Abstract
Krüppel-like factor 4 (KLF4) is a pleiotropic zinc finger transcription factor that regulates genes being involved in differentiation and cell-cycle control. Knock out studies revealed a critical function for KLF4 in the terminal differentiation of many epithelial cells. In testicular Sertoli cells, Klf4 is strongly inducible by the glycoprotein Follicle stimulating hormone (FSH). Since KLF4 is essential for postnatal survival in mice, we deleted Klf4 specifically in Sertoli cells using the Cre/loxP system. Importantly, around postnatal day 18, a critical period of terminal Sertoli cell differentiation, mutant seminiferous tubules exhibited a disorganized germinal epithelium and delayed lumen formation. The ultrastructural finding of highly vacuolized Sertoli cell cytoplasm and the identification of differentially expressed genes, which are known to play roles during vesicle transport and fusion or for maintenance of the differentiated cell state, suggest impaired apical secretion of the Sertoli cell. Interestingly, a high proportion of all identified genes was localized in a small subregion of chromosome 7 suggesting coordinated regulation. Intriguingly, adult mutant mice are fertile and show normal testicular morphology, although the testosterone levels are decreased. In summary, KLF4 plays a significant role for proper and timely Sertoli cell differentiation in pubertal mice.
Keyterms: Krüppel-like factor 4, Sertoli cell, testis, spermatogenesis, epithelial cell polarity, exocytosis, vesicle, gene cluster, differentiation
Introduction
Testicular development is dependent on Sertoli cell fate determination, which occurs around embryonic day 11–12 in the mouse (Buehr et al., 1993; Schmahl et al., 2000). This initial event is critically dependent on the transcription factors SRY, Sox9 and others (Cupp and Skinner, 2005). During testicular development immature Sertoli cells rapidly proliferate and eventually differentiate to become mature, fully differentiated Sertoli cells, which no longer proliferate. This Sertoli cell state is achieved by the orchestrated expression of many regulatory proteins (Chaudhary and Skinner, 2005; Kim et al., 2007; Sridharan et al., 2007). The zinc finger transcription factor Krüppel-like factor 4 (KLF4) is an important differentiation factor for several epilthelia. Deletion of Klf4 in keratinocytes severely impairs skin function resulting in early postnatal death (Segre et al., 1999). Absence of KLF4 from the colonic mucosal epithelium led to a dramatic reduction in the number of goblet cells (Katz et al., 2002) and gastric epithelium lacking KLF4 exhibited altered proliferation and eventually a precancerous stage (Katz et al., 2005). Recently, KLF4 has also been shown to be an important regulator of haematopoiesis (Feinberg et al., 2007; Klaewsongkram et al., 2007). Moreover, this transcription factor has been implicated in breast and bladder carcinogenesis (Foster et al., 2000; Ohnishi et al., 2003). KLF4 can function as a tumor suppressor or an oncogene depending on the molecular context (Rowland et al., 2005) and also plays an essential role during the artificial reprogramming of differentiated somatic cells to pluripotent cells (Okita et al., 2007; Takahashi and Yamanaka, 2006; Wernig et al., 2007). We have shown that Klf4 is strongly expressed in the adult mouse testis with highest expression in postmeiotic round spermatids (Behr and Kaestner, 2002; Godmann et al., 2005). Furthermore, our results and other studies showed that Klf4 is also expressed in Sertoli cells (Godmann et al., 2005; Hamil and Hall, 1994; McLean et al., 2002; Sadate-Ngatchou et al., 2004), which constitute together with germ cells the germinal epithelium. Sertoli cells show highest proliferative activity around birth (Cupp and Skinner, 2005). In the neonatal and young postnatal testis they make up around 45% of all testicular cells (Vergouwen et al., 1993) and approximately 90% of the cells in the seminiferous tubules (Ellis et al., 2004). Subsequently, Sertoli cells still proliferate with a decreasing rate and finally cease to proliferate around postnatal day 18 (Vergouwen et al., 1993; Vergouwen et al., 1991). With progression of postnatal testicular development, germ cells and Leydig cells exhibit strong proliferation and represent increasing proportions of all testicular cells (Vergouwen et al., 1993; Vergouwen et al., 1991). Therefore, Sertoli cells make up only a few percent of all testicular cells in the adult mouse testis (deduced from (Tegelenbosch and de Rooij, 1993; Vergouwen et al., 1993)) and 12% of the cells in the seminiferous tubules in postnatal day 31 mouse testes (Ellis et al., 2004). It has been shown that Klf4 expression can be rapidly (within 2 hours) and strongly (20- to 50-fold) induced by Follicle stimulating hormone (FSH) in maturing rat Sertoli cells (Hamil and Hall, 1994; McLean et al., 2002). The striking importance of KLF4 in several cell types and its strong inducibility in Sertoli cells by FSH prompted us to investigate the role of KLF4 in Sertoli cells by Sertoli cell-specific deletion of Klf4 using the Cre/loxP system.
We show that maturation of the Sertoli cells appears to be delayed as indicated by aberrant histology of the maturing testes, retarded formation of the tubular lumen and strongly increased vacuolization of Sertoli cell cytoplasm. Moreover, gene expression profiling using whole genome microarrays revealed candidate genes that probably provide the molecular links between the lack of the transcription factor KLF4 and the observed phenotype at postnatal day 18. Intriguingly, adult testis histology was normal and the mutant mice were fertile. However, adult males had significantly lower testosterone levels compared to controls.
Materials and Methods
Derivation of Sertoli cell-specific Klf4 knock out (SC-Klf4-ko) mice
The SC-Klf4-ko mice were obtained using the Cre/loxP technology. Mice derived from a C57BL/6 background carrying floxed Klf4 exons 2 to 4 (Godmann et al., 2005) have been described (Klf4-loxP/loxP mice; (Katz et al., 2002)). These mice were crossed with C57-B6/SJL transgenic mice expressing the Cre recombinase under the control of the Sertoli cell-specific Anti Müllerian hormone (AMH) gene promoter (Lecureuil et al., 2002). Thus, mice studied were on a mixed genetic background. We used Cre-negative Klf4loxP/loxP mice as controls (termed lox) and Cre-positive Klf4loxP/loxP mice as mutants (ko or SC-Klf4-ko; see also Comparison of transgenic mice carrying floxed Klf4 alleles with wildtype mice in results). Genotyping was performed by multiplex polymerase chain reaction using tail DNA as described below (all primers used in this study are listed in supplemental material, Tab.1). The mice were maintained under controlled conditions of temperature (21°C), light (12L:12D), and 55% humidity with food and water ad libitum. The animals were housed in the central animal facility of the University of Essen Medical School under conditions according to German animal legislation and in accordance with accepted standards of humane animal care. To collect organ and blood samples, mice were weighed, anesthetized with Isofluran (CuraMED Pharma, Karlsruhe, Germany) and killed by decapitation.
