1996; Herson & Ashford, 1997; Mendez & Penner, 1998; Barros et al. 2001). These ionic mechanisms of NVI are schematically depicted in Fig. 1 (lower right). Under ATP-deficient conditions, furthermore, such cell swelling must persist, since the following RVD process cannot be activated, because the VSOR anion channel activity is inhibited by reduction of intracellular ATP per se and by elevation of the intracellular free Mg2+ level (Oiki et al. 1995; Okada, 1997). In fact, marked inhibition of both RVD and VSOR Cl− channel activity was observed under hypoxia or mitochondrial inhibition in mouse cerebellar granule neurons (Patel et al. 1998). ATP depletion or metabolic inhibition thus induces persistent cell swelling and finally cell rupture, and a strong hypotonic challenge may override the RVD activity, thereby eventually bringing about cell rupture (Fig. 1, lower right and upper right to middle right).
Apoptotic volume decrease (AVD)
In contrast to necrotic cell death, cell shrinkage is a major hallmark of programmed cell death or apoptosis (Wyllie et al. 1980). The apoptotic cell shrinkage occurs in two distinct stages: the first phase starting before cell fragmentation or formation of the apoptotic body and the second phase associated with cell fragmentation (Benson et al. 1996). The early-phase shrinkage is termed apoptotic volume decrease (AVD) (Maeno et al. 2000). AVD proceeds under normotonic conditions without inducing (or by over-riding) the RVI mechanism, presumably by activation of volume-regulatory K+ and/or Cl− channels (Maeno et al. 2000), as schematically illustrated in Fig. 1 (lower left). When RVI is over-ridden by AVD or suppressed by some other mechanism, apoptotic cell death ensues (lower left to middle left). Also, persistent physical shrinkage forced by hypertonic stress leads to apoptotic death in a variety of cell types (Bortner & Cidlowski, 1996; Matthews & Feldman, 1996; Orlov et al. 1996; Singleton et al. 1996; Qin et al. 1997; Edwards et al. 1998; Bilney & Murray, 1998; Rasola et al. 1999; Morales et al. 2000). Bortner & Cidlowski (1996) showed that hypertonic stress can induce apoptosis in cells that lack the RVI mechanism (Fig. 1, upper left to middle left).
The control of cell volume is essential not only for maintaining physiological cell activities but also for cell survival. Both apoptotic and necrotic cell death are associated with impairments or alterations of cell volume regulation.
RVD facilitation mediated by G protein-coupled receptors
Application of GTPγS was found to facilitate the RVD process in cervical cancer cells, whereas the GTP analogue failed to affect RVD of normal or human papilloma virus (HPV)-immortalized cervical cells (Shen et al. 1998). The activity of volume-sensitive Cl− channels was enhanced by GTPγS in some cell types (Doroshenko & Neher, 1992; Fahlke et al. 1992; Nilius et al. 1994a; Shen et al. 1996; Mitchell et al. 1997; Voets et al. 1998; Shimizu et al. 2000) but not in others (Botchkin & Matthews, 1993; Ackerman et al. 1994; Miley et al. 1999). These facts suggest that heterotrimeric or monomeric G proteins are often (but not always) involved in upregulation (but not in activation mechanisms per se) of RVD or volume-sensitive Cl− channels, depending on the cell type and on the availability of ligands. Recently, Nilius et al. (1999) have shown that activation of a small monomeric G protein, Rho, is involved in the activation cascade of volume-sensitive Cl− channels. Also, there has been ample evidence for an involvement of heterotrimeric G proteins, such as leukotriene D4 receptor (Lambert et al. 1987; Lambert, 1989; Jørgensen et al. 1996), thrombin receptor (Manolopoulos et al. 1997) and endothelin ETA1 receptor (Du & Sorota, 2000), in the control of volume-regulatory K+ and Cl− channels.
Taken together, there is a possibility that RVD is facilitated by activation either K+ or Cl− channels due to stimulation of G protein-coupled receptors by some ubiquitous ligands which are normally available during osmotic swelling in any type of cell. Recently, we have tested this possibility regarding receptors for ATP and Ca2+ in human epithelial Intestine 407 cells.
ATP receptor-mediated upregulation of volume-regulatory K+, but not Cl−, channels
Osmotic cell swelling has been found to induce the release of ATP from many cell types (Wang et al. 1996; Roman et al. 1997; Feranchak et al. 1998; Hazama et al. 1998, 1999; Mitchell et al. 1998; Taylor et al. 1998; Musante et al. 1999; Van der Wijk et al. 1999; Hazama et al. 2000b). It was clearly demonstrated not only in cystic fibrosis transmembrane conductance regulator (CFTR)-lacking Intestine 407 cells (Hazama et al. 1998, 1999; Van der Wijk et al. 1999) but also in CFTR-transfected C127 cells (Hazama et al. 2000b), that neither the CFTR nor the VSOR anion channel serves as the ATP-releasing pathway, as summarized elsewhere (Okada et al. 2000).
By a biosensor technique, the extracellular ATP concentration in the immediate vicinity of the cell surface was observed to reach over 10 μm, which is high enough to stimulate purinergic receptors (Dubyak & El-Moatassim, 1993), in Intestine 407 cells (Hazama et al. 1999). Functional expression of P2Y2 purinergic receptors in Intestine 407 cells was evidenced by fura-2 studies (Dezaki et al. 2000b). Reverse transcription-polymerase chain reaction (RT-PCR) studies demonstrated expression of the transcripts of P2Y2 receptors in this cell line (K. Dezaki & Y. Okada, unpublished observations). Thus, there is a possibility that ATP released from swollen cells stimulates P2Y2 receptors, thereby modulating RVD. This inference was, in fact, proved by the following four observations in human Intestine 407 cells (Dezaki et al. 2000b). (1) RVD was largely inhibited by extracellular application of an ATP-hydrolysing enzyme, apyrase (Fig. 2A, ♦). (2) An antagonist of P2 purinergic receptors, suramin, also suppressed RVD (Fig. 2A, •). (3) Extracellular application of ATP accelerated RVD at micromolar concentrations (Fig. 2A, ▴). (4) Facilitation of RVD was also produced in Intestine 407 cells upon hypotonic challenge in the presence of UTP (Fig. 2A, ▿). Similar observations have been reported in other cell types by applying apyrase (Wang et al. 1996; Roman et al. 1997, 1999; Light et al. 1999; Hazama et al. 2000b), suramin (Wang et al. 1996; Roman et al. 1997, 1999) and ATP (Light et al. 1999).
Figure 2. Effects of stimulation and inhibition of P2 purinergic receptors (A) and stimulation of CaR (B) on RVD observed in the presence (1 mm: A) and nominal absence (B) of extracellular Ca2+ in Intestine 407 cells.

A hypotonic challenge (70 % osmolality) was applied at time zero. Cell volume was normalized to that before the hypotonic challenge. Data represent the mean ±s.e.m. (vertical bars) of 5-10 observations. (Data in A are adapted from Dezaki et al. 2000b.)
Recently, Fitz and collaborators put forward an attractive hypothesis that stimulation of P2 receptors by released ATP is a causative factor for the activation of VSOR Cl− channels, based on their observations that apyrase, suramin and another blocker of P2 receptors, reactive blue 2, prevented activation of swelling-activated Cl− currents in hepatic cells (Wang et al. 1996; Roman et al. 1997, 1999). However, suramin and reactive blue 2 are known to directly block preactivated volume-sensitive Cl− currents (Galietla et al. 1997; Van der Wijk et al. 1999). Also, extracellular application of ATP has never been observed to induce activation of VSOR Cl− currents (Nilius et al. 1994b; Gschwentner et al. 1995; Jackson & Strange, 1995; Tsumura et al. 1996; Van den Wijk et al. 1999; Okada et al. 2000). In addition, no basal activity of VSOR Cl− channels was observed in C127/CFTR cells, which exhibit significant background ATP-release activity under isotonic conditions (Hazama et al. 2000b). Furthermore, block of swelling-induced ATP release by Gd3+, apyrase or anti-ATP-release antibodies failed to inhibit swelling-induced activation of VSOR Cl− currents in Intestine 407 cells (Hazama et al. 1999; Van der Wijk et al. 1999) and C127/CFTR cells (Hazama et al. 2000a,b; Okada et al. 2000). Thus, it appears that swelling-induced ATP release is not involved in the activation of the VSOR Cl− channel.
