Abstract
The condensin complex has a fundamental role in chromosome dynamics. In this study, we report that accumulation of Schizosaccharomyces pombe condensin at mitotic kinetochores and ribosomal DNAs (rDNAs) occurs in multiple steps and is necessary for normal segregation of the sister kinetochores and rDNAs. Nuclear entry of condensin at the onset of mitosis requires Cut15/importin α and Cdc2 phosphorylation. Ark1/aurora and Cut17/Bir1/survivin are needed to dock the condensin at both the kinetochores and rDNAs. Furthermore, proteins that are necessary to form the chromatin architecture of the kinetochores (Mis6, Cnp1, and Mis13) and rDNAs (Nuc1 and Acr1) are required for condensin to accumulate specifically at these sites. Acr1 (accumulation of condensin at rDNA 1) is an rDNA upstream sequence binding protein that physically interacts with Rrn5, Rrn11, Rrn7, and Spp27 and is required for the proper accumulation of Nuc1 at rDNAs. The mechanism of condensin accumulation at the kinetochores may be conserved, as human condensin II fails to accumulate at kinetochores in hMis6 RNA interference–treated cells.
Introduction
Clarifying the mechanism of chromosomal DNA compaction is essential for understanding how the nucleus is made within the eukaryotic cell. Even in the simplest eukaryotic organisms, such as yeast, chromosomal DNA is compacted ∼1,000-fold in the interphase nucleus, which contains decondensed chromosomes. In mitosis, chromosomal DNA is condensed, but the actual compaction is several to 10-fold more than that of interphase chromosomes. The structural integrity and strength of the condensed mitotic chromosomes may be important for making chromosomes able to withstand the pulling force and movement toward the opposite spindle poles. If this is the case, mitotic condensation might not occur only for the compaction process but might also serve to provide the structural integrity and mode of packing appropriate for the sister chromatid separation and chromosome transportation that occur in anaphase. Understanding the higher order structure of mitotic chromosomes is vital for understanding the mechanism of chromosome segregation.
Mitotic condensation occurs along the entire chromosomal DNA structure. Two distinct chromosomal domains are of interest. One is the centromere/kinetochore where kinetochore microtubules associate, and the other is the nucleolus-organizing ribosome RNA–encoding DNA (ribosomal DNA [rDNA]) repeats. During mitosis, several proteins are newly associated with the centromeric chromatin that contains centromere protein A (CENP-A), a histone H3 variant, and form the mitosis-specific higher order structure that is called the kinetochore (Rieder and Salmon, 1998). In mitosis, the nucleolus in higher eukaryotes is degraded and reassembled after chromosome segregation. In fungi, however, the nucleolus does not degrade during mitosis, raising an interesting question regarding the mechanism of nucleolar segregation (Strunnikov, 2005).
In this study, we examine how condensin, which strongly binds to chromosomes upon entry into mitosis, participates in the mitotic structural formation of kinetochores and rDNA repeats. Condensin consists of five subunits, two of which are called the structural maintenance of chromosome (SMC) subunits and are essential for chromosome condensation and segregation in mitosis. In spite of the wealth of information obtained by investigating the condensin complex in budding yeast (Strunnikov et al., 1995; Freeman et al., 2000), fission yeast (Saka et al., 1994; Sutani et al., 1999; Aono et al., 2002), fly (Steffensen et al., 2001), worm (Hagstrom et al., 2002), and vertebrates (Hirano and Mitchison, 1994; Saitoh et al., 1994; Hirano et al., 1997; Ono et al., 2003; Wignall et al., 2003), an understanding of its functions in chromosome condensation and segregation at the molecular level is surprisingly meager.
Results
Condensin is enriched in two distinct domains of the mitotic nucleus
To observe the mitotic localization of condensin in living cells, the GFP-tagged condensin gene Cnd1-GFP integrated into the chromosome was expressed under the native promoter. Cnd1, a non-SMC subunit of condensin (Sutani et al., 1999), is similar to human hCAP-D2 and Saccharomyces cerevisiae Ycs4. The integrated Schizosaccharomyces pombe strain also carried a plasmid that expressed the RFP-tagged centrosome marker Sad1 so that the two colors, green Cnd1 and red Sad1, were visualized in the same cells. Sad1 served as a marker for mitotic progression, as it localized at the centrosome-equivalent spindle pole bodies (SPBs) and also at the nuclear membrane throughout the cell cycle (Hagan and Yanagida, 1995).
Videos of cells that expressed both Cnd1-GFP and Sad1-RFP were made using a DeltaVision microscope. Images of Cnd1-GFP at 2-min intervals are shown in Fig. 1 A (top), and merged images with Sad1-RFP are also shown (Fig. 1 A, bottom). In late G2 cells displaying one SPB, the Cnd1-GFP signals were dispersed in the nucleus and cytoplasm. In mitotic cells having the separated SPBs, however, the signals became intense in nuclei.
Figure 1.
Mitotic nuclear accumulation of condensin and its loss in two mutants. (A) Time-lapse images of the wild-type S. pombe cultured at 36°C. Cells expressed the condensin subunit Cnd1-GFP (green) and the SPB and nuclear envelope protein Sad1-RFP (red). In the inset, the arrowheads indicate the two enriched nuclear domains. The arrow in the merged images shows the condensin in the middle of the two separated daughter nuclei. (bottom) The mitotic nucleus containing Cut14-GFP and stained with the ethidium bromide–DAPI mixture, which revealed the nucleolar domain in the mitotic nucleus. (B and C) Two example cells in metaphase at 1-min intervals are shown that expressed Cnd1-GFP Sad1-RFP (B) or Cut14-GFP Sad1-RFP (C). (D) Time-lapse images for a wild-type cell carrying plasmid REP81-Cut3WT (left) or REP81-Cut3 T19A (right). (E and F) Time-lapse images of mutant cut15-85 that expressed Cut14-GFP (E) or Cnd1-GFP (F) and Sad1-mCherry. (A and D–F) The numbers in the panels indicate time in minutes. Bars: (A, top; and B–F) 10 μm; (A, bottom) 2 μm.
The Cnd1-GFP signals were enriched in the nuclei from prophase to metaphase until telophase, with two intense domains clearly seen until metaphase (Fig. 1 A, arrowheads in the inset at 12 min). The images of two other metaphase nuclei taken at 1-min intervals are shown (Fig. 1 B and Videos 1 and 2, available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1). One of the two intense domains was between the separated SPBs in metaphase, which is similar to the localization of centromere/kinetochore, and the other domain resembled the nucleolus. For comparison, the nucleolar domain in the mitotic nucleus, which was revealed by the double stain of ethidium bromide–DAPI (Umesono et al., 1983), is shown together with the signal of the other condensin subunit Cut14-GFP (hCAP-E/Smc2; see below). The ethidium bromide–enhanced nucleolus was merged with the larger condensin-positive domain (Fig. 1 A, bottom right). Mis12-RFP (a centromere/kinetochore marker similar to budding yeast Mtw1 and human hMis12; Goshima et al., 1999; Aoki et al., 2006) signals were the same as the smaller dotlike signals of mitotic Cnd1-GFP (Fig. 2 A). These distinct domains were always observed in metaphase but were no longer discernible in anaphase. In addition, a subpopulation of condensin remained in the middle of the separated daughter nuclei in late anaphase (Fig. 1 A, arrow) and disappeared in telophase. To determine whether the aforementioned localization dynamics were the same for the other subunit, we made another strain that expressed chromosomally integrated GFP-Cut14 and plasmid-borne Sad1-RFP, both under the native promoter. The localization pattern of Cut14-GFP (Videos 3 and 4) and that of the two intense metaphase domains were the same (Fig. 1 C).
Figure 2.