Serum hormones
Blood was collected for serum hormone analysis, stored at 4°C overnight and finally centrifuged (20 min, 3000 rpm, 4°C). The serum was stored at −20°C until assayed. Serum FSH and testosterone levels were determined as described (Bartlett et al., 1989; Chandolia et al., 1991). Plasma thyroid hormone (T3) concentration was determined by RIA using commercial kits according to the manufacturer’s instructions (Diagnostic Products, Los Angeles, USA). Statistical analyses were performed as described below.
Tissue processing for immunohistochemistry
Testes were fixed in Bouin’s solution and paraffin-embedded. Specimens were sectioned at 7 μm, rehydrated and microwaved in 0.01 M sodium citrate buffer, pH 6.0, for 10 min for antigen retrieval. Endogenous peroxidase was inhibited by incubation with peroxidase blocking reagent (DakoCytomation Carpinteria, CA, USA, LSAB+ system-HRP, K0679). Unspecific binding of the first antibody was blocked by a 30 minute incubation step in 0.5% (w/v) BSA in 0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, pH 7.6 (TBS). All incubation steps were performed in a humified chamber and incubations with the primary antibodies were performed overnight at 4°C. DakoCytomation Universal LSAB Plus-kit (K0679) including biotinylated second antibody polymer and horseradish peroxidase (HRP) conjugated streptavidin was used for detection of bound primary antibody. 3,3′-diaminobenzidine (DAB) chromogen was used as substrate for the HRP and Mayer’s hematoxylin as counterstain. Control stains were carried out omitting the primary antibody. Antibodies used in this study were: Anti Müllerian Hormone (C-20), rabbit polyclonal, dilution 1:1000 (Santa Cruz Biotechnology; Heidelberg, Germany), androgen receptor (N-20), rabbit polyclonal, 1:800 (Santa Cruz Biotechnology, Heidelberg, Germany), cleaved caspase 3, rabbit polyclonal, 1:100 (Cell Signaling Technology, Danvers, USA), p27kip1, mouse monoclonal, 1:40 (Novocastra, Menarini Diagnostics, Neuss, Germany), and PCNA (PC10), mouse monoclonal, 1:100 (Novocastra, Menarini Diagnostics, Neuss, Germany).
Determination of proliferating and apoptotic cells in seminiferous tubules
In order to analyze the number of proliferating cells in the seminiferous tubules of postnatal day 12, 15, and 18 control and mutant mice, PCNA immunohistochemical staining was performed on Bouin-fixed and paraffin-embedded testes sections. PCNA-positive Sertoli cell nuclei were counted in 30 round tubular cross sections (10 from 3 mice each) and calculated as positive Sertoli cell nuclei/tubule. Apoptotic cells in the seminiferous tubules of postnatal day 18 control and mutant mice were detected by TUNEL assay (In situ cell death detection kit, AP, Roche Applied Science, Mannheim, Germany) on Bouin-fixed and paraffin-embedded testes following the manufacturer’s instructions. TUNEL positive seminiferous tubules were counted and calculated as number per 100 tubule cross sections. Furthermore, the number of TUNEL positive cells per positive seminiferous tubule was determined. Statistical analyses were performed as described below.
Transmission electron microscopy
For transmission electron microscopy, testes were removed, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.4 for 4 h at 4°C. Tissues were washed for several times in 0.1 M sodium cacodylate and postfixed in 1% osmium tetroxide in 0.1 M sodium cacodylate for 2.5 h in the dark at room temperature. Then, samples were washed three times for 30 min at room temperature in 0.1 M sodium cacodylate, and, after dehydration in a graded ethanol-series followed by two changes of propylene oxide (20 min, room temperature) and three changes of a mixture of propylene oxide and epon (propylene oxide: epon; 1:2, 1:1, 2:1; each for 2 h at room temperature), samples were embedded in epon. Semi-thin sections (0.5 μm) were stained with 1 % methylene blue/1% azurII blue/1% toluidine blue/1% sodiumtetraborat in ddH2O. Ultra-thin sections (85 nm) were further treated with 1% uranyl acetate and 0.4% lead citrate and mounted on 200-mesh copper grids, double-stained with uranyl acetate and lead citrate and examined with a Zeiss 902 A at 80 kV (Carl Zeiss, Jena, Germany).
Morphology and quantitative histological analysis
Testicular semi-thin sections of postnatal day 16, p18, p20 and adult control (Cre-neg/Klf4loxP/loxP) and Sertoli cell-specific Klf4 knock out mice (AMH-Cre/Klf4loxP/loxP) were examined with a Zeiss Axiophot microscope. Analysis of images was performed with Eclipsenet software version 1.20 (Laboratory imaging s.r.o., Praha, Czech Republic). The smallest and largest diameter of each seminiferous tubule’s cross sections were measured and only those tubules, whose smallest diameter was at least 98% of the largest diameter (= 100%) were considered as circular and included in the analysis. The smallest diameter of a circular tubular cross section was used to calculate the diameter of control and mutant tubular cross sections. Diameters were analyzed for each group and expressed as the mean ± SD.
An area of a circular cross section of the seminiferous tubule was classified as a lumen by its central localization and a minimum pixel number of 4500 pixels (for calculation of the number of pixels please see below). All tissue spaces that were directly associated with the central lumen were also considered as part of a lumen and excluded from further calculation of “tissue spaces”. Hence, tubules with a central space of at least 4.500 pixels were defined as “lumen-positive” and termed open. All other circular cross sections without a lumen were termed closed. Circular cross sections without a lumen were counted and calculated as number per total circular seminiferous tubule’s cross sections of a testis.
The seminiferous epithelium of a circular tubule’s cross section was termed disorganized when the tubules lacked the typical architecture of the epithelium with clearly distinct cell boundaries of premeiotic and meiotic germ cells (please see Fig. 2 and 3 for illustration purposes). Circular cross sections with a disorganized epithelium were counted and calculated as number per total circular seminiferous tubule’s cross sections of a testis.