Osmotic cell swelling brings about an increase in the intracellular free Ca2+ concentration ([Ca2+]i) in many cell types (see McCarty & O'Neil, 1992). Intestine 407 cells also respond to a hypotonic challenge with an [Ca2+]i rise (Hazama & Okada, 1990a) due to both Ca2+ influx via swelling-activated Ca2+-permeable cation channels (Okada et al. 1990) and Ca2+ release from intracellular Ca2+ stores (Hazama & Okada, 1990b). Swelling-induced ATP release has recently been shown to augment the Ca2+ response to a hypotonic challenge via stimulation of P2Y2 receptors in Intestine 407 cells (Dezaki et al. 2000b). We have recently found that an inhibitor of phospholipase C (PLC), U-73122, but not its inactive stereoisomer U-73343, abolishes the ATP-evoked [Ca2+]i rise in Intestine 407 cells (K. Dezaki & Y. Okada, unpublished observations).
Human Intestine 407 cells respond to hypotonic stress with parallel activation of a Ca2+-dependent K+ conductance and a Ca2+-independent VSOR Cl− conductance (Hazama & Okada, 1988; Kubo & Okada, 1992). Thus, it is suggested that this cell line lacks Ca2+-activated Cl− channels. This was supported by the observation by Tilly and collaborators (Tilly et al. 1994) that 125I− efflux was not stimulated by Ca2+-mobilizing hormones in Intestine 407 cells. Actually, a cytosolic Ca2+ rise induced by extracellular application of ATP was found to bring about the activation of K+ currents but never Cl− currents (Dezaki et al. 2000b). Our recent single-channel recordings and RT-PCR study have revealed the existence of inwardly rectifying intermediate-conductance Ca2+-activated K+ channels and expression of mRNA for the intermediate-conductance Ca2+-activated K+ (IK1) channel in Intestine 407 cells (Wang et al. 2001). The involvement of IK1 in RVD was also shown in T lymphocytes (Khanna et al. 1999). Taken together, it is concluded that swelling-induced ATP release facilitates RVD by augmenting cytosolic Ca2+ mobilization via G protein-coupled, PLC-linked P2Y2 receptors, thereby stimulating IK1 channel currents but not Cl− channel currents in the epithelial cell line, as schematically depicted in Fig. 3 (left half).
Figure 3. Schematic model of the molecular mechanism of RVD and its control mediated by G protein-coupled receptors, P2Y2R and CaR, in Intestine 407 cells.

Gq and Gs represent G proteins coupled to phospholipase C (PLC) and adenylate cyclase (AC), respectively. PMCA represents the plasmalemmal Ca2+ pump. (See text for details.)
Ca2+-sensing receptor-mediated upregulation of volume-sensitive Cl−, but not K+, channels
The plasma or extracellular Ca2+ concentration ([Ca2+]o) is normally maintained within a narrow range around 1 mm. However, the microenvironmental [Ca2+]o may considerably increase during injury of the cell or tissue. Recently, it has been shown that the local [Ca2+]o around a cell that is responding to a stimulus with a cytosolic Ca2+ rise may be increased by active extrusion of Ca2+ (mainly via the plasma membrane Ca2+ pump, PMCA) and thereby exceed the level sufficient to activate the G protein-coupled, extracellular Ca2+/polyvalent cation-sensing receptor (Ca2+-sensing receptor, CaR), which is known to be expressed in a wide variety of mammalian cell types (see Brown et al. 1998), on that cell and adjacent cells (Hofer et al. 2000). Therefore, Ca2+ extruded from a stimulated cell or released from an injured cell may serve not only as a paracrine signal to nearby cells but also as an autocrine signal to this Ca2+-releasing cell itself, if the cells express the CaR.
In Intestine 407 cells, expression of CaR transcripts and that of protein has recently been evidenced by RT-PCR and Western blot analysis, respectively (Shimizu et al. 2000). Also, stimulation of the CaR by a divalent mineral cation, Mg2+ (50 mm), or an organic polycation, neomycin (0.5 mm), markedly facilitated RVD observed in the absence of extracellular Ca2+ in the cells, as shown in Fig. 2B.
Since a cytosolic Ca2+ rise was never observed upon stimulation with extracellular 50 mm Mg2+ or 0.5 mm neomycin in Intestine 407 cells (K. Dezaki, T. Shimizu & Y. Okada, unpublished observations), CaR-mediated facilitation of RVD cannot be explained by upregulation of Ca2+-activated K+ channels. It can be deduced that upregulation of VSOR Cl− channels is responsible for CaR-mediated facilitation of RVD in Intestine 407 cells, based on the following two observations (Shimizu et al. 2000). (1) Whole-cell VSOR Cl− currents were augmented by extracellular application of CaR agonists such as Ca2+, Mg2+, La3+ and spermine. (2) A non-hydrolysable analogue of GDP, GDPβS, which competes with GTP for the nucleotide binding sites on G proteins, abolished the effect of extracellular Ca2+ on VSOR Cl− currents, whereas a non-hydrolysable GTP analogue, GTPγS, augmented the VSOR Cl− current and abolished the upregulating effect of extracellular Ca2+. It is noteworthy that similar extracellular Ca2+-dependent activation of VSOR Cl− currents was observed in epithelial cells of kidney distal convoluted tubules (Rubera et al. 1997) and osteoclasts (Sakai et al. 1999), and that expression of the CaR has been demonstrated in both cell types (Riccardi et al. 1996; Kameda et al. 1998; Kanatani et al. 1999).
Activation of the CaR is known to result in cell type-specific coupling to intracellular signalling cascades including Ca2+, cAMP and mitogen-activated protein (MAP) kinase pathways (see Brown et al. 1998). It is suggested that cAMP is the intracellular messenger for CaR-mediated upregulation of volume-sensitive Cl− channels in Intestine 407 cells (Shimizu et al. 2000). This is in good agreement with previous reports of cAMP-induced upregulation of VSOR Cl− currents in many cell types, such as canine (Sorota, 1992; Du & Sorota, 1997) and human cardiac myocytes (Oz & Sorota, 1995), rat hepatocytes (Meng & Weinman, 1996), rat carotid body type I cells (Carpenter & Peers, 1997) and human prostate cancer epithelial cells (Shuba et al. 2000). In Intestine 407 cells, the cAMP-dependent VSOR Cl− current component may not represent ClC-3 channel activity, which is known to be downregulated by cAMP via protein kinase A (Nagasaki et al. 2000). Also, an involvement of CFTR can be ruled out for the following reasons: (1) Intestine 407 cells lack CFTR expression (Hazama et al. 1998), (2) cAMP stimulation failed to activate the Cl− current under isotonic conditions (Shimizu et al. 2000) and (3) the cAMP effect on VSOR Cl− current was not abolished by an inhibitor of protein kinase A (Shimizu et al. 2000).
Taken together, it is reasonable to conclude that stimulation of CaR by Ca2+ ions extruded actively from osmotically swollen cells or released from injured cells induces upregulation of VSOR Cl− channels via a G protein-mediated increase in intracellular cAMP in human epithelial cells, as schematically illustrated in Fig. 3 (right half).