Cnp1, Mis6, and Mis13 are required for localizing condensin at the kinetochore. (A) Cells cultured at 26°C in EMM2 were observed for the colocalization of Cnd1-GFP with Mis12-RFP, a centromere/kinetochore protein. The numbers in the right panels indicate time in minutes. The enlarged images of Cnd1-GFP (left), Mis12-RFP (middle), and the merged images (right) at 13 min are shown in the insets. (B) Cut14-GFP and Sad1-RFP were observed in the wild-type, mis6-302, cnp1-1, mis13-1, mis16-53, and mis18-262 mutants cultured at 26°C and were shifted to 36°C for 8 h. (C) Chromosomally integrated Cnp1/CENP-A–GFP expressed under the native promoter was observed in the wild-type and cut14-208 mutant cultured at 36°C for 2 h. (D) A ChIP assay was performed using extracts of block-released nda3-311 mutant that expressed Cut14-Flag. The probes were from the central centromere, cnt1 (c10, c9, and c7.5), imr1 (c4 and c1), the outer centromere dg, and the noncentromeric lys1 +. WCE, whole cell extract; IP, immunoprecipitate. Quantitative data are shown at the bottom (see Results for details). (E) A ChIP assay was performed using extracts of block-released cdc25-22 mutant that expressed Cut14-Flag. Extracts of cdc25 cells (blocked in G2 at 36°C and released to 26°C) were prepared at 0 min (G2 phase), 25 min (prometaphase), 50 min (meta/anaphase), and 75 min (telophase or G1/S phase). The probes used were from the central centromere, cnt1 (c9), imr1 (c4), and the three arm probes arm1 (SPBC28F2.08c coding region), arm2 (SPBC29B5 noncoding region), and arm3 (ars2004). In the bottom panel, the percent frequencies of different types of cells are indicated.(F) A ChIP assay was performed using extracts of asynchronous wild-type, mis6-302, and cnp1-1 mutants that expressed Cut14-Flag. These strains were cultured at 26°C and were shifted to 36°C for 8 h. Real-time PCR was performed using the primers of cnt1, imr1, lys1 +, and rDNA (N1). The levels of precipitated cnt1 and imr1 were diminished in cnp1-1 and mis6-302 mutants. Because the asynchronous cultures were used, the differences between wild-type and mutant cells were relatively small. Error bars represent SD. Bars: (A and C) 10 μm; (B) 2 μm.
We examined whether these domains were present in a dominant-negative Cut3-T19A that failed to enter the nucleus during mitosis and produced a phenotype similar to that of a temperature-sensitive (ts) mutant (Sutani et al., 1999). Cut3 is similar to human hCAP-C and S. cerevisiae Smc4. In cells that mildly overexpressed Cut3-T19A (using REP81 plasmid), Cut14-GFP signals did not enter the mitotic nucleus (Fig. 1 D, right). Cut3-T19A was nonphosphorylatable by Cdc2 kinase and could not rescue the ts cut3 phenotype (Sutani et al., 1999). Wild-type overproduced Cut3 formed a normal complex with Cut14-GFP that was enriched in the two nuclear domains in metaphase (Fig. 1 D, left).
In another mutant in importin α (cut15-85), chromosomes did not condense, revealing a cut phenotype resembling the condensin mutant (Matsusaka et al., 1998). No signal for Cut14-GFP was observed in the mitotic nuclei (Fig. 1 E), indicating that the lack of condensation was caused by the absence of condensin in the mitotic nuclei. In the same mutant, the non-SMC subunit Cnd1-GFP was also not enriched in any domain of the mitotic nuclei (Fig. 1 F). The previous results that Cut3 was abundant in the mitotic nuclei of cut15-85 (Matsusaka et al., 1998) are likely caused by nonspecific binding of the polyclonal antibodies against a contaminating antigen.
The other domain is overlapped with the kinetochores
To examine whether the other enriched domain was a centromere/kinetochore, we constructed double-labeled cells using the plasmid-borne Mis12-RFP gene under the native promoter. Live images showed that the Mis12-RFP dotlike images overlapped with the dotlike portions of condensin subunit Cnd1-GFP in metaphase (Fig. 2 A). In the enlarged frame (Fig. 2 A, 13 min), the kinetochore signals of Mis12-RFP completely overlapped with the three dot signals of Cnd1-GFP.
Condensin's kinetochore localization requires Cnp1, Mis6, and Mis13
We examined whether the kinetochore localization of condensin depended on the presence of certain kinetochore proteins. To this end, a strain that expressed Cut14-GFP and Sad1-RFP was crossed with mis6-302, cnp1-1, mis13-1, mis16-53, and mis18-262, and the resulting strains were cultured. Cnp1 is centromeric histone H3, similar to vertebrate CENP-A and S. cerevisiae Cse4, whereas Mis6 is needed for recruiting Cnp1 and is similar to vertebrate CENP-I. Mis13 (S. cerevisiae Dsn1 and human hMis13; Obuse et al., 2004; Kiyomitsu et al., 2007) is the subunit of the Mis12 complex. Mis16 and Mis18 are required for chromatin priming of the CENP-A loading and correspond to human RbAp46/48 and hMis18, respectively (Hayashi et al., 2004; Fujita et al., 2007). In Fig. 2 B, the kinetochore signals of condensin were diminished in the mis6-302, cnp1-1, and mis13-1 backgrounds at 36°C, whereas the signals of the other rDNA domain remained intense. In the wild type, mis16-53, and mis18-262, however, both domains were enriched by Cut14-GFP. Condensin accumulation at the kinetochores thus required Cnp1, Mis6, and Mis13 (central centromeric proteins throughout the cell cycle) but not Mis16 and Mis18 (not present at the kinetochore during mitosis; Fujita et al., 2007).
In the reverse experiment, mitotic kinetochore localization of Cnp1-GFP was observed in cut14-208 (Fig. 2 C), but missegregation occurred, suggesting that condensin did not affect the recruitment of Cnp1. This is quite different from the case of budding yeast (Yong-Gonzalez et al., 2007), in which condensin is required for Cse4 localization at the centromere.
Mitotic condensin is bound to the central centromeric chromatin
The S. pombe centromere consists of the central core (cnt and imr) and the outer repetitive region (otr; Takahashi et al., 1992). Chromatin immunoprecipitation (ChIP) was performed to determine the centromeric region to which condensin was bound in metaphase, anaphase, and G1/S phase. We constructed a strain that expressed chromosomally integrated Cut14-Flag under the native promoter and then crossed the resulting strain with a cold-sensitive nda3-311 that was reversibly defective in β tubulin (Hiraoka et al., 1984). Cells of nda3-311 cultured at 20°C for 8 h failed to produce the spindle, resulting in mitotic arrest. Then, by releasing the cells to 36°C, the spindle was rapidly assembled, and, 3 min later, the cells were in metaphase. After 15 min and 30 min, the cells were in anaphase and G1/S phase, respectively (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1). Five probes (c10, c9, c7.5, c4, and c1) from the central cnt and imr and one (dg) from the outer region were used (Table S1). Pericentromeric lys1 + was also used. The central region is associated with Cnp1, Mis6, and Bub1 (Saitoh et al., 1997).
Cut14-Flag (+) was coprecipitated with the central probes (but not with the outer dg or lys1 +) in metaphase and anaphase but was greatly diminished in G1/S phase (Fig. 2 D). The control that did not express Cut14-Flag (2) showed no coprecipitated DNA. Condensin accumulation in the central centromere peaked in metaphase. Basically identical results were obtained in the cdc25 mutant block-release experiment (Fig. 2 E). At the timing of anaphase, ∼50 min after the temperature release, the maximal association of Cut14-Flag at the centromere occurred. It is interesting to note that association of condensin with the arm regions was negligible in prometaphase but increased from anaphase to telophase.
We then tested whether the levels of kinetochore DNA in ChIP were altered using the asynchronous cultures of wild type, mis6-302, and cnp1-1 that were chromosomally integrated with Cut14-Flag. As shown in Fig. 2 F, condensin enrichment at cnt1 and imr1 was reduced in mis6-302 and cnp1-1 but not in wild type. At lys1+, such reduction was not observed. In the rDNA N1 region, the ChIP signals increased, suggesting that Cut14-GFP unbound to the kinetochore in mis6 and cnp1 mutants might associate with the rDNA.
Kinetochore accumulation of human condensin II requires hMis6
In human cells, there are two condensin complexes, I and II, and II is enriched in the kinetochores during mitosis (Ono et al., 2003, 2004). We evaluated whether kinetochore localization of condensin II is dependent on the presence of hMis6/CENP-I using CENP-A as the centromere/kinetochore marker (Fig. 3). The RNAi method was applied to hMis6 as described previously (Goshima et al., 2003), and the localization of hCAP-H2, a component of condensin II, was determined using the metaphase chromosome spread method (Ono et al., 2003). Fig. 3 A (right) shows three chromosomes that are derived from those framed in the left panel (bottom right). Kinetochore localization of hCAP-H2 was abolished by hMis6 RNAi, whereas the arm localization of hCAP-H2 remained. The control RNAi (Fig. 3 B) revealed that the kinetochore localization of hCAP-H2 overlapped with CENP-A (Ono et al., 2004). The percent frequencies of chromosomes that showed the hCAP-H2 signals on kinetochores were quantified (Fig. 3 C). The kinetochore signals of hMis6 were diminished by RNAi as reported previously (Fig. 3 D; Goshima et al., 2003).