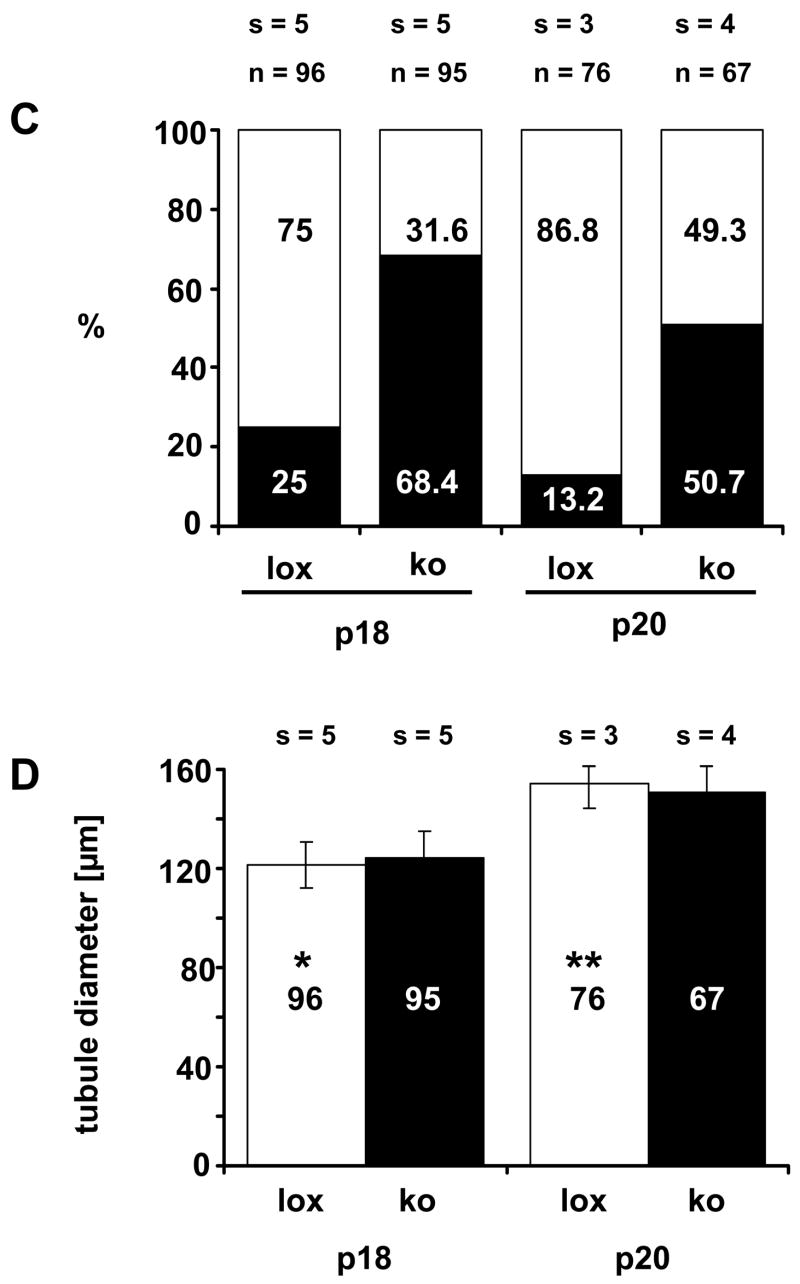
Figure 2.
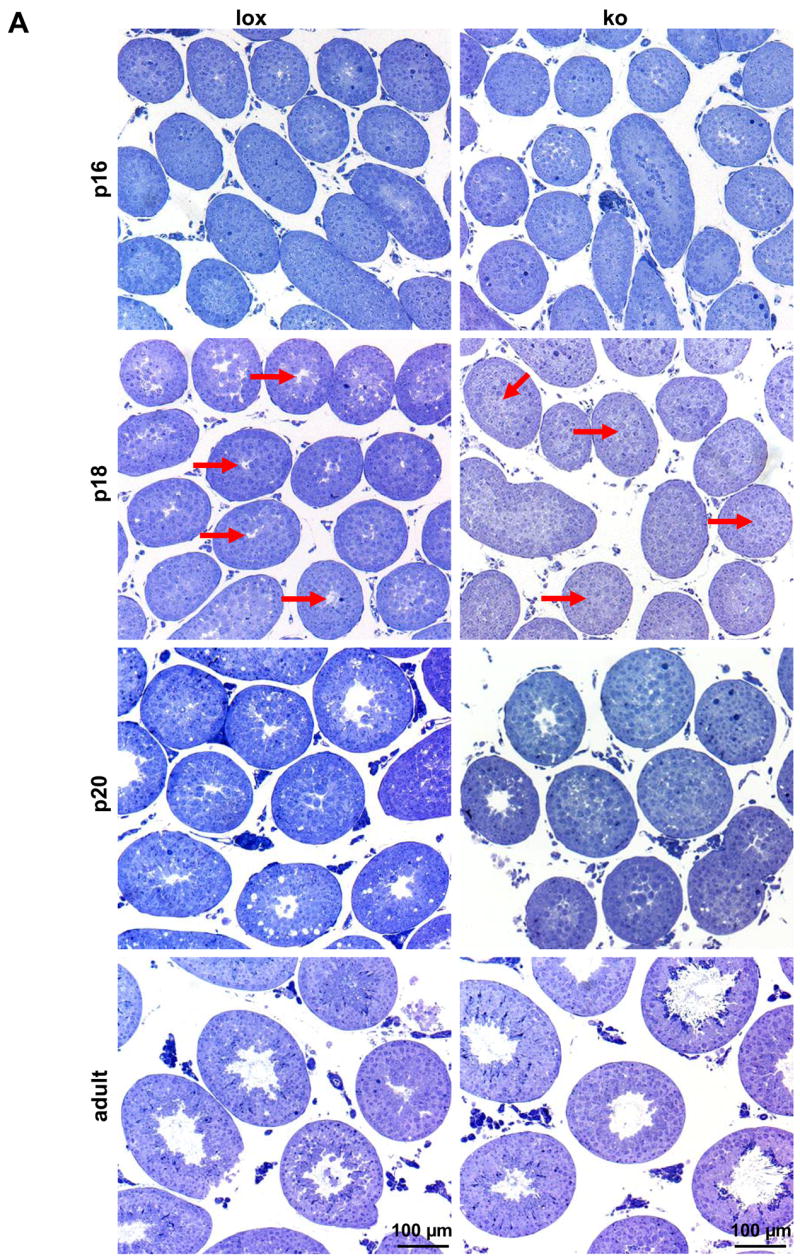
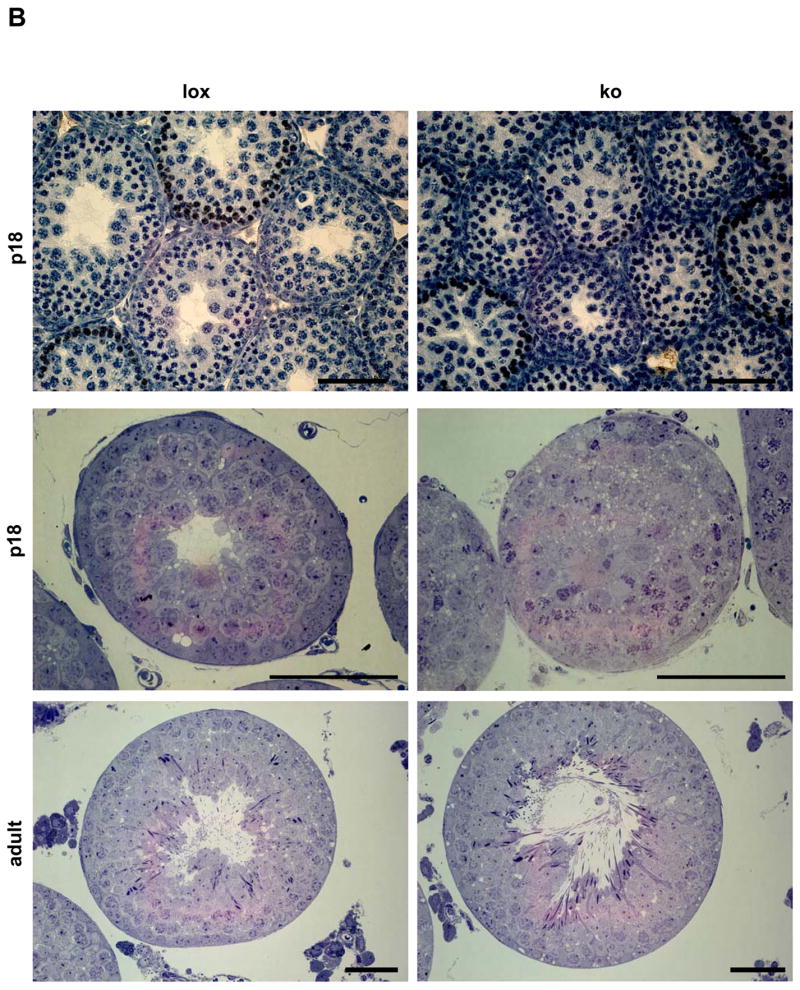
(a) Testicular histology of AMH-Cre/Klf4loxP/loxP mice (ko) on semi-thin sections during the phase of terminal differentiation of Sertoli cells (postnatal day 16 (p16)–20 (p20)) and in adulthood compared to their control (lox) littermates. Red arrows point at the centers of p18 tubules, which are mainly open in controls but still closed in most mutants. (b) Top panel: A typical control and a mutant testis at p18 after fixation in Bouin’s solution. Basically the same situation can be seen as in semi-thin sectioned tissues: most of the tubules in the control are open at p18 while the majority of the tubules in the mutants are still closed. For more details please see results. Middle panel: A typical p18 tubule of a mutant and a control testis. The control shows a regularly ordered germinal epithelium and an established lumen. Relatively few tissue spaces are present. In contrast, the mutant tubule exhibits numerous vacuoles and no lumen. Also, the mutant epithelium appears to be disorganized. Bottom panel: Semi-thin sections of typical tubules from a mutant and a control testis. Both tubules show a normally arranged epithelium and a lumen. The adult mutant tubule does not exhibit increased vacuolization. The bar represents in all pictures 50 μm. (c) Quantification of the percentage (%) of open (white bar) and closed tubules (black bar) in p18 and p20 testes from control (lox) and mutant (ko) mice. Numbers on the bars indicate the percentage (%), numbers above the bars indicate the numbers (n) of analysed tubules and the number (s) of evaluated testis samples. (d) Quantification of the diameter of the tubules in p18 and p20 testes