AVD induction mediated by death receptors
In contrast to growth factor receptors, which support cell survival, some members of the tumour necrosis factor (TNF) receptor family, also called the death receptor, trigger the cell death programme in response to extracellular death signals and are characterized by an intracellular death domain that serves to recruit some adaptor proteins (such as TRADD and FADD) and cysteine proteases (such as caspase-8) (see Wallach et al. 1999). The best-characterized death receptors are Fas (also called Apo1 or CD95) and TNFR1 (also known as p55 or CD120a) (Smith et al. 1994; Nagata, 1997). Fas is expressed in various human cells (see Pinkoski et al. 2000) and is activated through its trimerization by the binding of Fas ligand (FasL), which is expressed on the plasma membrane of activated T lymphocytes and many other cells (Nagata & Golstein, 1995). Trimerization of TNFR1 is induced by an inflammatory polypeptide cytokine, TNFα, that is released by activated macrophages and T cells as well as many transformed cells (Smith et al. 1994), and thereafter triggers the operation of two opposite signalling pathways: an apoptotic pathway activating caspases and a cell death protecting pathway activating MAP kinase and nuclear factor-κB (NF-κB) (Xia et al. 1999). In the presence of an inhibitor of de novo protein synthesis, such as cycloheximide (CHX) and actinomycin D, stimulation of TNF receptors can therefore trigger apoptosis.
Apoptosis is defined by a characteristic series of biochemical events including caspase activation, and morphological changes including cell shrinkage. The downstream signalling pathway of Fas or TNFR1 responsible for the mechanism of apoptotic cell shrinkage is not as yet known precisely. However, there are in general two possibilities, though not mutually exclusive, for induction of cell shrinkage: release of osmotically active particles along with osmotically obligated water from the cell by activation of channels or transporters, and remodelling of the cell construction or exocytosis-mediated water displacement by caspase-induced cytoskeletal protein cleavage. Recently, we have carried out studies to answer the following two questions about the induction mechanism of AVD, which starts before cell fragmentation or apoptotic body formation. (1) Does the AVD mechanism co-opt normal volume regulatory systems? (2) Is AVD a downstream or upstream event of the activation of executor caspase-3?
Fas/TNF receptor-mediated AVD induction coupled to RVD facilitation
Stimulation of TNFR1 by TNFα (with CHX) resulted in a significant reduction of mean cell volume within 2 h in neuronal PC12 (Fig. 4A, top), NG108-15, histiocytic monoblastoid lymphoma U937 and epithelial HeLa cells (Maeno et al. 2000). AVD was also found to be induced by Fas ligation by anti-Fas antibody within 2 h in U937 cells, as shown in Fig. 4A (bottom). Similar early AVD induction by Fas ligation was observed in Jurkat T cells (Lang et al. 1998b; Bortner & Cidlowski, 1999). The time course of RVD after osmotic swelling under hypotonic stress was found to be hastened in AVD-exhibiting cells within 2 h after stimulation with TNFα (plus CHX) (Maeno et al. 2000). As shown in Fig. 4B (top), TNFα-stimulated PC12 cells responded to a hypotonic challenge with distinct RVD, whereas control PC12 cells exhibited little RVD. Facilitation of RVD was also observed in human U937 cells after a 2 h induction of apoptosis by Fas ligation (Fig. 4B, bottom). RVD facilitation coupled to AVD induction was found to be induced by apoptotic induction with the bacterial alkaloid staurosporine (STS) within 2 h in U937, HeLa, PC12 and NG108-15 cells (Maeno et al. 2000). This is at variance with a previous report by Benson et al. (1996) that no alteration in RVD rate was observed in lymphoblastoid CEM-C7A cells after 24 h treatment with dexamethasone. However, there is the possibility that RVD facilitation had taken place during a much earlier phase of apoptotic cell shrinkage before the activation of cellular processes of cell fragmentation had begun in CEM-C7A cells.
Figure 4. Death receptor-mediated AVD induction and RVD facilitation.
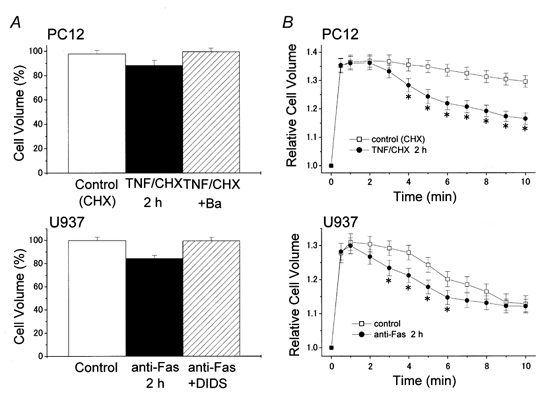
Induction of AVD (A) and facilitation of RVD (B) by 2 h treatment with 10 ng ml−1 TNFα (plus 1 μg ml−1 CHX) in PC12 cells (top) or 0.5 μg ml−1 anti-Fas antibody in U937 cells (bottom), and prevention of AVD induction (A) by simultaneous treatment with a K+ channel blocker, Ba2+ (5 mm), in PC12 cells (top) or a Cl− channel blocker, DIDS (0.5 mm), in U937 cells (bottom). Cell volume measurements were carried out by an electronic cell-sizing technique after washing out any drugs, and the data were normalized by those measured immediately before apoptotic (A) or hypotonic stimulation (B). Data represent the mean ±s.e.m. (vertical bars) of 10 observations. * P < 0.05 vs. corresponding control. (Data from PC12 cells were described in the text, although not shown, in Maeno et al. 2000.)
An involvement of volume-regulatory K+ channels in AVD induction was suggested by the observation that AVD was abolished by a K+ channel blocker (Ba2+ or quinine) in U937, HeLa, PC12 and NG108-15 cells treated with TNFα or STS (Maeno et al. 2000). As shown in Fig. 4A (top), pretreatment with Ba2+ (5 mm) prevented AVD induction in PC12 cells after stimulation of TNFR1. AVD was also reported to be blocked by a K+ channel blocker (tetrapentylammonium) in UV-irradiated human myeloid HL-60 cells (McCarthy & Cotter, 1997) and by other K+ channel blockers (4-aminopyridrine (4AP), sparteine and quinidine) in apoptotic human eosinophils (Beauvais et al. 1995). Stimulation with TNFα was actually found to activate 4AP-sensitive K+ channel currents in rat cortical neurons (Houzen et al. 1997) and Ba2+- and quinine-sensitive K+ currents in rat hepatoma HTC cells (Nietsch et al. 2000). Also, upregulation of TEA-sensitive delayed K+ currents and that of 4AP-sensitive K+ currents was found to be induced by other apoptotic stimuli in neurons (Yu et al. 1997; Colom et al. 1998) and myeloblastic cells (Wang et al. 1999), respectively. In contrast, Fas ligation was observed to suppress a voltage-dependent K+ channel (Kv1.3) in Jurkat T cells (Szabòet al. 1996). However, it was recently reported that the volume-regulatory K+ channel in T cells is the clotrimazole-sensitive IK1 but not the agitoxin 2-sensitive Kv1.3 (Khanna et al. 1999).
Maeno et al. (2000) showed that AVD induction and RVD facilitation induced by TNFα or STS were prevented by pretreatment with blockers of VSOR Cl− channels such as NPPB, DIDS, SITS, niflumic acid, glibenclamide and phloretin, in four different cell types. DIDS also abolished Fas ligation-induced AVD induction (Fig. 4A, bottom) in U937 cells. Actually, we have recently observed, using a Cl−-selective fluorescent dye, a significant decrease in the intracellular Cl− concentration in HeLa cells within 1-2 h after treatment with STS and its abolition by NPPB (Dezaki et al. 2000a). In addition, activation of outwardly rectifying Cl− currents was observed upon apoptotic induction by Fas ligation in Jurkat T cells (Szabòet al. 1998) and by TNFα in HTC hepatoma cells (Nietsch et al. 2000). VSOR-like Cl− currents were also activated by other apoptotic inducers in rat hepatocytes (Meng et al. 1997) and Xenopus oocytes (Souktani et al. 2000).