Figure 3.
RNAi of hMis6 diminishes condensin II at human kinetochores. (A and B) Kinetochore localization of hCAP-H2 on the spread metaphase chromosomes is diminished in cells after RNAi knockdown. Metaphase spread chromosomes were prepared in HeLa-transfected cells for 48 h by siRNA of hMis6 (A) or luciferase (B; control). Cells were stained by Hoechst33342 for DNA (blue), anti–CENP-A antibody for the centromere/kinetochore (red), and anti–hCAP-H2 for the condensin (green) antibodies. The enlarged images correspond to the dashed boxes in the left panel. (C) The percent frequencies of metaphase chromosomes showing the hCAP-H2 signals on kinetochores; the control was 98.2% (n = 112), whereas depleted hMis6 was only 18.3% (n = 131). (D) HeLa transfected by siRNA of luciferase or hMis6 were fixed for 48 h and stained by Hoechst33342 (blue), anti-hMis6 (red), and anti–hCAP-H2 (green) antibodies. Bars, 10 μm.
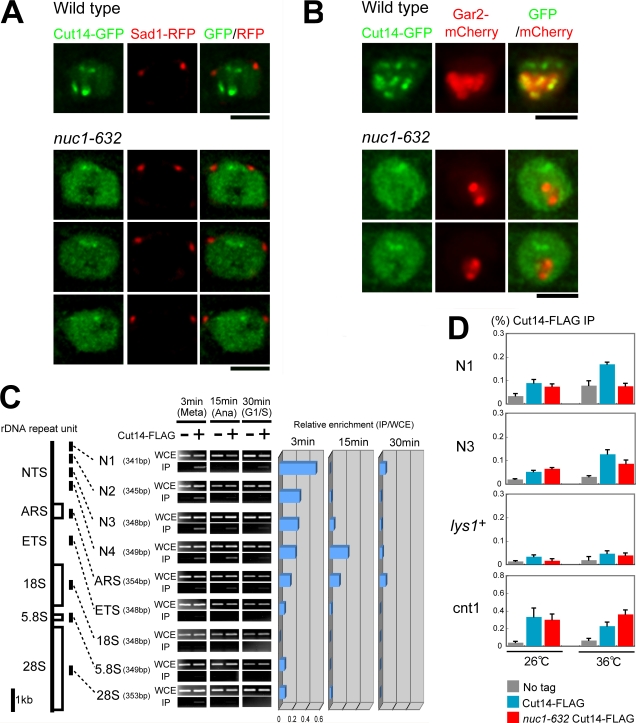
Condensin accumulation at rDNAs requires Nuc1
We hypothesized that condensin was enriched along the rDNAs, as the cytological behavior of the second domain highly resembled that of the nucleolus. Thus, we examined whether condensin was normally enriched during mitosis in nuc1-632, a ts mutant defective in the large subunit of RNA polymerase I. Nuc1 affects the higher order structure of rDNA repeats, and its inactivation causes the nucleolar collapse (Hirano et al., 1989). We constructed a nuc1-632 mutant that expressed Cut14-GFP. As shown in Fig. 4 A, the Cut14-GFP signals in the kinetochore were not affected, but those at the presumed rDNAs were greatly diminished in nuc1-632 cultured at 36°C for 6 h. There was a tendency that the nuclear signals of condensin in nuc1 mutant were diffused in the nucleoplasm. We then used the nucleolar marker of Gar2 (required for ribosome RNA processing; Gulli et al., 1995)–monomeric Cherry (mCherry) and observed it together with Cut14-GFP in the mitotic nuc1 mutant (Fig. 4 B). The part of condensin signals that overlapped with Gar2-mCherry disappeared in nuc1-632.
Figure 4.
Nuc1 is required for localizing condensin at rDNAs. (A) Localization of Cut14-GFP was examined in the nuc1-632 mutant cultured at 36°C for 6 h. (B) Localization of Cut14-GFP was examined in the nuc1-632 mutant with the nucleolar protein Gar2-mCherry. (C) A ChIP assay was performed using a block-release nda3-311 mutant that expressed Cut14-Flag. Nine DNA probes derived from the rDNA repeat unit were used: NTS (N1–N4), ARS, external transcribed sequence, and 18S, 5.8S, and 28S coding sequences. WCE, whole cell extract; IP, immunoprecipitate. The negative control (−) is the strain that did not carry Cut14-Flag. Quantitative enrichment is shown on the right. (D) A ChIP assay was performed using extracts of asynchronous wild-type and nuc1-632 mutant that expressed Cut14-Flag. The wild-type and nuc1-632 mutant were cultured at 26°C and shifted to 36°C for 6 h, and immunoprecipitation was performed. Real-time PCR was used to amplify and quantify DNAs with the PCR primers (N1, N3, lys1 +, and cnt1). Error bars represent SD. Bars, 2 μm.
Association of condensin with rDNAs peaks in metaphase
To determine whether condensin physically interacts with rDNAs, ChIP was performed using the block-release experiment of nda3-311 mutant that expressed Cut14-Flag and the nine probes in the rDNA unit (Fig. 4 C): four nontranscribing sequences (NTSs; N1, N2, N3, and N4), autonomously replicating sequence (ARS), external transcribed sequence, and 18S, 5.8S, and 28S rRNA coding sequences (Table S1). The binding of condensin to NTS and ARS was intense in metaphase but diminished in G1/S phase. There was no association of condensin with the transcribed regions throughout mitosis.
We then performed the Cdc25 block-release experiment followed by the ChIP assay using extracts of cdc25 mutant at 0 and 50 min (the period from metaphase to anaphase) after the temperature shift. Condensin was bound to the rDNA noncoding N1 region at 50 min but not to the coding 28S (Fig. S1 B). We then examined whether the degree of condensin association with rDNAs was diminished in the asynchronous nuc1-632 mutant culture by ChIP experiment. The binding of Cut14-Flag to NTS (N1 and N3) was reduced in the nuc1 mutant at restrictive temperature (Fig. 4 D).
Condensin accumulation at rDNAs requires Acr1 (Spbc17D1.04)
We isolated a ts mutant from a collection of ∼1,000 randomly mutagenized strains (Hayashi et al., 2004) through the search of strains that exhibited the phenotype similar to that of nuc1-632. One strain, 936, was defective in condensin accumulation at rDNAs (Fig. 5 A). Plasmids carrying Spbc17D1.04 (GenBank/EMBL/DDBJ accession no. CAA20428.1) in chromosome II completely rescued the ts phenotype. Tetrad dissection of the cross between 936 and mad2Δ deletion indicated that they were tightly linked (1.2 cM). The mad2 + gene is only 38 kb apart from Spbc17D1.04, so the 936 strain was the most likely mutant in the Spbc17D1.04 gene that encoded a 29-kD protein classified as a sequence orphan in the Sanger Center S. pombe database. The Cys58 residue was changed to Tyr58 in Spbc17D1.04. We hereafter refer to Spbc17D1.04 as Acr1 (accumulation of condensin at rDNA 1). Gene disruption showed that the acr1 + gene was essential for viability (unpublished data). A high-copy suppressor encoding Tbp1/Spac29E6.08 was isolated (unpublished data). Tbp1 is the TATA-binding transcription initiation factor (Mitsuzawa et al., 2001).
Figure 5.
Enrichment of condensin at rDNAs requires a nucleolar protein, Acr1, which interacts with the rDNA upstream activator complex. (A) Cut14-GFP was observed in mitotic cells of the acr1-936 mutant and the control wild type cultured at 36°C for 4 h. (B) A ChIP assay was performed using extracts of asynchronous wild-type and acr1-936 mutant that expressed Cut14-Flag. The wild-type and acr1-936 mutant were cultured at 26°C and shifted to 36°C for 4 h, and immunoprecipitation was performed. Real-time PCR was used to amplify and quantify DNAs with the PCR primers (N1, N2, N3, lys1 +, and cnt1). Error bars represent SD. (C) Localization of Acr1 was examined in the strain that expressed Acr1-GFP. (D) A ChIP assay was performed using the strain that expressed Acr1-Flag. Two rDNA probes (N2 and 18S) and four control probes (cnt1, imr1, dg, and lys1 +) were used. (E, top) Preparation of proteins coprecipitated with Acr1-Flag and the Flag-only control for liquid chromatography–tandem mass spectrometry. The positions of identified protein names are indicated. The two bands were obtained for Acr1-Flag (Fig. S2 D, available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1). (bottom) Four proteins (Rrn5, Rrn11, Rrn7, and Spp27) were coprecipitated with Acr1-Flag. emPAI, exponentially modified protein abundance index (Ishihama et al., 2005). Bars: (A) 2 μm; (C) 10 μm.