from control (lox, white bars) and mutant (ko, black bars) mice. While in p18 testes the mutant tubules had a slightly, yet significantly increased diameter, at p20 the mutant tubules showed a significantly reduced diameter. Only strictly circular cross sections (smallest diameter at least 98% of the largest diameter) were selected for analysis to exclude the possibility of evaluating sagittal sections. Numbers of the analyzed tubules are indicated on the bar and numbers (s) above the bar refer to the evaluated testis samples. Results were expressed as the mean ± SD as indicated by error bars. *p<0.033, **p<0.043.
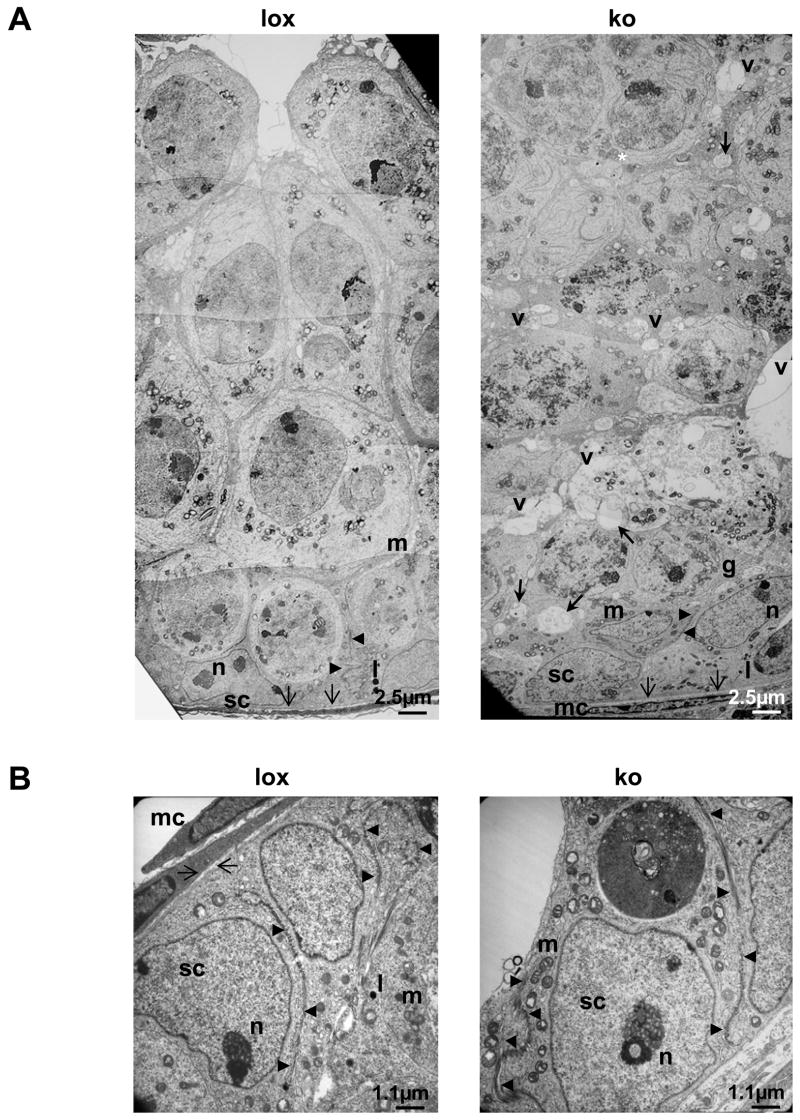
Figure 3.
(a) Ultrastructural analysis of the germinal epithelium at postnatal day 18 (p18) in AMH-Cre/Klf4loxP/loxP and control mice by transmission electron microscopy (2500x). The germinal epithelium is shown from the basal lamina (arrows at the bottom) up to the center of the tubule in controls (lox) and mutant mice (ko). While most tubules in the controls had developed a lumen (upper part of the left picture) and exhibit a properly ordered epithelium, the mutant tubule appears disorganized. Cell boundaries are hardly visible and no lumen had formed in the center of the tubule (asterisk). Moreover, numerous vacuoles (v) delimited by membranous structures can be seen (bold arrows). The blood-testis barrier appears to be normal in both genotypes (arrow heads). (b) Higher magnification (6000x) of the basal part of the germinal epithelium in AMH-Cre/Klf4loxP/loxP (ko) and control mice (lox) confirms that the blood-testis-barrier (arrow heads) has been formed at p18 in control (lox) and mutant (ko) mice. G: Golgi apparatus; l: lysosome; m: mitochondria; mc: myoid cell; n: nucleolus; sc: Sertoli cell; v: vacuole.