The activation mechanisms of AVD-inducing K+ and Cl− channels are unknown. However, an involvement of some protein tyrosine kinases (PTKs) may be suggested, because PTK activation was found to be associated not only with apoptotic induction by Fas ligation (Eischen et al. 1994; Atkinson et al. 1996; Schlottmann et al. 1996) and other stimuli (Qin et al. 1997; Yoshida et al. 2000), but also with RVD induction after a hypotonic challenge (Tilly et al. 1993; Lepple-Wienhues et al. 1998). It is conceivable that activation or regulation of VSOR Cl− channels involves PTKs for the following five reasons. (1) Inhibitors of PTKs inhibited VSOR channel activity in a variety of cell types (Tilly et al. 1993; Sorota, 1995; Crépel et al. 1998; Lepple-Wienhues et al. 1998; Voets et al. 1998; Thoroed et al. 1999; Shuba et al. 2000). (2) Inhibitors of protein tyrosine phosphatases potentiated VSOR channel activity in some cells (Tilly et al. 1993; Voets et al. 1998; Shuba et al. 2000). (3) Introduction of a member of the PTK family, p56lck, into Jurkat T cells triggered activation of a VSOR-like Cl− channel, whereas swelling-induced Cl− channel activation was abolished in p56lck-deficient cells (Lepple-Wienhues et al. 1998). (4) Tyrosine phosphorylation of another member of the PTK family, p125FAK, was observed in association with the activation of a VSOR Cl− conductance in Intestine 407 cells (Tilly et al. 1996). (5) Stimulation of the epidermal grown factor receptor (EGFR), which possesses intrinsic protein tyrosine kinase activity, potentiated swelling-induced efflux of 125I and 86Rb from Intestine 407 cells (Tilly et al. 1993). Also, it is noted that stimulation of TNFα receptors upregulates EGFR activity (Bird & Saklatvala, 1990; Kalthoff et al. 1993).
Taken together, it is suggested that AVD induction involves ion transport mechanisms normally responsible for RVD, and that some signals downstream of the death receptors may shift the set-point of the volume sensitivity of the volume-regulatory ion channels below the normal cell volume.
AVD-inducing process as an early prerequisite for apoptosis
AVD is known to precede most other morphological alterations during the apoptotic process (Klassen et al. 1993; Chang et al. 2000). Since a number of cytoskeletal proteins, including fodolin and actin, are cleaved by caspases during apoptosis (see Nicholson & Thornberry, 1997; Tan & Wang, 1998), it has been generally conceived that caspase-induced cytoskeleton disassembly is responsible for apoptotic cell shrinkage (Huppertz et al. 1999). A broad-spectrum caspase inhibitor, zVAD-fmk, was found to consistently block apoptotic cell shrinkage in ML-1 cells after a 4 h exposure to etoposide (Wolf et al. 1997), in thymocytes after a 4 h exposure to glucocorticoids (Hughes & Cidlowski, 1998), in Jurkat T cells after a 24 h exposure to anti-Fas antibody (Bortner & Cidlowski, 1999) and in B lymphoma cells after a 48 h exposure to transforming growth factor (TGF)β (Schrantz et al. 1999). In these studies, however, the effect of zVAD-fmk on the earlier phase of apoptotic shrinkage was not examined, and no quantitative or statistical evaluation was made of cell size changes.
Our recent study demonstrated that the early-phase apoptotic shrinkage, AVD, induced by 2 h exposure to STS was not significantly blocked by the general caspase inhibitors zVAD-fmk or zD-dcb in U937 cells (Maeno et al. 2000). Similar AVD was found to start as early as 30 min after stimulation with TNFα in U937 cells, and zVAD-fmk failed to abolish AVD induction, although the caspase inhibitor completely abolished activation of caspase-3 (E. Maeno & Y. Okada, unpublished observations). The time course of AVD induction distinctly preceded those of caspase-3 activation and cell death induction in TNFα- or STS-treated U937 cells (Maeno et al. 2000).
Both TNFα- and STS-induced AVD started within 30-60 min and thus preceded cytochrome c release and DNA laddering in four different cell lines (Maeno et al. 2000). Cytochrome c release, caspase-3 activation and DNA laddering were all blocked by a Cl− channel blocker (DIDS, NPPB or phloretin), or by a K+ channel blocker (Ba2+ or quinine), in four different cell types stimulated either by TNFα or by STS (Maeno et al. 2000). Apoptotic cell death was finally rescued by the Cl− or K+ channel blocker in U937, HeLa, PC12 and NG108-15 cells (Maeno et al. 2000). This is in good agreement with previous observations that apoptotic cell death was blocked by a K+ channel blocker (TEA, tetrapentylammonium or 4AP) in neurons, thymocytes and leukaemia cells stimulated by several different apoptotic inducers (Yu et al. 1997; Colom et al. 1998; Dallaporta et al. 1999; Wang et al. 1999), and that DNA laddering and annexin V binding were partially suppressed by a low concentration of a Cl− channel blocker (glibenclamide, IAA or DPC) in Fas-stimulated Jurkat T cells (Szabòet al. 1998).
On balance, it appears that AVD is an event upstream of cytochrome c release, caspase-3 activation and DNA laddering. Also, AVD or the AVD-inducing ionic process is likely to be an early, though may not be the earliest, prerequisite to following apoptotic events leading to cell death.
Conclusions and future investigations
Cell volume regulation is an important cell function supporting other cell functions and cell survival. However, much remains to be clarified about the molecular mechanisms of volume regulation. In particular, the cloning or molecular identification of the VSOR Cl− channel, the swelling-activated cation channel and the ATP-release pathway is required to answer how cells sense changes in their volume. Also, further studies on the signalling pathways involved in volume regulation are of great importance. Some G protein-coupled receptors, including P2Y2R and CaR, can potentiate RVD by augmenting volume-regulatory K+ and/or Cl− channel activities. Under certain pathophysiological conditions, however, the volume-regulatory machinery may turn into a volume-disordering machinery that is associated with cell death. Death receptor stimulation with FasL or TNFα triggers the AVD process, which is an early prerequisite to apoptosis, by upregulating volume-regulatory K+ and/or Cl− channels. Thus, there is a possibility that the activation mechanism of volume-regulatory K+ or Cl− channels involves a common signal(s) downstream from AVD-triggering death receptors and RVD-facilitating G protein-coupled receptors. Future studies of the ‘Yin-Yang’ roles of cell volume regulation machinery will open an avenue towards understanding the physiology of cell death and cell survival, which are two sides of the same coin.
Acknowledgments
We thank R. Z. Sabirov for discussion, M. Ohara and S. Tanaka for technical assistance, and T. Okayasu for secretarial help.