To observe the localization of condensin, Cut14-GFP and Sad1-RFP were expressed in acr1-936, which was cultured at 36°C for 4 h. The Cut14-GFP signals were normal for kinetochore localization but were diminished in the nucleolar localization in mitotic cells (Fig. 5 A), like in nuc1. Thus, Acr1 was required for the nucleolar localization of condensin but not for the kinetochores. ChIP was performed to examine whether condensin was associated with noncoding N1, N2, and N3 in wild-type and acr1-936 mutant. Results (Fig. 5 B) show that the association with N1, N2, and N3 took place in wild type but was diminished in acr1-936. Association with the lys1 + gene was not diminished, whereas association with centromeric DNA increased in acr1-936. A possible reason for this is that the unbound Cut14-Flag to rDNA in acr1 mutant might associate with the centromeric cnt1 DNA.
Acr1-GFP, which was constructed and chromosomally integrated under the native promoter, showed nucleolar localization throughout the cell cycle (Fig. 5 C). A ChIP assay was performed using chromosomally integrated Flag-tagged Acr1 under the native promoter. Probes of the noncoding N2 and the coding 18S intensely coprecipitated with Acr1-Flag, but the control probes did not (Fig. 5 D), suggesting that Acr1 was bound to both transcribed and nontranscribed regions of rDNA.
Acr1 associates with the upstream activation factor complex
To identify Acr1-interacting proteins, we performed mass spectrometry using the Flag-tagged Acr1 strain. Proteins precipitated with anti-Flag antibodies were subjected to mass spectroscopic analysis (Obuse et al., 2004). Four proteins (Rrn5, Rrn11, Rrn7, and Spp27) coprecipitated with Acr1 (Fig. 5 E). To confirm that Acr1 is bound to Rrn5, a strain containing Myc-tagged Rrn5 and Acr1-Flag was made and used for immunoprecipitation. Results (Fig. S2 D, available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1) established that Acr1-Flag is stably bound to Rrn5-Myc. Three of the Acr1-coprecipitated proteins (Rrn5, Rrn11, and Rrn7) form the RNA polymerase I core transcription factor (Liu et al., 2002). In budding yeast, similar ones were identified as the upstream binding factor of rDNA (upstream activation factor; Keys et al., 1996). Spp27 is similar to human SMARCD1 (identity of 45%) and budding yeast Uaf30 (identity of 34%), both of which are implicated in transcriptional regulation (Siddiqi et al., 2001). Careful homology search indicated that Acr1 was weakly similar to S. cerevisiae RRN9, a component of the upstream activation factor complex (Fig. S2, E and F). Thus, Acr1 may not be an orphan. The Rrn9-like proteins contained two conserved domains (Fig. S2, E and F).
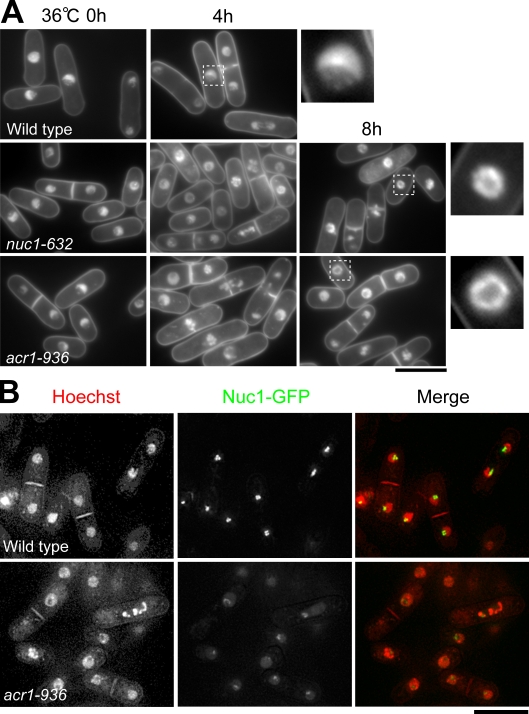
The phenotypes of acr1-936
Both mitotic and interphase phenotypes are seen in acr1-936 (Fig. 6 and Fig. S2, A–C). When mutant cells cultured at 26°C were shifted to 36°C, the cell number increase decayed around 8 h, and cell viability decreased 4–6 h after the shift (Fig. S2 A, left). Condensed chromosomes (∼10%) and lagging chromosomes (∼5%) were mixed with interphase phenotypes that displayed the ringlike nuclear chromatin shape (∼40%; Fig. 6 A, insets). These phenotypes were similar to those in nuc1-632. The frequencies of condensed and lagging chromosomes peaked around 8 h at 36°C (Fig. S2 A, right). Another prominent phenotype was the absence of a long extended spindle (Fig. S2, B and C). The maximum spindle length was 3–5 μm, which is in sharp contrast to the 3–11-μm anaphase spindle in wild-type mitosis.
Figure 6.
acr1-936 had both mitotic and interphase defects. (A) DAPI-stained wild-type, nuc1-632, and acr1-936 cells that were cultured at 36°C for 4–8 h. The insets correspond to the white dashed boxes in the left panels. (B) Localization of Nuc1 was dispersed in the nucleus in the acr1-936 mutant. Bars, 10 μm.
We tested the hypothesis that Nuc1 localization requires Acr1 by observing Nuc1-GFP in acr1-936. The Nuc1-GFP signals were dispersed in the nucleus and were greatly diminished in the nucleolus (Fig. 6 B). Thus, Acr1 is needed for recruiting Nuc1.
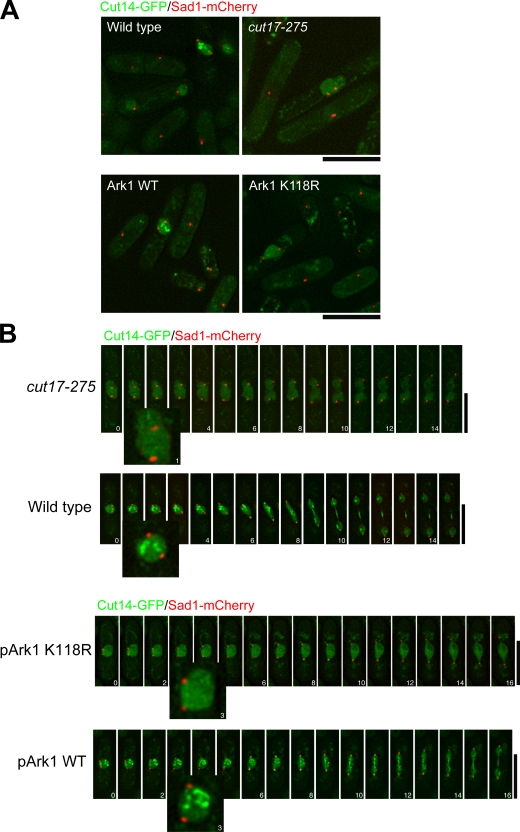
Cut17/Bir1/survivin and Ark1/aurora are needed for condensin's accumulation at kinetochores and rDNAs
The aforementioned results suggested that condensin accumulation at the two distinct nuclear domains might be independent, requiring different gene functions. Therefore, we examined whether there was a mutant defective in condensin accumulation at both domains after condensin entered the nucleus. The cut17/bir1/survivin mutant was a candidate because it is defective in chromosome condensation and segregation (Morishita et al., 2001). Indeed, condensin did not accumulate at either the kinetochores or rDNAs in cut17-275 cultured at 36°C for 3 h, but it did enter the mitotic nucleus (Fig. 7 A, top). In mitosis, intense signals were seen in distinct domains in the wild-type nucleus but were diffused in the cut17 nucleus. The signals were scarcely observed in the interphase nuclei (showing one Sad1-mCherry signal). This was consistent with the previous finding that Cut3 failed to accumulate in the mitotic nucleus by expression of the aurora kinase-dead Ark1-K118R (Petersen and Hagan, 2003).
Figure 7.
Condensin entered the nucleus but failed to accumulate at the two distinct domains in the cut17 mutant and the dominant-negative Ark1/aurora cells. (A, top) Wild-type and cut17-275 mutant cells that expressed Cut14-GFP and Sad1-mCherry were cultured at 26°C, shifted to 36°C for 3 h, and observed. (bottom) The wild-type cells carrying the inducible plasmid REP1-Ark1WT or REP1-Ark1-K118R were cultured in the absence of thiamine at 26°C. (B) Time-lapse series of the Cut14-GFP and Sad1-mCherry images in the wild-type or cut17-275 cells cultured at 36°C for 3 h (top) and in the cells expressing wild-type Ark1 or the dominant-negative Ark1 K118R are shown. Bars, 10 μm.