Tissue spaces (defined as all areas represented by less than 3.500 pixels and randomly distributed over the germinal epithelium) visible by light microscopy on semi-thin sections were vacuoles, spaces left behind by degenerated cells and intercellular spaces. There were no objects in the range between 3.500 and 4.500 pixels allowing clear distinction between a lumen and a tissue space. In order to determine the area of tissue spaces within the germinal epithelium per seminiferous tubule’s cross sections in p18 control and mutant testes, 15 circular tubular cross sections of three different animals per group were randomly picked and measured using the Image processing and analysis IPA module of Simple PCI software Version 5.3.1 (Compix Inc., Imaging Systems, Cranberry Township, USA). Briefly, threshold ranges were selected to identify the regions of interest. Unwanted pixels (e.g. objects outside of a cross section and the tubule’s lumen) were excluded by setting measurement ranges. First, the total area of an entire circular cross section was determined and then the numbers of pixels of each qualified object as well as the number of all selected objects per cross section were collected and expressed as the mean ± SD. Data of mutant mice were compared to the corresponding controls.
Quantitative histological analysis was performed on three p16 control animals and two p16 mutant animals, at p18 five controls and five Sertoli cell-specific knock out mice have been analysed and three p20 control testes were compared to four mutant testes. Statistical significance was analyzed with an unpaired Student’s t test. A value of p < 0.05 was considered significant. Tissue sample analysis has been performed in a blinded way.
Statistical Analyses
If not otherwise mentioned, in all experiments presented a minimum of three control mice (Cre-neg/Klf4loxP/loxP) and three Sertoli cell-specific Klf4 knock out mice (AMH-Cre/Klf4loxP/loxP) mice per group have been investigated. Results were expressed as the mean ± SD. Statistical significance was analyzed by Students’ unpaired t test. A value of p < 0.05 was considered significant.
RNA Isolation and RT-PCR
Total RNA was extracted using peqGOLD-TriFast (peqLab Biotechnology GmbH, Erlangen, Germany) following manufacturer’s instructions. For oligonucleotide microarray analysis, total RNA was additionally purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). In case of RT-PCR, total RNA was treated with DnaseI (RNeasy Mini Kit, Qiagen) and reverse transcribed with oligo-d(T) primers using the Omniscript reverse transcriptase (Qiagen). For analysis of cDNA 2 μl of a reverse-transcription reaction were added to 3 μl 10×PCR-buffer (including 1.5 mM MgCl2), 0.5 μl dNTPs (10 mM each), 0.5 μl of each primer (10 μM), 0.5 μl polymerase (2.5 U; BioTherm DNA-polymearse, GeneCraft, Lüdinghausen, Germany) and ddH20 to a final volume of 30 μl. PCR conditions for all primer pairs used were 1 × 94 °C for 4 min; 35 × 94 °C for 40 s, 60 °C for 25 s, 72 °C for 90 s; 1 × 72 °C for 10 min. PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. Primers are listed in supplemental material, table 1.
PCR on genomic DNA - Genotyping
Mice were genotyped by multiplex PCR using the PCR mixture as described for RT-PCR except that the cDNA was replaced by 2 μl of genomic DNA. Primer sequences and PCR conditions for the Klf4 gene were: mmKlf4-ex1-fw1 ctg ggc ccc cac att aat gag; mmKlf4-GSP2-re1 gtc gct gac agc cat gtc aga ctc g; mmKlf4-intr3-re2 cag agc cgt tct ggc tgt ttt; 1 × 94°C for 4 min; 32 × 94°C for 40 sec, 60°C for 25 sec, 72°C for 1 min; 1× 72°C for 10 min; and for Cre recombinase and β-Actin: CRE-26 cct gga aaa tgc ttc tgt ccg; CRE-36 cag ggt gtt ata agc aat ccc; Act1 acc ttc aac acc ccm gcc atg tac g (m=a/c); Act2 ctr atc cac atc tgc tgg aag gtg g (r=a/g); 1 × 94°C for 2 min; 35 × 94°C for 40 sec, 61°C for 25 sec, 72°C for 1 min 30 sec; 1 × 72°C for 10 min. Genomic DNA was obtained either by Proteinase K digestion of the tissue in non-ionic detergent (NID) buffer (50 mM KCl, 10 mM Tris/Cl, pH 8.3, 2 mM MgCl2, 0.1 mg/ml gelatin, 0.45% nonidet P40, 0.45% Tween 20), 56°C overnight, followed by Proteinase K heat inactivation (15 min, 95°C) or in case of testis material, by using the Qiagen DNeasy Tissue Kit (Hilden, Germany) following the manufacture’s instructions.
Southern Blotting
Southern blotting was performed as previously described (Matzuk et al., 1992). To generate a specific Southern blot probe, a 1.8 kb genomic fragment upstream of the Klf4 gene was amplified using the AccuTaq LA DNA polymerase system (Sigma-Aldrich, Taufkirchen, Germany). Primer sequences and PCR conditions for the Klf4 gene were: mmKlf4-SB-fw1 agc gta agt ctg acg tca acg; mmKlf4-SB-re1 gct ctt gga atg cat ctc ttc c; 1 × 94°C for 2 min; 35 × 94°C for 30 sec, 61°C for 25 sec, 68°C for 2 min; 1 × 68°C for 10 min. The PCR product was subcloned into the pCRII vector, digested with EcoRI and PstI and gel purified as described above. The resulting 668 bp fragment located outside of the original targeting construct, was used as a probe and hybridized to XbaI-digested genomic DNA. A 6.9 kb fragment (representing the floxed Klf4 allele) and a 4.8 kb fragment (Klf4 null allele after homologous recombination) could be detected.
Microarray analysis
Testes from mice being homozygous for the floxed Klf4 allele (n = 4) and testes from mice with a homozygous Sertoli cell-specific deletion of the Klf4 allele (n = 4) were isolated at postnatal day 18. Total RNA was extracted and purified as described above. cDNA synthesis and synthesis of biotinylated cRNA were performed as done by Durig et al. (Durig et al., 2003). Each cRNA probe was hybridized to an Affymetrix Gene Chip Mouse Genome 430 2.0 Array (Affymetrix, High Wycombe, USA). This Array contains probe sets representing over 39.000 transcripts. For analysis: Using filter criteria of a 1.7 or greater fold change in expression, all “present calls” in at least one group and a p-value of < 0.05 generated from the Mann-Whitney test (equivalent to Wilcoxon rank sum test) produced a list of genes that were differentially expressed. A false discovery rate of 5% was used as a cut off for expressed genes. All genes which showed at least a 1.7-fold change in expression were considered as differentially expressed.
Promoter analysis
Promoters of KLF4 target genes identified by microarray analysis were analyzed in terms of putative KLF4 binding sites. The CACCC motif was found to bind KLF4 and other related transcription factors by Garrett-Sinha and colleagues (Garrett-Sinha et al., 1996; Shields and Yang, 1998). The RRGGYGY (G/AG/AGGC/TGC/T) motif was identified by Shields and Young (Shields and Yang, 1998). Both sequences are distinct from each other and do not overlap. Moreover, KLF4 appears to have different affinities to both binding motifs (Shields and Yang, 1998). Both binding sites were used to search the promoters (base pairs −1000 to −1 relative to the transcriptional start site) of all genes which showed at least a 3.0-fold change in expression. Promoter sequences were retrieved using the “Transcriptional Regulatory Element Database” (http://rulai.cshl.edu/cgi-bin/TRED/tred.cgi?process=home) (Jiang et al., 2007; Zhao et al., 2005). Further analyses were performed with the Sequencher Software, Gene Codes Cooperation, Ann Arbour, USA.