References
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. Journal of General Physiology. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken PG, Borgdorff AJ, Juta AJA, Kiehart DP, Somjen GG, Wadman WJ. Volume changes induced by osmotic stress in freshly isolated rat hippocampal neurons. Pflügers Archiv. 1998;436:991–998. doi: 10.1007/s004240050734. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Lobinowich ME, Osehobo EP. Evidence against volume regulation by cortical brain cells during acute osmotic stress. Experimental Neurology. 1997;143:300–312. doi: 10.1006/exnr.1996.6375. [DOI] [PubMed] [Google Scholar]
- Atkinson EA, Ostergaard H, Kane K, Pinkoski MJ, Caputo A, Olszowy MW, Bleackley RC. A physical interaction between the cell death protein Fas and the tyrosine kinase p59fyn. Journal of Biological Chemistry. 1996;271:5968–5971. doi: 10.1074/jbc.271.11.5968. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Progress in Neurobiology. 2000;62:215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Barros LF, Stutzin A, Calixto A, Catalan M, Castro J, Hetz C, Hermosilla T. Non-selective cation channels as effectors of free radical-induced rat liver cell necrosis. Hepatology. 2001;33:114–122. doi: 10.1053/jhep.2001.20530. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Ellory JC. The role of swelling-induced anion channels during neuronal volume regulation. Molecular Neurobiology. 1996;13:137–153. doi: 10.1007/BF02740638. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Mobasheri A, Errington R, Huang C-C, Al-Adawi S, Ellory JC. Inhibition of Na+, K+-ATPase activates swelling-induced taurine efflux in a human neuroblastoma cell line. Journal of Cellular Physiology. 1998;174:145–152. doi: 10.1002/(SICI)1097-4652(199802)174:2<145::AID-JCP1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Baumgarten CM, Feher JJ. Osmosis and the regulation of cell volume. In: Sperelakis N, editor. Cell Physiology Source Book. San Diego: Academic Press; 1998. pp. 253–292. [Google Scholar]
- Beauvais F, Michel L, Dubertret L. Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. Journal of Leukocyte Biology. 1995;57:851–855. doi: 10.1002/jlb.57.6.851. [DOI] [PubMed] [Google Scholar]
- Benson RSP, Heer S, Dive C, Watson AJM. Characterization of cell volume loss in CEM-C7A cells during dexamethasone-induced apoptosis. American Journal of Physiology. 1996;270:C1190–1203. doi: 10.1152/ajpcell.1996.270.4.C1190. [DOI] [PubMed] [Google Scholar]
- Bilney AJ, Murray AW. Pro- and anti-apoptotic effects of K+ in HeLa cells. FEBS Letters. 1998;424:221–224. doi: 10.1016/s0014-5793(98)00172-0. [DOI] [PubMed] [Google Scholar]
- Bird TA, Saklatvala J. Down-modulation of epidermal growth factor receptor affinity in fibroblasts treated with interleukin 1 or tumor necrosis factor is associated with phosphorylation at a site other than threonine 654. Journal of Biological Chemistry. 1990;265:235–240. [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. American Journal of Physiology. 1996;271:C950–961. doi: 10.1152/ajpcell.1996.271.3.C950. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Caspase independent/ dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. Journal of Biological Chemistry. 1999;274:21953–21962. doi: 10.1074/jbc.274.31.21953. [DOI] [PubMed] [Google Scholar]
- Botchkin LM, Matthews G. Chloride current activated by swelling in retinal pigment epithelium cells. American Journal of Physiology. 1993;265:C1037–1045. doi: 10.1152/ajpcell.1993.265.4.C1037. [DOI] [PubMed] [Google Scholar]
- Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing receptor: its role in health and disease. Annual Review of Medicine. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Peers C. Swelling- and cAMP-activated Cl- currents in isolated rat carotid body type I cells. Journal of Physiology. 1997;503:497–511. doi: 10.1111/j.1469-7793.1997.497bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Phelps PC, Berezesky IK, Ebersberger ML, Jr, Trump BF. Studies on the mechanisms and kinetics of apoptosis induced by microinjection of cytochrome c in rat kidney tubule epithelial cells (NRK-52E) American Journal of Pathology. 2000;156:637–649. doi: 10.1016/S0002-9440(10)64768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual Review of Neuroscience. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Churchwell KB, Wright SH, Emma F, Rosenberg PA, Strange K. NMDA receptor activation inhibits neuronal volume regulation after swelling induced by veratridine-stimulated Na+ influx in rat cortical cultures. Journal of Neuroscience. 1996;16:7447–7457. doi: 10.1523/JNEUROSCI.16-23-07447.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom LV, Diaz ME, Beers DR, Neely A, Xie W-J, Apple SH. Role of potassium channels in amyloid-induced cell death. Journal of Neurochemistry. 1998;70:1925–1934. doi: 10.1046/j.1471-4159.1998.70051925.x. [DOI] [PubMed] [Google Scholar]
- Crépel C, Panenka W, Kelly MEM, MacVicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. Journal of Neuroscience. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaporta B, Marchetti P, dePablo MA, Maisse C, Duc H-T, Métivier D, Zamzami N, Geuskens M, Kroemer G. Plasma membrane potential in thymocyte apoptosis. Journal of Immunology. 1999;162:6534–6542. [PubMed] [Google Scholar]
- Dezaki K, Maeno E, Okada Y. Fluorescence measurements of intracellular Cl- concentration during cell apoptosis. Japanese Journal of Physiology. 2000a;50(suppl):S37. Abstract. [Google Scholar]
- Dezaki K, Tsumura T, Maeno E, Okada Y. Receptor-mediated facilitation of cell volume regulation by swelling-induced ATP release in human epithelial cells. Japanese Journal of Physiology. 2000b;50:235–241. doi: 10.2170/jjphysiol.50.235. [DOI] [PubMed] [Google Scholar]
- Diener M. Segmental differences along the crypt axis in the response of cell volume to secretagogues or hypotonic medium in the rat colon. Pflügers Archiv. 1994;426:462–464. doi: 10.1007/BF00388312. [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. Journal of Physiology. 1992;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-Y, Sorota S. Modulation of dog atrial swelling-induced chloride current by cAMP: protein kinase A-dependent and -independent pathways. Journal of Physiology. 1997;500:111–122. doi: 10.1113/jphysiol.1997.sp022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-Y, Sorota S. Cardiac swelling-induced chloride current is enhanced by endothelin. Journal of Cardiovascular Pharmacology. 2000;35:769–776. doi: 10.1097/00005344-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Dubyak GP, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Duvall E, Wyllie AH. Death and the cell. Immunology Today. 1986;7:115–119. doi: 10.1016/0167-5699(86)90152-0. [DOI] [PubMed] [Google Scholar]
- Edwards YS, Sutherland LM, Power JHT, Nicholas TE, Murray AW. Osmotic stress induces both secretion and apoptosis in rat alveolar type II cells. American Journal of Physiology. 1998;275:L670–678. doi: 10.1152/ajplung.1998.275.4.L670. [DOI] [PubMed] [Google Scholar]
- Eggermont J, Buyse G, Voets T, Tytgat J, Droogmans G, Nilius B. Is there a link between protein pICln and volume-regulated anion channels? Biochemical Journal. 1998;331:347–349. doi: 10.1042/bj3310347u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen CM, Dick CJ, Leibson PJ. Tyrosine kinase activation provides an early and requisite signal for Fas-induced apoptosis. Journal of Immunology. 1994;153:1947–1954. [PubMed] [Google Scholar]
- Fahlke Ch, Zachar E, Häussler U, Rudel R. Chloride channels in cultured human skeletal muscle are regulated by G proteins. Pflügers Archiv. 1992;421:566–571. doi: 10.1007/BF00375052. [DOI] [PubMed] [Google Scholar]
- Feranchak AP, Roman RM, Schwiebert EM, Fitz JG. Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. Journal of Biological Chemistry. 1998;273:14906–14911. doi: 10.1074/jbc.273.24.14906. [DOI] [PubMed] [Google Scholar]
- Galietta LJV, Falzoni S, Divirgilio F, Romeo G, Zegarra-Moran O. Characterization of volume-sensitive taurine- and Cl--permeable channels. American Journal of Physiology. 1997;273:C57–66. doi: 10.1152/ajpcell.1997.273.1.C57. [DOI] [PubMed] [Google Scholar]
- Gschwenter M, Nagl UO, Wöll E, Schmarda A, Ritter M, Paulmichl M. Antisense oligonucleotides suppress cell-volume-induced activation of chloride channels. Pflügers Archiv. 1995;430:464–470. doi: 10.1007/BF00373882. [DOI] [PubMed] [Google Scholar]