To further study the possible role of Ark1, we constructed a strain that expressed Ark1-K118R (Fig. 7 A, bottom). Overproduced Ark1-K118R caused the strong dominant-negative effect in chromosome segregation. Nuclear signals of condensin Cut14-GFP in cells that expressed Ark1-K118R were diffused as in cut17 cells and were hardly observed in interphase. Mitotic enrichment of Cut14-GFP at the kinetochore and rDNA was not observed at all in Ark1-K118R–overproducing cells.
We then took videos of Cut14-GFP during mitosis in the cut17 mutant at 36°C or in cells overproducing Ark1-K118R at 26°C (Fig. 7 B). Enriched localization of Cut14-GFP at the centromere and rDNA was not seen during the entire mitotic stage. Localization of Cnp1, Mis6, Nuc1, and Acr1 was not affected at all in cut17 mutant cells (Fig. S3, A–D; available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1), suggesting that the influence of Cut17 on the localization of condensin was not through the location of these kinetochore and rDNA-binding proteins. Rather, Ark1/aurora might directly phosphorylate condensin for its recruitment.
Unequal segregation of sister kinetochores in a condensin mutant
The role of kinetochore- and rDNA-enriched condensin in chromosome segregation was investigated. Sister kinetochore separation and segregation have not been examined in S. pombe condensin mutants. Centromere-binding protein mutants invariably have unequal chromosome segregation (Nabeshima et al., 1998; Hayashi et al., 2004). Whereas the bulk of chromosome DNAs are not separated in condensin mutants, a portion of chromosomal DNAs, presumably centromeric, are separated by the spindle (Saka et al., 1994).
The Cen2-GFP strain was used as the centromere marker (Yamamoto and Hiraoka, 2003). Three mutants (cut14-208, mis6-302, and mis12-537) and the wild type were cultured at 36°C. Hoechst33342 (red) and Cen2-GFP (green) showed nuclear DNA and Cen2 DNA, respectively (Fig. 8 A). In the wild-type cells (n = 103), Cen2-GFP was equally segregated (1:1). In contrast, in the two mutants (mis6-302, n = 108; mis12-537, n = 109), there was unequal segregation of Cen2-GFP (2:0) in 66.7% and 18.3% of cells, respectively. DNA staining displayed the large and small daughter nuclei frequently observed in these mutants (Hayashi et al., 2004). In cut14-208 (n = 109), Cen2-GFP was segregated in a 1:1 ratio in 88.8% of the cells (Fig. 8 A, left) and in a 2:0 ratio in 11.2% of cells (Fig. 8 A, right). The fidelity of sister kinetochore segregation was reduced in cut14-208, as in mis12-537. Thus, the condensin defect decreased the fidelity of sister kinetochore segregation, whereas the bulk of the chromosomal DNA remained associated.
Figure 8.
Proper segregation of sister kinetochores and rDNAs requires condensin. (A) Cen2-GFP was used to examine the segregation phenotype of centromeric DNA in the condensin mutant cut14-208. The wild type, mis6-302, and mis12-537 were used as the control strains. These were first grown at 26°C and were shifted to 36°C for 2 h (wild type and cut14-208) or 8 h (mis6-302 and mis12-537). The frequency of equal or unequal segregation is shown at the bottom of the images. (B) Kymographs for Cen2-GFP are shown for the wild type and cut14-208, which also expressed Sad1-RFP for the SPB marker. Images were taken at 10-s intervals. The frequency of each phenotype is indicated in parentheses. The arrow in the bottom panel (cell 2) shows the two closely situated splits of Cen2-GFP signals upon the presumed onset of anaphase. (C) The rDNA repeat unit was used for the FISH probe in the wild-type and condensin mutant cells. DAPI and anti-Sad1 antibodies were used to stain DNA and to immunostain the SPBs, respectively. (D) Steps required for condensin to be accumulated at mitotic kinetochores and rDNAs in S. pombe. Cut15, importin α; Cut17, Bir1/survivin; Ark1, aurora; Cnp1, CENP-A; Mis6, CENP-I; Mis13, hMis13; Nuc1, pol I largest subunit; Acr1, accumulation of condensin at rDNA. Bars: (A) 5 μm; (B), 2 μm; (C) 10 μm.
To monitor the dynamics of sister kinetochores, kymographs of Cen2-GFP (green) were made for the wild-type and cut14-208 mutant at 36°C using Sad1-RFP (red) as the centrosome marker. The time course for normal sister kinetochore segregation was the same in two types of wild-type cells (Fig. 8 B). The splitting of sister kinetochores before the onset of anaphase was clearly observed in all of the wild-type cells. In contrast, such sister kinetochore splitting was often not observed before the onset of anaphase in 11 videos taken of cut14-208 (Fig. 8 B, bottom; cell 1). Of the 11 videos, 9 showed a 1:1 sister kinetochore separation in later mitosis. Of these nine, four did not show splitting in the metaphase. The remaining 2 of the 11 videos showed cells with a 2:0 ratio that did not exhibit sister kinetochore separation later in mitosis and also did not show splitting before the onset of anaphase, but two closely situated splits occurred near one of the SPBs upon the presumed onset of anaphase (Fig. 8 B, bottom; cell 2; arrow). Similar phenotypes were reported in the centromere mutants mis6 and mis12 (Goshima et al., 1999; Appelgren et al., 2003). The lack of nonsplitting in metaphase suggested that the normal bioriented sister kinetochores under tension did not form in the condensin mutant.
Condensin is required for the segregation of rDNAs
We then examined rDNAs in the condensin mutant at 36°C using the full-length 10.9-kb rDNA repeat unit as the FISH probe (Uzawa and Yanagida, 1992). DAPI and anti-Sad1 antibodies were used to stain the DNA and SPBs in fixed cells, respectively. The wild-type cells had normal separation (Fig. 8 C, top; third row) and segregation (Fig. 8 C, top; fourth row). In the cut14 condensin mutant at 36°C for 2 h, however, the rDNA FISH signals remained in the middle without any indication of segregation, whereas chromosomal DNA (DAPI) was partly extended by anaphase spindle elongation (Fig. 8 C, bottom; third and fourth rows). The nucleolar rDNA required condensin for proper separation in anaphase.
Discussion
The findings of this study indicate that S. pombe condensin is enriched at kinetochores and rDNAs in metaphase. ChIP experiments suggested that the accumulation was caused by the binding of condensin to the central centromeric chromatin and the noncoding region of rDNAs. We identified several essential proteins for recruiting and accumulating condensin at the kinetochores and rDNAs. The interaction of condensin with rDNAs or kinetochores was previously reported in various organisms (Freeman et al., 2000; Cabello et al., 2001; Aono et al., 2002; Bhalla et al., 2002; Hagstrom et al., 2002; Ono et al., 2003, 2004; Jager et al., 2005; Oliveira et al., 2005), but, to our knowledge, the specific enrichment factors for condensin are not well known.
As S. pombe performs closed mitosis, condensin must be moved into the nucleus upon entry into mitosis. We confirmed that Cdc2-dependent phosphorylation of a condensin subunit, Cut3/Smc4, at the T19 residue (Sutani et al., 1999) is essential for entry into the mitotic nucleus. In addition, Cut15, one of the two importin α proteins in S. pombe (Matsusaka et al., 1998; Umeda et al., 2005), is needed for condensin to be transported into the nucleus, probably through the importin role of Cut15. In contrast, Cut17/Bir1/survivin (Morishita et al., 2001) is required to recruit the condensin to the docking sites of both kinetochores and rDNAs. Consistently, overexpression of dominant-negative Ark1-K118R allowed the localization of Cut14-GFP in the nucleus but abolished the enriched localization of Cut14-GFP at the kinetochores and rDNAs. Therefore, we speculate that Cdc2 and Cut15/importin α are required for the nuclear localization of condensin, whereas Cut17/Bir1/survivin and Ark1/aurora are needed for mitotically enriched localization to the chromosomal docking sites. The similar phenotypes of the cut15 and cut17 mutants and dominant-negative Cut3-T19A and Ark1-K118R suggest that condensin recruitment is essential for proper condensation and segregation (Fig. 8 D). Using kymographs of Cen2-GFP and the FISH method, we could show that sister kinetochores fail to split in metaphase and that rDNAs do not segregate at all in a condensin mutant, respectively.