Results
Comparison of transgenic mice carrying floxed Klf4 alleles with wildtype mice
Klf4loxP/loxP mice have been used in other studies and no abnormalities of development, cellular function or histology have been reported for Cre-negative Klf4loxP/loxP mice indicating that the floxed Klf4 allele is functionally wildtype (Feinberg et al., 2007; Katz et al., 2005; Klaewsongkram et al., 2007; Swamynathan et al., 2007). To make sure that the floxed Klf4 allele is functionally indistinguishable from the wild-type allele we analyzed Bouin-fixed and paraffin-embedded testis sections of Klf4loxP/wt and Klf4loxP/loxP mice and compared their histology with testis sections of wildtype mice of the same age. We could not detect any histological differences between these groups (data not shown). These results indicate that also in the testis the Klf4loxP allele is functionally wildtype. Henceforth, we used Klf4loxP/loxP mice as controls.
Deletion of Klf4 in Sertoli cells
The specificity of Cre recombinase expression in the AMH-Cre line has been shown previously (Lecureuil et al., 2002) and this mouse line has successfully been used for complete Sertoli cell-specific gene deletion in vivo (Brehm et al., 2007; Chang et al., 2004; De Gendt et al., 2004; Huyghe et al., 2006). Deletion of Klf4 in Sertoli cells in this study was confirmed by genomic Southern blot analysis of postnatal day 4 (p4), p5 and adult testis (Fig. 1a). The 4.8 kb band indicating the absence of exons 2, 3 and 4 of the Klf4 gene (Godmann et al., 2005) occurred exclusively in the AMH-Cre/Klf4loxP/loxP mice, while Cre-negative Klf4 loxP/loxP did not exhibit this band. As expected from the proportions of the respective cell types in young (approximately half of the cells are Sertoli cells) and adult testis (only few percent Sertoli cells), we detected much stronger signals for the deleted Klf4 allele in p4 and p5 testes than in adult testes (Fig. 1a). As an additional control we performed genotyping PCR on genomic DNA isolated from p4 testis, adult epididymis including sperm cells, and tail from both Cre-negative Klf4loxP/loxP and AMH-Cre/Klf4loxP/loxPmice. As control for the mutant band we included tail DNA from a general Klf4 knock out mouse. We obtained a band representing the deleted Klf4 allele only in AMH-Cre/Klf4loxP/loxP p4 testis. No mutant band was obtained from sperm cell-containing epididymis from an AMH-Cre/Klf4loxP/loxP mouse indicating that no recombination occurred in germ cells (Fig. 1b).
Figure 1.
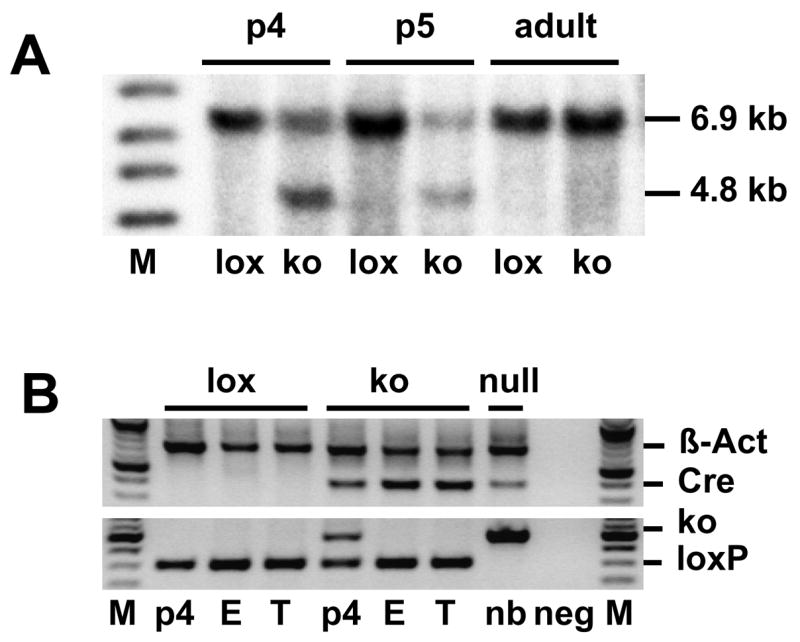
Sertoli cell-specific homologous recombination in testes of AMH-Cre/Klf4loxP/loxP mice. (a) Genomic Southern blot on DNA isolated from mutant (ko) and control (lox) testes from p4, p5 and adult mice, respectively. The 6.9 kb fragment represents the undeleted loxP allele and the 4.8 kb fragment indicates deletion of exons 2, 3, and 4 of the Klf4 gene. In control testes only the 6.9 kb band can be detected, while Cre-positive animals also exhibit the smaller 4.8 kb band. (b) Genotyping-PCR on genomic DNA isolated from p4 testis, adult epididymis (E) and tips of tails (T) from AMH-Cre/Klf4loxP/loxP mice (ko) and Cre-negative control mice (lox). This PCR confirmed that recombination did not occur in germ cells as DNA from adult epididymis (including all sperm cells stored in this organ) did not exhibit the band indicating recombination in these cells. The upper band in the upper panel shows β-Actin as an internal control. The lower band in the upper panel indicates Cre recombinase expression. The lower panel shows the Klf4 genotyping. M: size marker; p4: postnatal day 4 testis; E: epididymis; T: tail; neg: PCR without genomic DNA; null: Klf4 was deleted constitutively in all cells in a new born (nb) mouse.
Testis weight, Sertoli cell proliferation and fertility
Testicular weight was determined at postnatal days 9, 12, 15, 16, 18, 21, 24, 27, 56, 84, 168, and 365. No significant differences were found between mutant mice and controls (see supplemental material, Tab.2). Furthermore, the ratio of testis weight to body weight was also not significantly different (see supplemental material, Tab.2). Since testis weight is a reliable marker for Sertoli cell proliferation (Holsberger et al., 2005), the unchanged testis weights suggested that deletion of Klf4 in Sertoli cells did not influence Sertoli cell proliferation. In addition, we used antibody staining against proliferating cell nuclear antigen (PCNA) to analyze Sertoli cell proliferation (Levine et al., 2000) and found no significant differences between controls and mutants at p12 (2.38 ± 0.98 and 2.58 ± 0.98 (mean ± SD) stained Sertoli cell nuclei per round control and mutant tubule, respectively, p>0.48) and p15 (1.44 ± 0.51 and 1.44 ± 0.63 (mean ± SD) stained Sertoli cell nuclei per round tubule in both, mutant and controls, p=1) and no PCNA-positive Sertoli cell neither in mutants nor controls at p18, which is in agreement with previous investigations (Vergouwen et al., 1991). Moreover, the mutant mice were fertile and produced litters of the same size and at the same frequency as the controls. The pups of the matings of AMH-Cre/Klf4loxP/loxP × Klf4loxP/loxP exhibited a 1:1 ratio with respect to sex and presence/absence of the Cre transgene.