- Hazama A, Ando-Akatsuka Y, Fan H-T, Tanaka S, Okada Y. CFTR-dependent and -independent ATP release induced by osmotic swelling. In: Suketa Y, Carafoli E, Lazduvski M, Mikoshiba K, Okada Y, Wright EM, editors. Control and Disease of Sodium Dependent Transportation Proteins and Ion Channels. Amsterdam: Elsevier; 2000a. pp. 429–431. [Google Scholar]
- Hazama A, Fan H-T, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl- conductances in C127 cells. Journal of Physiology. 2000b;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Miwa A, Miyoshi T, Shimizu T, Okada Y. ATP release from swollen or CFTR-expressing epithelial cells. In: Okada Y, editor. Cell Volume Regulation: the Molecular Mechanism and Volume Sensing Machinery. Amsterdam: Elsevier; 1998. pp. 93–98. [Google Scholar]
- Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. Journal of Physiology. 1988;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Okada Y. Biphasic rises in cytosolic free Ca2+ in association with activation of K+ and Cl- conductance during the regulatory volume decrease in cultured human epithelial cells. Pflügers Archiv. 1990a;416:710–714. doi: 10.1007/BF00370619. [DOI] [PubMed] [Google Scholar]
- Hazama A, Okada Y. Involvement of Ca2+-induced Ca2+ release in the volume regulation of human epithelial cells exposed to a hypotonic medium. Biochemical and Biophysical Research Communications. 1990b;167:287–293. doi: 10.1016/0006-291x(90)91763-i. [DOI] [PubMed] [Google Scholar]
- Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line. Lack of correlation with volume-sensitive Cl- channels. Journal of General Physiology. 1999;114:525–533. doi: 10.1085/jgp.114.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Ashford MLJ. Activation of a novel non-selective cation channel by alloxan and H2O2 in the rat insulin-secreting cell line CRI-G1. Journal of Physiology. 1997;501:59–66. doi: 10.1111/j.1469-7793.1997.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Curci S, Doble MA, Brown EM, Soybel DI. Hyposmotically activated chloride channels in cultured rabbit non-pigmented ciliary epithelial cells. Nature Cell Biology. 2000;2:392–398. doi: 10.1038/35017020. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Pedersen SF. Sensors and signal transduction in the activation of cell volume regulatory ion transport system. In: Lang F, editor. Cell Volume Regulation. Basel: Karger; 1998. pp. 50–78. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiological Reviews. 1989;69:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Horie H, Ikuta S, Takenaka T, Ito S. Adaptation of cultured mammalian neurons to a hypotonic environment with age-related response. Brain Research. 1989;477:223–240. doi: 10.1016/0006-8993(89)91411-x. [DOI] [PubMed] [Google Scholar]
- Houzen H, Kikuchi S, Kanno M, Shinpo K, Tashiro K. Tumor necrosis factor enhancement of transient outward potassium currents in cultured rat cortical neurons. Journal of Neuroscience Research. 1997;50:990–999. doi: 10.1002/(SICI)1097-4547(19971215)50:6<990::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Jr, Cidlowski JA. Glucocorticoid-induced thymocyte apoptosis: protease-dependent activation of cell shrinkage and DNA degradation. Journal of Steroid Biochemistry and Molecular Biology. 1998;65:207–217. doi: 10.1016/s0960-0760(97)00188-x. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Frank H-G, Kaufmann P. The apoptosis cascade - morphological and immunohistochemical methods for its visualization. Anatomy and Embryology. 1999;200:1–18. doi: 10.1007/s004290050254. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Characterization of the voltage-dependent properties of a volume-sensing anion conductance. Journal of General Physiology. 1995;105:661–677. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen J, Lambert IH, Hoffmann EK. Role of LTD4 in the regulatory volume decrease in Ehrlich ascites tumor cells. Journal of Membrane Biology. 1996;151:159–173. doi: 10.1007/s002329900067. [DOI] [PubMed] [Google Scholar]
- Kalthoff H, Roeder C, Gieseking J, Humburg I, Schmiegel W. Inverse regulation of human ERBB2 and epidermal growth factor receptors by tumor necrosis factor α. Proceedings of the National Academy of Sciences of the USA. 1993;90:8972–8976. doi: 10.1073/pnas.90.19.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, Izumi N, Kawashima H, Ozawa H, Ikeda K, Kameda A, Hakeda Y, Kumegawa M. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochemical and Biophysical Research Communications. 1998;245:419–422. doi: 10.1006/bbrc.1998.8448. [DOI] [PubMed] [Google Scholar]
- Kanatani M, Sugimoto T, Kanzawa M, Yano S, Chihara K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochemical and Biophysical Research Communications. 1999;261:144–148. doi: 10.1006/bbrc.1999.0932. [DOI] [PubMed] [Google Scholar]
- Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Journal of Biological Chemistry. 1999;274:14838–14849. doi: 10.1074/jbc.274.21.14838. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Swelling and volume control in brain astroglial cells. Advances in Comparative and Environmental Physiology. 1991;9:82–117. [Google Scholar]
- Klassen NV, Walker PR, Ross CK, Cygler J, Lach B. Two-stage cell shrinkage and the OER for radiation-induced apoptosis of rat thymocytes. International Journal of Radiation Biology. 1993;64:571–581. doi: 10.1080/09553009314551791. [DOI] [PubMed] [Google Scholar]
- Koliwad SK, Elliott SJ, Kunze DL. Oxidized glutathione mediates cation channel activation in calf vascular endothelial cells during oxidant stress. Journal of Physiology. 1996;495:37–49. doi: 10.1113/jphysiol.1996.sp021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annual Review of Physiology. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- Kubo M, Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. Journal of Physiology. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert IH. Leukotriene-D4 induced cell shrinkage in Ehrlich ascites tumor cells. Journal of Membrane Biology. 1989;108:165–176. doi: 10.1007/BF01871027. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Hoffmann EK, Christensen P. Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. Journal of Membrane Biology. 1987;98:247–256. doi: 10.1007/BF01871187. [DOI] [PubMed] [Google Scholar]
- Lang F. Cell Volume Regulation. Basel: Karger; 1998. [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiological Reviews. 1998a;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lang F, Madlung J, Uhlemann AC, Risler T, Gulbins E. Cellular taurine release triggered by stimulation of the Fas(CD95) receptor in Jurkat lymphocytes. Pflügers Archiv. 1998b;436:377–383. doi: 10.1007/s004240050646. [DOI] [PubMed] [Google Scholar]
- Leaf A. Maintenance of concentration gradients and regulation of cell volume. Annals of the New York Academy of Sciences. 1959;72:396–404. doi: 10.1111/j.1749-6632.1959.tb44168.x. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Marsh SJ, Brown DA. A swelling-activated chloride current in rat sympathetic neurones. Journal of Physiology. 1997;501:555–564. doi: 10.1111/j.1469-7793.1997.555bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabò I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. Journal of Cell Biology. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidofsky SD, Roman RM. Alanine uptake activates hepatocellular chloride channels. American Journal of Physiology. 1997;273:G849–853. doi: 10.1152/ajpgi.1997.273.4.G849. [DOI] [PubMed] [Google Scholar]
- Light DB, Capes TL, Gronau RT, Adler MR. Extracellular ATP stimulates volume decrease in Necturus red blood cells. American Journal of Physiology. 1999;277:C480–491. doi: 10.1152/ajpcell.1999.277.3.C480. [DOI] [PubMed] [Google Scholar]
- Lippmann BJ, Yang R, Barnett DW, Misler S. Pharmacology of volume regulation following hypotonicity-induced cell swelling in clonal N1E115 neuroblastoma cells. Brain Research. 1995;686:29–36. doi: 10.1016/0006-8993(95)00447-x. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiological Reviews. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lomneth R, Medrano S, Gruenstein EI. The role of transmembrane pH gradients in the lactic acid induced swelling of astrocytes. Brain Research. 1990;523:69–77. doi: 10.1016/0006-8993(90)91636-u. [DOI] [PubMed] [Google Scholar]
- McCarthy JV, Cotter TG. Cell shrinkage and apoptosis: a role for potassium and sodium ion efflux. Cell Death and Differentiation. 1997;4:756–770. doi: 10.1038/sj.cdd.4400296. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiological Reviews. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Mackert B-M, Staub F, Peters J, Baethmann A, Kempski O. Anoxia in vitro does not induce neuronal swelling or death. Journal of the Neurological Sciences. 1996;139:39–47. [PubMed] [Google Scholar]