Mutant analyses show that the centromere/kinetochore proteins Cnp1, Mis6, and Mis13 are required for the accumulation of condensin at the kinetochores. In their mutants, condensin accumulated at the rDNAs but not at the kinetochores, suggesting that they are kinetochore-specific enrichment factors for condensin. We examined whether the same is true in human cells using hMis6/CENP-I RNAi. Kinetochore localization of condensin II is greatly diminished in hMis6 RNAi, suggesting that Mis6, which was originally identified as a recruitment factor of Cnp1/CENP-A (Takahashi et al., 2000) and was recently reported to be needed for the recruitment of newly synthesized CENP-A in vertebrate cells (Okada et al., 2006), is required for the accumulation of condensin at the kinetochores in both S. pombe and human cells.
However, our results are against a simple hypothesis that Cnp1 is needed and is sufficient for recruitment of condensin. Condensin recruitment was abolished in the mis13 mutant, which is independent of the Cnp1/CENP-A pathway (Hayashi et al., 2004). In mis16 and mis18 mutants, which are defective in priming centromeric chromatin to recruit Cnp1/CENP-A (Hayashi et al., 2004; Fujita et al., 2007), condensin was located at the centromere/kinetochore. In mis16 and mis18 mutant cells, newly synthesized Cnp1 may not be recruited, but preexisting Cnp1 remains at the centromere/kinetochore. Furthermore, Mis16/RbAp46 and Mis18 were absent in the mitotic kinetochore of S. pombe and human cells (Fujita et al., 2007). We suppose that mitotic condensin requires several centromere/kinetochore proteins to be properly recruited. The kinetochore docking site for mitotic condensin is not identified, but it may be a mitotically modified molecule or mitosis-specific chromosome DNA topology at the kinetochore. The formation of such a site requires multiple centromeric/kinetochore proteins, including Cnp1/CENP-A, Mis6, and Mis13. Understanding the nature of the docking site definitively requires further investigation.
In the nuc1 and acr1/rrn9 mutants, condensin is enriched at the kinetochores but not at the rDNAs: ts nuc1 mutant cells had a collapsed nucleolus, showing a ringlike nuclear chromatin structure (Hirano et al., 1989). We show that Acr1 is a nucleolar protein that binds to subunits of the rDNA upstream transcription activator complex and shares two conserved domains with Rrn9-like proteins in fungi. The phenotypes of acr1/rrn9 at 36°C were mixed with the mitotic defects in the absence of full spindle extension and the collapsed nucleolus. Nuc1 localization at rDNAs was diminished in the acr1 mutant so that Nuc1 and Acr1 seemed to share a common function. We do not consider these proteins to be recruitment factors for condensin but regard them as the chromatin architectural proteins that are required for the formation of the docking site at rDNA for condensin. It remains to be determined whether the nature of the docking sites at rDNAs and the kinetochore share something in common.
The levels of kinetochore- and rDNA-bound condensin were highest in metaphase, decreased in anaphase, and negligible in G1/S phase. Because the intracellular levels of condensin were not altered during the cell cycle (Sutani et al., 1999), the decrease should represent the dissociation of condensin from the docking sites. We hypothesize that the association-dissociation cycle of condensin is regulated by two mitotically activated kinases, Cdc2 and aurora/Ark1 (Cut17/Bir1 is the subunit of the holoenzyme). Although Cdc2 directly phosphorylates condensin, it remains to be determined whether aurora directly regulates condensin. The involvement of aurora for the mobilization of condensin to the mitotic chromosome was previously reported in fly and yeast (Giet and Glover, 2001; Morishita et al., 2001; Petersen and Hagan, 2003).
In vertebrate cells, aurora B is required for the kinetochore localization of condensin II (Ono et al., 2004). This is consistent with the finding that S. pombe condensin's recruitment to the centromere/kinetochore requires Cut17/Bir1 and Ark1 but leaves a question about the role of condensin accumulation for rDNA segregation. In vertebrate cells, condensin's enrichment at rDNAs and the implication of aurora in rDNA segregation are unclear. In S. cerevisiae, however, Ipl1/aurora is required for the condensin enrichment in and the segregation of rDNAs (Lavoie et al., 2004; Sullivan et al., 2004). In fungi, condensin and aurora kinase may be critical for mitotic segregation of the nucleolus, which remains as the bulky intranuclear structure during anaphase. In vertebrates, the nucleolus is degraded and disappears during mitosis and may not require the massive accumulation of condensin at rDNAs in mitosis.
A possibility is that aurora directly affects the association of condensin with mitotic chromosomes. In S. pombe, Cnp1, Mis6, Nuc1, and Acr1 were normally localized in cut17-275 mutants, suggesting that the requirement for Ark1/aurora and Cut17/survivin in condensin's recruitment to kinetochores and rDNAs is not via the direct interactions with these proteins. Ark1/aurora may confer the ability to interact with the mitotic kinetochore and rDNA chromatin on the condensin complex by direct phosphorylation.
The kymograph result suggested that there was a defect in the kinetochore–microtubule interaction in the condensin mutant, as the nonsplitting kinetochore was stuck to one spindle pole during the period of metaphase; the sister kinetochores did not appear to be under the normal tension in metaphase. Kinetochore microtubules might require the assistance of condensin to be properly attached to the kinetochore. Alternatively, bioriented sister kinetochore formation might be defective in the absence of condensin. Wignall et al. (2003) showed that depletion of condensin in frog extracts inhibited microtubule growth and organization around chromosomes, reducing the percentage of sperm nuclei capable of forming spindles and causing dramatic defects in anaphase chromosome segregation. Similar findings were made using RNAi of human cells (Ono et al., 2004). The kinetochore-driven spindle assembly seems to become defective if condensin is depleted. However, we observed no extensive defect in the S. pombe spindle formation in condensin mutant cells within the resolution of light microscopy. In S. pombe, the spindle may form in the absence of proper interaction between the kinetochore and the kinetochore microtubule. Alternatively, the phenotypic difference might be caused by variations in inactivating condensin (ts mutation versus depletion).
How, then, does mitotic condensin accumulate at the kinetochores and rDNAs? One hypothesis is that kinetochores and rDNAs contain many docking sites, although their nature is unknown. To examine this issue, it is important to determine the abundance of condensin at these sites in comparison with other chromosomal regions. One estimate for the number of condensin complexes per cell is ∼3,000 in the postreplicative state (Sutani et al., 1999). We reestimated this number and obtained basically the same number (unpublished data). Therefore, each condensin localizes with the mean ∼8-kb intervals when condensin is maximally bound to the chromosomes (the postreplicative cell contains 24 Mbp). Our estimate based on ChIP is that approximately three condensins may be present in the 3-kb noncoding rDNA upstream in metaphase, as only three primers in 1-kb intervals in the noncoding region could precipitate condensin. There are ∼150 rDNA repeats, so ∼900 condensins may be bound to rDNAs in the postreplicative mitotic cells (3 × 150 × 2). We presumed that each kinetochore (central centromere) DNA contained ∼30 or more condensin, as all of the ChIP primers constructed every 0.5-kb interval in the 15-kb-long central centromere region could precipitate condensin. As S. pombe has three centromeres, ∼180 (90 × 2) condensins may be accumulated in the kinetochore DNAs. If these rough calculations are valid, approximately one third of the whole condensin is bound to the kinetochores and rDNAs. The docking sites for condensin should be abundant in these regions. One condensin per 0.5 kb and 1 kb for kinetochores and rDNAs, respectively, is 16- and 8-fold more than that in the other regions on average. The defective phenotypes of the condensin mutant strongly suggest that enriched condensin is important for proper segregation of the sister kinetochores and rDNAs. Wang et al. (2005) reported that one condensin was present at ∼2.0-kb intervals in the rDNA repeat region of S. cerevisiae, whereas one condensin was present at 10.7-kb intervals in the whole genome. We speculate that condensin forms the supramolecular protein–DNA complex at kinetochores and rDNAs in metaphase that contain the docking site that might share a common structural feature.