AMH-Cre/Klf4loxP/loxP mice exhibit delayed lumen formation of the seminiferous tubules
Detailed histological analyses of semi-thin sectioned testes around the time of terminal Sertoli cell proliferation (p16, 18, and 20) and in adult mice (p56) revealed significant differences between the two groups during puberty (Fig. 2). At p16 almost no tubules exhibited a lumen when examined on semi-thin sections, neither in the controls nor in the mutant mice (Fig. 2a). Postnatal day 18 is of special interest with regard to Sertoli cell differentiation since at this time point proliferation of Sertoli cells ceases (Vergouwen et al., 1991), the blood-testis barrier has formed, the Sertoli cells increase apical secretion and the seminiferous tubules’ lumina open. The histological differences between the groups were most pronounced in p18 semi-thin sectioned testes, which clearly showed a developmental delay with regard to lumen formation (Fig. 2a, b, c). At p18 75% of the tubules in the controls had developed a lumen. In striking contrast, in the mutant mice only 32% of the tubules exhibited a lumen (Fig. 2c). At p20 87% of the tubules in the controls exhibited a lumen while only half of the mutant tubules showed a lumen. When we determined the average diameter of opened tubules in p18 testes, we found a small yet significant increase in tubule diameter in mutant mice compared to controls (124.6 μm ± 10.5 vs. 121.5 μm ± 9.1 (mean ± SD); p < 0.033), even though fewer mutant tubules had developed a lumen at this stage (Fig. 2d). In contrast, in p20 mutant testes the tubules exhibited a slightly but significantly decreased average diameter (154.47 μm ± 9.88 vs. 150.64 μm ± 12.33 (mean ± SD); p < 0.042).
The seminiferous epithelium of p18 AMH-Cre/Klf4loxP/loxP mice appears frequently disorganized and is characterized by vacuolization
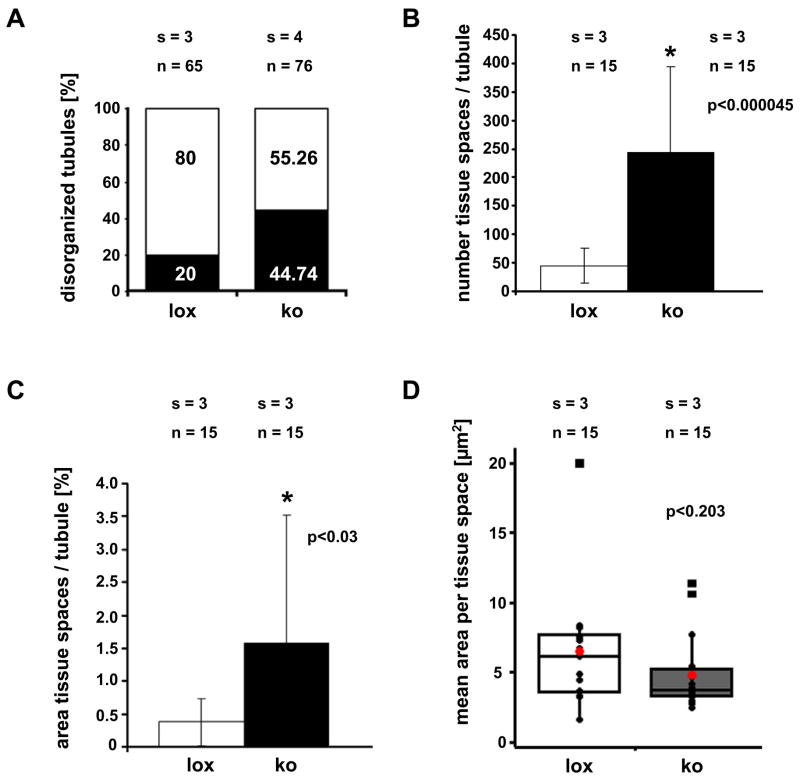
Detailed histological analysis of semi-thin sectioned p18 tissues by light microscopy and ultra-thin sectioned tissues by transmission electron microscopy additionally revealed that the germinal epithelium in the mutant mice was frequently disorganized (Fig. 2b, 3). While 44.74% of circular seminiferous tubule’s cross sections of p18 mutant mice showed a disorganized epithelium only 20% of control circular seminiferous tubule’s cross sections did not show the epithelial architecture considered as organized with clearly visible cell boundaries (Fig. 4a). The seminiferous epithelium of p18 mice usually includes tissue spaces being composed of vacuoles of different sizes, degenerating cells and intercellular spaces. The number of tissue spaces was dramatically increased in p18 mutant seminiferous tubules compared to the corresponding controls (243.74 ± 150.69 vs. 45 ± 30.4 (mean ± SD); p<0.000045) (Fig. 4b). This is also reflected by a significantly increased portion of the area per tubule which is covered by tissue spaces in the mutants (1.6% ± 1.9 vs. 0.4% ± 0.36 (mean ± SD); p<0.03) (Fig. 4c). However, the mean area per tissue space is not significantly different between the two groups (Fig. 4d). Ultrastructural analyses revealed that the small tissue spaces appeared to be mainly vacuoles present in the Sertoli cell cytoplasm (Fig. 3). These vacuoles were delimited by membranes and sometimes contained organelles. However, larger organelles like mitochondria, the Golgi apparatus and lysosomes appeared to be morphologically normal in mutant cells. Also the cytoplasm of neighboring germ cells appeared normal. Vacuoles could be found in the apical, middle, and basal part of Sertoli cells. As concluded from transmission electron microscopy, the blood-testis barrier seemed to be correctly established (Fig. 3b). In order to investigate whether the higher number of tissue spaces was caused by a higher number of apoptotic cells a TUNEL assay was performed on p18 testes of Sertoli cell-specific Klf4 knock out and control mice. The analyses revealed no statistically significant difference between the percentages of TUNEL positive tubules in mutant mice (40.34 ± 12.58 (mean ± SD)) versus controls (29.34 ±4.04 (mean ± SD); p > 0.223). Although we found morphological differences between mutant and wildtype testes we could not confirm developmental immaturity of the Sertoli cells as judged from expression of marker proteins (supplemental material Fig. 1–3). Anti Müllerian hormone (AMH) is a marker protein for immature Sertoli cells that is extinguished as the cells mature (Sharpe et al., 2003). However, this marker was not detectable after p15 by immunohistochemistry in neither mutant nor control testes, indicating that AMH expression is not affected by the lack of KLF4. We also analyzed expression of the androgen receptor and the cell cycle regulator p27kip1 (Sharpe et al., 2003). Both proteins are considered as markers for differentiated Sertoli cells. Again, we found no differences in the expression of both markers during postnatal testicular development (supplemental material Fig. 2 and 3). In adult testes we could not observe any differences with regard to vacuolization of the cytoplasm or (dis-) organization of the germinal epithelium (Fig. 2b). Hence, the phenotype described in the p18 testes is transient.
Figure 4.