- Macknight ADC, Leaf A. Regulation of cellular volume. Physiological Reviews. 1977;57:510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Macleod J, Hamilton JR. Separate K+ and Cl- transport pathways are activated for regulatory volume decrease in jejunal villus cells. American Journal of Physiology. 1991;260:G405–415. doi: 10.1152/ajpgi.1991.260.3.G405. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proceedings of the National Academy of Sciences of the USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos GV, Prenen J, Droogmans G, Nilius B. Thrombin potentiates volume-activated chloride currents in pulmonary artery endothelial cells. Pflügers Archiv. 1997;433:845–847. doi: 10.1007/s004240050354. [DOI] [PubMed] [Google Scholar]
- Matthews CC, Feldman EL. Insulin-like growth factor I rescues SH-SY5Y human neuroblastoma cells from hyperosmotic induced programmed cell death. Journal of Cellular Physiology. 1996;166:323–331. doi: 10.1002/(SICI)1097-4652(199602)166:2<323::AID-JCP10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mendez F, Penner R. Near-visible ultraviolet light induces a novel ubiquitous calcium-permeable cation current in mammalian cell lines. Journal of Physiology. 1998;507:365–377. doi: 10.1111/j.1469-7793.1998.365bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-J, Carruth MW, Weinman SA. Leukotriene D4 activates a chloride conductance in hepatocytes from lipopolysacchaide-treated rats. Journal of Clinical Investigation. 1997;99:2915–2922. doi: 10.1172/JCI119486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-J, Weinman SA. cAMP- and swelling-activated chloride conductance in rat hepatocytes. American Journal of Physiology. 1996;271:C112–120. doi: 10.1152/ajpcell.1996.271.1.C112. [DOI] [PubMed] [Google Scholar]
- Miley HE, Brown PD, Best L. Regulation of a volume-sensitive anion channel in rat pancreatic β-cells by intracellular adenine nucleotides. Journal of Physiology. 1999;515:413–417. doi: 10.1111/j.1469-7793.1999.413ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proceedings of the National Academy of Sciences of the USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Zhang JJ, Wang L, Jacob TJC. Volume-sensitive chloride current in pigmented ciliary epithelial cells: role of phospholipases. American Journal of Physiology. 1997;272:C212–222. doi: 10.1152/ajpcell.1997.272.1.C212. [DOI] [PubMed] [Google Scholar]
- Morales MP, Gálvez A, Eltit JM, Ocaranza P, Díaz-Araya G, Lavandero S. IGF-1 regulates apoptosis of cardiac myocyte induced by osmotic-stress. Biochemical and Biophysical Research Communications. 2000;270:1029–1035. doi: 10.1006/bbrc.2000.2550. [DOI] [PubMed] [Google Scholar]
- Mounfield PR, Robson L. The role of Ca2+ in volume regulation induced by Na+-coupled alanine uptake in single proximal tubule cells isolated from frog kidney. Journal of Physiology. 1998;510:145–153. doi: 10.1111/j.1469-7793.1998.145bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante L, Zegarra-Moran O, Montaldo PG, Ponzoni M, Galietta LJV. Autocrine regulation of volume-sensitive anion channels in airway epithelial cells by adenosine. Journal of Biological Chemistry. 1999;274:11701–11707. doi: 10.1074/jbc.274.17.11701. [DOI] [PubMed] [Google Scholar]
- Nagasaki M, Ye L, Duan D, Horowitz B, Hume JR. Intracellular cyclic AMP inhibits native and recombinant volume-regulated chloride channels from mammalian heart. Journal of Physiology. 2000;523:705–717. doi: 10.1111/j.1469-7793.2000.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nicholson D, Thornberry NA. Caspases: killer proteases. Trends in Biochemical Sciences. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor α. Journal of Biological Chemistry. 2000;276:20556–20561. doi: 10.1074/jbc.M002535200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Progress in Biophysics and Molecular Biology. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Oike M, Zahradnik I, Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. Journal of General Physiology. 1994a;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Sehrer J, Droogmans G. Permeation properties and modulation of volume-activated Cl--currents in human endothelial cells. British Journal of Pharmacology. 1994b;112:1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. Journal of Physiology. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Walters RJ, Valverde MA, Sepúlveda FV. Regulatory volume increase after hypertonicity- or vasoactive-intestinal-peptide-induced cell-volume decrease in small-intestinal crypts is dependent on Na+-K+-2Cl- cotransport. Pflügers Archiv. 1993;423:67–73. doi: 10.1007/BF00374962. [DOI] [PubMed] [Google Scholar]
- Oiki S, Kubo M, Okada Y. Mg2+ and ATP-dependence of volume-sensitive Cl- channels in human epithelial cells. Japanese Journal of Physiology. 1995;44(suppl 2):S77–S79. [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y. Cell Volume Regulation: The Molecular Mechanism and Volume Sensing Machinery. Amsterdam: Elsevier; 1998a. [Google Scholar]
- Okada Y. Cell volume-sensitive chloride channels. In: Lang F, editor. Cell Volume Regulation. Basel: Karger; 1998b. pp. 21–33. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hazama A, Abdullaev I, Tanaka S, Ando-Akatsuka Y, Shimizu T, Sabirov RZ, Hayashi S, Fan H-T. Cell volume-sensitive Cl- channels and ATP release. In: Suketa Y, Carafoli E, Lazduvski M, Mikoshiba K, Okada Y, Wright EM, editors. Control and Disease of Sodium Dependent Transportation Proteins and Ion Channels. Amsterdam: Elsevier; 2000. pp. 261–264. [Google Scholar]
- Okada Y, Hazama A, Yuan W-L. Stretch-induced activation of Ca2+-permeable ion channels is involved in the volume regulation of hypotonically swollen epithelial cells. Neuroscience Research. 1990;12:S5–13. doi: 10.1016/0921-8696(90)90004-m. [DOI] [PubMed] [Google Scholar]
- Okada Y, Oiki S, Hazama A, Morishima S. Criteria for the molecular identification of the volume-sensitive outwardly rectifying Cl- channel. Journal of General Physiology. 1998;112:365–367. doi: 10.1085/jgp.112.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov SN, Dam T-V, Tremblay J, Hamet P. Apoptosis in vascular smooth muscle cells: role of cell shrinkage. Biochemical and Biophysical Research Communications. 1996;221:708–715. doi: 10.1006/bbrc.1996.0661. [DOI] [PubMed] [Google Scholar]
- Oz MC, Sorota S. Forskolin stimulates swelling-induced chloride current, not cardiac cystic fibrosis transmembrane-conductance regulator current, in human cardiac myocytes. Circulation Research. 1995;76:1063–1070. doi: 10.1161/01.res.76.6.1063. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Maar TE, Morán J. Cell volume regulation in cultured cerebellar granule neurons. Journal of Neuroscience Research. 1993;34:219–224. doi: 10.1002/jnr.490340209. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO Journal. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkoski MJ, Brunner T, Green DR, Lin T. Fas and Fas ligand in gut and liver. American Journal of Physiology. 2000;278:G354–366. doi: 10.1152/ajpgi.2000.278.3.G354. [DOI] [PubMed] [Google Scholar]
- Qin S, Minami Y, Kurosaki T, Yamamura H. Distinctive functions of Syk and Lyn in mediating osmotic stress- and ultraviolet C irradiation-induced apoptosis in chicken B cells. Journal of Biological Chemistry. 1997;272:17994–17999. doi: 10.1074/jbc.272.29.17994. [DOI] [PubMed] [Google Scholar]
- Quinn RH, Pierce SK. The ionic basis of the hypo-osmotic depolarization in neurons from the opisthobranch mollusc Elysia Chlorotica. Journal of Experimental Biology. 1992;163:169–186. doi: 10.1242/jeb.163.1.169. [DOI] [PubMed] [Google Scholar]
- Rasola A, Far DF, Hofman P, Rossi B. Lack of internucleosomal DNA fragmentation is related to Cl- efflux impairment in hematopoietic cell apoptosis. FASEB Journal. 1999;13:1711–1723. doi: 10.1096/fasebj.13.13.1711. [DOI] [PubMed] [Google Scholar]
- Riccardi D, Lee W-S, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. American Journal of Physiology. 1996;271:F951–956. doi: 10.1152/ajprenal.1996.271.4.F951. [DOI] [PubMed] [Google Scholar]
- Robson L, Hunter M. Stimulation of Na+-alanine cotransport activates a voltage-dependent conductance in single proximal tubule cells isolated from frog kidney. Journal of Physiology. 1999;517:193–200. doi: 10.1111/j.1469-7793.1999.0193z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl- secretion in cultured human and rat biliary epithelial cells. American Journal of Physiology. 1999;276:G1391–1400. doi: 10.1152/ajpgi.1999.276.6.G1391. [DOI] [PubMed] [Google Scholar]
- Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz JG. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. Journal of Biological Chemistry. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Bostel S, Dubois JM. Evidence for several mechanisms of volume regulation in neuroblastoma×glioma hybrid. Neuroscience. 1999;88:307–317. doi: 10.1016/s0306-4522(98)00236-x. [DOI] [PubMed] [Google Scholar]
- Rubera I, Tauc M, Poujeol C, Bohn MT, Bidet M, DeRenzis G, Poujeol P. Cl- and K+ conductances activated by cell swelling in primary cultures of rabbit distal bright convoluted tubules. American Journal of Physiology. 1997;273:F680–697. doi: 10.1152/ajprenal.1997.273.5.F680. [DOI] [PubMed] [Google Scholar]
- Sakai H, Nakamura F, Kuno M. Synergetic activation of outwardly rectifying Cl- currents by hypotonic stress and external Ca2+ in murine osteoclasts. Journal of Physiology. 1999;515:157–168. doi: 10.1111/j.1469-7793.1999.157ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottmann KE, Gulbin E, Lau SM, Coggeshall KM. Activation of Src-family tyrosine kinases during Fas-induced apoptosis. Journal of Leukocyte Biology. 1996;60:546–554. doi: 10.1002/jlb.60.4.546. [DOI] [PubMed] [Google Scholar]
- Schrantz N, Blanchard DA, Auffredou M-T, Sharma S, Leca G, Vazquez A. Role of caspases and possible involvement of retinoblastoma protein during TGFβ-mediated apoptosis of human B lymphocytes. Oncogene. 1999;18:3511–3519. doi: 10.1038/sj.onc.1202718. [DOI] [PubMed] [Google Scholar]
- Shen M-R, Chou C-Y, Wu M-L, Huang K-E. Differential osmosensing signalling pathways and G-protein involvement in human cervical cells with different tumor potential. Cellular Signalling. 1998;10:113–120. doi: 10.1016/s0898-6568(97)00115-0. [DOI] [PubMed] [Google Scholar]
- Shen M-R, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. Journal of Physiology. 2000;529:385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M-R, Wu S-N, Chou C-Y. Volume-sensitive chloride channels in the primary culture cells of human cervical carcinoma. Biochimica et Biophysica Acta. 1996;1315:138–144. doi: 10.1016/0925-4439(95)00115-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Morishima S, Okada Y. Ca2+-sensing receptor-mediated regulation of volume-sensitive Cl- channels in human epithelial cells. Journal of Physiology. 2000;528:457–472. doi: 10.1111/j.1469-7793.2000.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba YM, Prevarskaya N, Lemonnier L, VanCoppenolle F, Kostyuk PG, Mauroy B, Skryma R. Volume-regulated chloride conductance in the LNCaP human prostate cancer cell line. American Journal of Physiology. 2000;279:C1144–1154. doi: 10.1152/ajpcell.2000.279.4.C1144. [DOI] [PubMed] [Google Scholar]
- Singleton JR, Dixit VM, Feldman EL. Type I insulin-like growth factor receptor activation regulates apoptotic proteins. Journal of Biological Chemistry. 1996;271:31791–31794. doi: 10.1074/jbc.271.50.31791. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circulation Research. 1992;70:679–687. doi: 10.1161/01.res.70.4.679. [DOI] [PubMed] [Google Scholar]
- Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflügers Archiv. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- Souktani R, Berdeaux A, Ghaleh B, Giudicelli JF, Guize L, LeHeuzey JY, Henry P. Induction of apoptosis using sphingolipids activates a chloride current in Xenopus laevis oocytes. American Journal of Physiology. 2000;279:C158–165. doi: 10.1152/ajpcell.2000.279.1.C158. [DOI] [PubMed] [Google Scholar]
- Strange K. Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln. Journal of General Physiology. 1998;111:617–622. doi: 10.1085/jgp.111.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Szabò S, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, Schlottmann K, Pongs O, Lang F. Tyrosine phosphorylation-dependent suppression of voltage-gated K+ channel in T lymphocytes upon Fas stimulation. Journal of Biological Chemistry. 1996;271:20465–20469. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- Szabò S, Lepple-Wienhues A, Kaba KN, Zoratti M, Gulbins E, Lang F. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proceedings of the National Academy of Sciences of the USA. 1998;95:6169–6174. doi: 10.1073/pnas.95.11.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Wang JYJ. The caspase-RB connection in cell death. Trends in Cell Biology. 1998;8:116–120. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. American Journal of Physiology. 1998;275:C1391–1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Thoroed SM, Brvan-Sisneros A, Doroshenko P. Protein phosphotyrosine phosphatase inhibitors suppress regulatory volume decrease and the volume-sensitive Cl- conductance in mouse fibroblasts. Pflügers Archiv. 1999;438:133–140. doi: 10.1007/s004240050890. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, Tertoolen LGJ, Morii N, Saitoh Y, Narumiya S, deJonge H. Activation of the osmo-sensitive chloride conductance involves p21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Molecular Biology of the Cell. 1996;7:1419–1427. doi: 10.1091/mbc.7.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, vandenBerghe N, Bot AGM, deJonge HR. Ca2+-mobilizing hormones potentiate hypotonicity-induced activation of ionic conductances in Intestine 407 cells. American Journal of Physiology. 1994;267:C1271–1278. doi: 10.1152/ajpcell.1994.267.5.C1271. [DOI] [PubMed] [Google Scholar]
- Tilly BC, vandenBerghe N, Tertoolen LGJ, Edixhoven MJ, deJonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. Journal of Biological Chemistry. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Tosteson DC, Hoffman JF. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. Journal of General Physiology. 1960;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura T, Oiki S, Ueda S, Okuma M, Okada Y. Sensitivity of volume-sensitive Cl- conductance in human epithelial cells to extracellular nucleotides. American Journal of Physiology. 1996;271:C1872–1878. doi: 10.1152/ajpcell.1996.271.6.C1872. [DOI] [PubMed] [Google Scholar]
- Van V, deJonge HR, Tilly BC. Osmotic cell swelling-induced ATP release mediates the activation of extracellular signal-regulated protein kinase (Erk)-1/2 but not the activation of osmo-sensitive anion channels. Biochemical Journal. 1999;343:579–586. doi: 10.1042/0264-6021:3430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. Journal of Physiology. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Wei L, SmetDe P, VanDriessche W, Eggermont J, Droogmans G, Nilius B. Downregulation of volume-activated Cl- currents during muscle differentiation. American Journal of Physiology. 1997;272:C667–674. doi: 10.1152/ajpcell.1997.272.2.C667. [DOI] [PubMed] [Google Scholar]
- Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annual Review of Immunology. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Wang J, Morishima S, Okada Y. A volume-regulatory, Ca2+-activated, intermediate-conductance K+ channel in human epithelial cells. Japanese Journal of Physiology. 2001;51(suppl) (Abstract, in the Press) [Google Scholar]
- Wang L, Xu D, Dai W, Lu L. An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. Journal of Biological Chemistry. 1999;274:3678–3685. doi: 10.1074/jbc.274.6.3678. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proceedings of the National Academy of Sciences of the USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CM, Reynolds JE, Morana SJ, Eastman A. The temporal relationship between protein phosphatase, ICE/CED-3 proteases, intracellular acidification, and DNA fragmentation in apoptosis. Experimental Cell Research. 1997;230:22–27. doi: 10.1006/excr.1996.3401. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: The significance of apoptosis. International Review of Cytology. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Weichselbaum R, Kharbanda S, Kufe D. Role of lyn tyrosine kinase as a regulator of stress-activated protein kinase activity in response to DNA damage. Molecular and Cellular Biology. 2000;20:5370–5380. doi: 10.1128/mcb.20.15.5370-5380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, Yeh C-H, Sensi SL, Gwag BJ, Canzoniero MT, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-α inhibits apoptosis in human endothelial cells. Journal of Biological Chemistry. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]