Materials and methods
Strains, plasmids, and media
An S. pombe haploid wild-type strain 972 h − and its derivative mutant strains were used. The acr1-936 ts mutant was isolated from a library of 1,015 ts mutants (Hayashi et al., 2004). The GFP-tagged cut14 + and cnd1 + genes were described previously (Sutani et al., 1999). The chromosomally integrated strains with the epitope (3Flag, 8Myc, GFP, and mCherry)-tagged Cut14, Sad1, Gar2, and Acr1 were made in the same manner. Plasmids carrying the Sad1-RFP and Mis12-RFP genes were used for the SPB and kinetochore markers, respectively (Aoki et al., 2006). The Cnp1/CENP-A–GFP strain was described previously (Takahashi et al., 2000). The Cen2-GFP strain was previously constructed (Yamamoto and Hiraoka, 2003) and was provided by the Yeast Genetic Resource Center (http://yeast.lab.nig.ac.jp). The Nuc1-GFP strain was a gift from Y. Hiraoka (Kobe Advanced ICT Research Center, National Institute of Information and Communications Technology, Kobe, Japan). The pRep81-Cut3WT and T19A plasmids were described previously (Sutani et al., 1999). The pRep1-Ark1WT and K118R plasmids were gifts from I. Hagan (Paterson Institute for Cancer Research, University of Manchester, Manchester, UK;Petersen and Hagan, 2003). The culture media used for S. pombe were complete YPD, SPA sporulation medium, and minimal EMM2 medium (Saka et al., 1994). The cell number was measured using a hematology analyzer (Sysmex F-800; Toa Medical Electronics). HeLa cells were grown at 37°C in DME (Invitrogen) supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% antibiotic-antimycotic.
Live cell analysis
S. pombe cells were cultured at 26°C in EMM2 medium and were shifted to 36°C for the appropriate duration. Before observation under a microscope (DeltaVision; Applied Precision), exponentially growing cells were transferred to a glass-bottomed dish (IWAKI Glass) coated with concanavalin A (Wako). Time-lapse images were recorded by the 3D microscopy system using the DeltaVision system. The objective lenses used were oil immersion lens (PlanApo 60× or UplanSApo 100× NA 1.4; Olympus). For observations of the GFP-tagged proteins with RFP or mCherry-tagged proteins, three optical sections were collected at 0.5- or 1-min intervals at 26°C or 36°C. The vertical separations between these sections were 0.5 μm. For observation of the Cen2-GFP signals, the real-time z-sweep acquisition protocol based on OAI technology (Applied Precision) was used in 1.5-μm optical space. Image projection and deconvolution were performed using an imaging workstation (SoftWoRx; Applied Precision).
Immunofluorescence microscopy and FISH method
The procedures for DAPI staining, immunofluorescence microscopy, and FISH were previously described (Hagan and Hyams, 1988; Uzawa and Yanagida, 1992). Antibodies used were anti-Sad1 (Hagan and Yanagida, 1995) for SPB staining and TAT1 for staining α tubulin (a gift from K. Gull, Biological Laboratory, University of Kent, Canterbury, UK). Double staining of chromatin and the nucleolus region was previously described (Umesono et al., 1983). Immunofluorescence in HeLa cells and primary antibodies against hMis6 and CENP-A were previously described (Goshima et al., 2003). Anti–hCAP-H2 antibody (Ono et al., 2003) was a gift from T. Hirano (Chromosome Dynamics Laboratory, RIKEN Discovery Research Institute, Saitama, Japan). A microscope (Axiovert 200M; Carl Zeiss, Inc.) was used to observe the HeLa cells. Images of the spread chromosome were acquired at 0.2-μm steps in the z axis and were deconvolved using AxioVision imaging software (Carl Zeiss, Inc.).
ChIP assay
The ChIP method was performed as previously described (Saitoh et al., 1997) with slight modifications. Immunoprecipitation was performed using anti-Flag M2 antibody (Sigma-Aldrich). Real-time PCR was performed on the Exicycler (Bioneer). The PCR primers used are shown in Table S1.
RNAi method
The siRNA for hMis6 RNAi was previously described (Goshima et al., 2003). The cell culture and transfection procedures were based on those previously described (Goshima et al., 2003) using Oligofectamine (Invitrogen).
Mitotic chromosome spread
Chromosome spread analysis was performed as previously described (Ono et al., 2003) with slight modifications. HeLa cells transfected with siRNA were incubated for 48 h. 100 ng/ml nocodazole was added, and cells were incubated for 4 h more. Cells were swollen in a hypotonic solution (1:6 diluted PBS with Milli-Q water [Millipore]) followed by spreading the cells with Cytospin 4 (Thermo Fisher Scientific) at 1,300 rpm for 10 min.
Mass spectrometry
The procedures used here were performed essentially as previously described (Ohta et al., 2002). For immunoprecipitation of Acr1, the extraction buffer was modified (25 mM Tris-HCl, pH 7.5, 15 mM EGTA, 15 mM MgCl2, 60 mM β-glycerophosphate, 15 mM p-nitrophenylphosphate, 0.5 mM Na3VO4, 0.1 mM NaF, 0.1% NP-40, 1 mM PMSF, 1% trasylol, and protease inhibitor cocktail [Sigma-Aldrich]). Immunopurified samples were separated on a 12.5% SDS-PAGE gel, and the region of the gel containing proteins from 250K to 20K was cut at ∼1–2-mm intervals. After in-gel digestion with modified trypsin (Roche), the resulting peptides were analyzed by online liquid chromatography–tandem mass spectrometry on a mass spectrometer (Finnigan LTQ; Thermo Fisher Scientific). All tandem mass spectra were searched against the S. pombe nonredundant protein database, including common contaminants such as trypsin and keratin, with the Mascot program (Matrix Science Ltd.).
Online supplemental material
Videos 1–4 show images of Cnd1- or Cut14-GFP taken with Sad1-RFP using a DeltaVision microscope (Videos 1 and 2 for Cnd1-GFP and Sad1-RFP; Videos 3 and 4 for Cut14-GFP and Sad1-RFP). Fig. S1 shows mitotic phenotypes of nda3-311 Cut14-Flag and ChIP assay in the cdc25-22 mutant. Fig. S2 shows the phenotypes of acr1 mutant cells and the interaction of Rrn5-Myc with Acr1-Flag that contained the domains conserved in Rrn9 family proteins. Fig. S3 shows that the localization of Cnp1, Mis6, Nuc1, and Acr1 proteins was normal in the cut17-275 mutant. Table S1 shows PCR primers for the ChIP assay. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200708170/DC1.
Supplemental Material
Acknowledgments
We are greatly indebted to Tatsuya Hirano for anti–hCAP-H2 antibodies, Yasushi Hiraoka for yeast strains, Iain Hagan for pArk1 plasmids, and Minoru Yoshida for a series of plasmids.
This work was supported by a specially promoted research grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan as well as by the Japan Science and Technology Corporation.
Abbreviations used in this paper: ARS, autonomously replicating sequence; CENP, centromere protein; ChIP, chromatin immunoprecipitation; mCherry, monomeric Cherry; NTS, nontranscribing sequence; rDNA, ribosomal DNA; SMC, structural maintenance of chromosome; SPB, spindle pole body; ts, temperature sensitive.
References
- Aoki, K., Y. Nakaseko, K. Kinoshita, G. Goshima, and M. Yanagida. 2006. CDC2 phosphorylation of the fission yeast dis1 ensures accurate chromosome segregation. Curr. Biol. 16:1627–1635. [DOI] [PubMed] [Google Scholar]
- Aono, N., T. Sutani, T. Tomonaga, S. Mochida, and M. Yanagida. 2002. Cnd2 has dual roles in mitotic condensation and interphase. Nature. 417:197–202. [DOI] [PubMed] [Google Scholar]
- Appelgren, H., B. Kniola, and K. Ekwall. 2003. Distinct centromere domain structures with separate functions demonstrated in live fission yeast cells. J. Cell Sci. 116:4035–4042. [DOI] [PubMed] [Google Scholar]
- Bhalla, N., S. Biggins, and A.W. Murray. 2002. Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell. 13:632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello, O.A., E. Eliseeva, W.G. He, H. Youssoufian, S.E. Plon, B.R. Brinkley, and J.W. Belmont. 2001. Cell cycle-dependent expression and nucleolar localization of hCAP-H. Mol. Biol. Cell. 12:3527–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, L., L. Aragon-Alcaide, and A. Strunnikov. 2000. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 149:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y., T. Hayashi, T. Kiyomitsu, Y. Toyoda, A. Kokubu, C. Obuse, and M. Yanagida. 2007. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev. Cell. 12:17–30. [DOI] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., S. Saitoh, and M. Yanagida. 1999. Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 13:1664–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., T. Kiyomitsu, K. Yoda, and M. Yanagida. 2003. Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J. Cell Biol. 160:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli, M.P., J.P. Girard, D. Zabetakis, B. Lapeyre, T. Melese, and M. Caizergues-Ferrer. 1995. gar2 is a nucleolar protein from Schizosaccharomyces pombe required for 18S rRNA and 40S ribosomal subunit accumulation. Nucleic Acids Res. 23:1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I.M., and J.S. Hyams. 1988. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89:343–357. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and M. Yanagida. 1995. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129:1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom, K.A., V.F. Holmes, N.R. Cozzarelli, and B.J. Meyer. 2002. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 16:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, T., Y. Fujita, O. Iwasaki, Y. Adachi, K. Takahashi, and M. Yanagida. 2004. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 118:715–729. [DOI] [PubMed] [Google Scholar]
- Hirano, T., and T.J. Mitchison. 1994. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 79:449–458. [DOI] [PubMed] [Google Scholar]
- Hirano, T., G. Konoha, T. Toda, and M. Yanagida. 1989. Essential roles of the RNA polymerase I largest subunit and DNA topoisomerases in the formation of fission yeast nucleolus. J. Cell Biol. 108:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, T., R. Kobayashi, and M. Hirano. 1997. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 89:511–521. [DOI] [PubMed] [Google Scholar]
- Hiraoka, Y., T. Toda, and M. Yanagida. 1984. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 39:349–358. [DOI] [PubMed] [Google Scholar]
- Ishihama, Y., Y. Oda, T. Tabata, T. Sato, T. Nagasu, J. Rappsilber, and M. Mann. 2005. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics. 4:1265–1272. [DOI] [PubMed] [Google Scholar]
- Jager, H., M. Rauch, and S. Heidmann. 2005. The Drosophila melanogaster condensin subunit Cap-G interacts with the centromere-specific histone H3 variant CID. Chromosoma. 113:350–361. [DOI] [PubMed] [Google Scholar]
- Keys, D.A., B.S. Lee, J.A. Dodd, T.T. Nguyen, L. Vu, E. Fantino, L.M. Burson, Y. Nogi, and M. Nomura. 1996. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 10:887–903. [DOI] [PubMed] [Google Scholar]
- Kiyomitsu, T., C. Obuse, and M. Yanagida. 2007. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell. 13:663–676. [DOI] [PubMed] [Google Scholar]
- Lavoie, B.D., E. Hogan, and D. Koshland. 2004. In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 18:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M., A. Guo, B. Boukhgalter, K. Van Den Heuvel, M. Tripp, and L. Pape. 2002. Characterization of the fission yeast ribosomal DNA binding factor: components share homology with Upstream Activating Factor and with SWI/SNF subunits. Nucleic Acids Res. 30:5347–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka, T., N. Imamoto, Y. Yoneda, and M. Yanagida. 1998. Mutations in fission yeast Cut15, an importin alpha homolog, lead to mitotic progression without chromosome condensation. Curr. Biol. 8:1031–1034. [DOI] [PubMed] [Google Scholar]
- Mitsuzawa, H., H. Seino, F. Yamao, and A. Ishihama. 2001. Two WD repeat-containing TATA-binding protein-associated factors in fission yeast that suppress defects in the anaphase-promoting complex. J. Biol. Chem. 276:17117–17124. [DOI] [PubMed] [Google Scholar]
- Morishita, J., T. Matsusaka, G. Goshima, T. Nakamura, H. Tatebe, and M. Yanagida. 2001. Bir1/Cut17 moving from chromosome to spindle upon the loss of cohesion is required for condensation, spindle elongation and repair. Genes Cells. 6:743–763. [DOI] [PubMed] [Google Scholar]
- Nabeshima, K., T. Nakagawa, A.F. Straight, A. Murray, Y. Chikashige, Y.M. Yamashita, Y. Hiraoka, and M. Yanagida. 1998. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol. Biol. Cell. 9:3211–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse, C., O. Iwasaki, T. Kiyomitsu, G. Goshima, Y. Toyoda, and M. Yanagida. 2004. A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6:1135–1141. [DOI] [PubMed] [Google Scholar]
- Ohta, S., Y. Shiomi, K. Sugimoto, C. Obuse, and T. Tsurimoto. 2002. A proteomics approach to identify proliferating cell nuclear antigen (PCNA)-binding proteins in human cell lysates. Identification of the human CHL12/RFCs2-5 complex as a novel PCNA-binding protein. J. Biol. Chem. 277:40362–40367. [DOI] [PubMed] [Google Scholar]
- Okada, M., I.M. Cheeseman, T. Hori, K. Okawa, I.X. McLeod, J.R. Yates III, A. Desai, and T. Fukagawa. 2006. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat. Cell Biol. 8:446–457. [DOI] [PubMed] [Google Scholar]
- Oliveira, R.A., P.A. Coelho, and C.E. Sunkel. 2005. The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis. Mol. Cell. Biol. 25:8971–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, T., A. Losada, M. Hirano, M.P. Myers, A.F. Neuwald, and T. Hirano. 2003. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 115:109–121. [DOI] [PubMed] [Google Scholar]
- Ono, T., Y. Fang, D.L. Spector, and T. Hirano. 2004. Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell. 15:3296–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, J., and I.M. Hagan. 2003. S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 13:590–597. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., and E.D. Salmon. 1998. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, N., I.G. Goldberg, E.R. Wood, and W.C. Earnshaw. 1994. ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J. Cell Biol. 127:303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, S., K. Takahashi, and M. Yanagida. 1997. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 90:131–143. [DOI] [PubMed] [Google Scholar]
- Saka, Y., T. Sutani, Y. Yamashita, S. Saitoh, M. Takeuchi, Y. Nakaseko, and M. Yanagida. 1994. Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 13:4938–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi, I.N., J.A. Dodd, L. Vu, K. Eliason, M.L. Oakes, J. Keener, R. Moore, M.K. Young, and M. Nomura. 2001. Transcription of chromosomal rRNA genes by both RNA polymerase I and II in yeast uaf30 mutants lacking the 30 kDa subunit of transcription factor UAF. EMBO J. 20:4512–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen, S., P.A. Coelho, N. Cobbe, S. Vass, M. Costa, B. Hassan, S.N. Prokopenko, H. Bellen, M.M. Heck, and C.E. Sunkel. 2001. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol. 11:295–307. [DOI] [PubMed] [Google Scholar]
- Strunnikov, A.V. 2005. A case of selfish nucleolar segregation. Cell Cycle. 4:113–117. [DOI] [PubMed] [Google Scholar]
- Strunnikov, A.V., E. Hogan, and D. Koshland. 1995. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev. 9:587–599. [DOI] [PubMed] [Google Scholar]
- Sullivan, M., T. Higuchi, V.L. Katis, and F. Uhlmann. 2004. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 117:471–482. [DOI] [PubMed] [Google Scholar]
- Sutani, T., T. Yuasa, T. Tomonaga, N. Dohmae, K. Takio, and M. Yanagida. 1999. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., S. Murakami, Y. Chikashige, H. Funabiki, O. Niwa, and M. Yanagida. 1992. A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell. 3:819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., E.S. Chen, and M. Yanagida. 2000. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 288:2215–2219. [DOI] [PubMed] [Google Scholar]
- Umeda, M., S. Izaddoost, I. Cushman, M.S. Moore, and S. Sazer. 2005. The fission yeast Schizosaccharomyces pombe has two importin-alpha proteins, Imp1p and Cut15p, which have common and unique functions in nucleocytoplasmic transport and cell cycle progression. Genetics. 171:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono, K., Y. Hiraoka, T. Toda, and M. Yanagida. 1983. Visualization of chromosomes in mitotically arrested cells of the fission yeast Schizosaccharomyces pombe Curr. Genet. 7:123–128. [DOI] [PubMed] [Google Scholar]
- Uzawa, S., and M. Yanagida. 1992. Visualization of centromeric and nucleolar DNA in fission yeast by fluorescence in situ hybridization. J. Cell Sci. 101:267–275. [DOI] [PubMed] [Google Scholar]
- Wang, B.D., D. Eyre, M. Basrai, M. Lichten, and A. Strunnikov. 2005. Condensin binding at distinct and specific chromosomal sites in the Saccharomyces cerevisiae genome. Mol. Cell. Biol. 25:7216–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall, S.M., R. Deehan, T.J. Maresca, and R. Heald. 2003. The condensin complex is required for proper spindle assembly and chromosome segregation in Xenopus egg extracts. J. Cell Biol. 161:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., and Y. Hiraoka. 2003. Monopolar spindle attachment of sister chromatids is ensured by two distinct mechanisms at the first meiotic division in fission yeast. EMBO J. 22:2284–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Gonzalez, V., B.D. Wang, P. Butylin, I. Ouspenski, and A. Strunnikov. 2007. Condensin function at centromere chromatin facilitates proper kinetochore tension and ensures correct mitotic segregation of sister chromatids. Genes Cells. 12:1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.