Quantitative histological analyses of semi-thin sectioned tissues at postnatal day 18 (p18) in mutant (ko) and control (lox) mice. (a) Percentage (%) of normally structured (white bar) and disorganized tubules (black bar) in p18 testes from control (lox) and mutant (ko) mice. (b) Quantification of the number of tissue spaces per circular cross section in control (lox) and mutant (ko) testes. The mutant testes show a significantly (p > 0.000045) higher number of tissue spaces as defined in materials and methods. (c) Percentage (%) of the area covered by tissue spaces in the germinal epithelium of p18 control (lox) and mutant (ko) testes. A centrally located lumen was excluded from the calculation. The area of the germinal epithelium covered by tissue spaces is significantly (p < 0.03) higher in the mutants than in the controls. (d) The mean area of tissue spaces per circular tubule’s cross section in mutants and controls. Each black circle in this box plot diagram represents a data point. The mean area covered by each tissue space is not significantly (p < 0.203) different between mutants and controls. Mean values are shown as red circles, vertical bars indicate SD. Black squares depict outliers as classified by Minitab 15 software (State College PA, USA). (n) Number of analyzed circular tubule’s cross sections, (s) number of evaluated tissue samples. Results were expressed as the mean ± SD. P values are given in each figure and statistical significance is indicated by asterisks.
Significantly reduced testosterone levels in adult mutant mice
Testosterone (T) plays a crucial role during spermatogenesis, although the androgen receptor is expressed in the testis mainly in the somatic Sertoli cells, Leydig cells and peritubular myoid cells, but not in germ cells. Androgen receptor function in Sertoli cells is essential for complete spermatogenesis (Chang et al., 2004; De Gendt et al., 2004). We analyzed T concentrations in the serum of adult age matched control (n = 32) and mutant (n = 28) mice (age range 56–365 days). While the controls had average T concentrations of 21.12 ± 29.56 nMol/l (mean ± SD), the mutant mice displayed a significantly (p < 0.05) reduced average level of 8.69 ± 13.95 nMol/l (mean ± SD) (Tab.1). However, no differences could be observed in the expression of the androgen receptor as revealed by micorarray analysis (see below) and immunohistochemistry (supplemental material).
Follicle stimulation hormone (FSH) and triiodothyronine (T3) levels
Since Sertoli cell proliferation and maturation are dependent on the gonadotropin follicle stimulation hormone (FSH) (Dierich et al., 1998; Kumar et al., 1997; Sairam and Krishnamurthy, 2001) and since Klf4 itself is regulated by FSH (Hamil and Hall, 1994; McLean et al., 2002; Sadate-Ngatchou et al., 2004), we measured FSH at postnatal days 16, 18, 56, 84, 168, and 365 in mutant and control males (total n mutant = 37, total n controls = 40, more details see Tab.2). However, there were no significant differences between the FSH levels in mutant and control mice. Even the strong increase in FSH during puberty between p16 and p18 in the controls is paralleled in the mutant mice indicating that the endocrine feedback-loop between the Sertoli cell and the pituitary is unaffected in the mutants with respect to FSH. This is also corroborated by the fact that testicular inhibin mRNA levels were unchanged between the mutant and the control group as revealed by microarray analysis. In addition to FSH, we determined the levels of the hormone triiodothyronine (T3), which also influences Sertoli cell function (Buzzard et al., 2003; Cooke et al., 1994; Holsberger et al., 2003; Orth et al., 2000). However, T3 levels were almost the same between both groups (supplemental material Tab.3). Moreover, neither FSH- nor thyroid hormone receptor mRNA were changed between the mutant and the control group as judged from expression profiling (see below).
Microarray analysis revealed potential candidate genes for the observed phenotypes
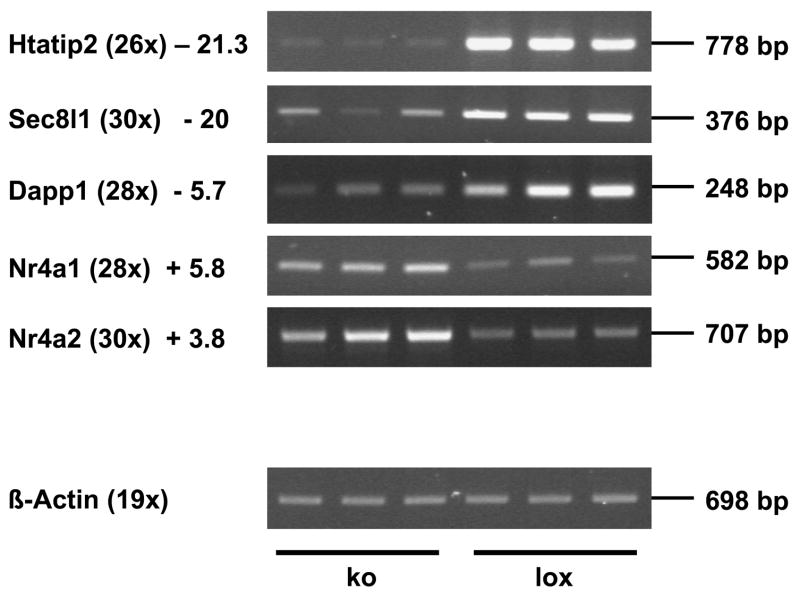
Since KLF4 is a transcription factor, we were interested in KLF4-dependent genes which can provide a link between the lack of KLF4 and the observed phenotype. We took 51 genes into detailed analyses that were significantly (more than 1.7-fold) up- or down-regulated in the absence of KLF4 (Tab.3 and Fig. 5). Two genes exhibited 20-fold reduced signal intensity: Htatip2 (=TIP30) and Sec8l1. Htatip2/TIP30 plays a role in tumor susceptibility (Ito et al., 2003) and possibly in the maintenance of the differentiated phenotype of epithelial cells (Lindfors et al., 2001). Sec8l1 is involved in directed vesicle transport, vesicle fusion and exocytosis. Interestingly, in the mutant mice there were two other genes down-regulated, which are also involved in vesicle transport: Hps5 (4.1-fold reduced) and Dapp1 (5.56-fold reduced). We identified also several genes that were up-regulated in response to the lack of KLF4. Interestingly, two nuclear hormone receptor family members, Nr4a1 (5.8-fold increased) and Nr4a2 (3.8-fold increased), which contain a zinc finger binding domain and are FSH-inducible in Sertoli cells (McLean et al., 2002), were among the genes exhibiting strongest up-regulation in the mutant Sertoli cells.
Figure 5.
Verification of the changed expression of selected genes in mutant testes by RT-PCR. Reduced expression of Htatip2, Sec8l1, and Dapp1 was confirmed as well as increased abundance of the mRNAs for the nuclear orphan receptors Nr4a1 and Nr4a2. β-Actin was used as internal control. Fold changes revealed by microarray analyses are shown next to the corresponding gene. Numbers in parentheses indicate the numbers of PCR cycles. Bp: base pair; ko: AMH-Cre/Klf4loxP/loxP; lox: control mice (Klf4loxP/loxP).
We performed an in silico search for putative binding sites for KLF4 (Garrett-Sinha et al., 1996; Shields and Yang, 1998) in a 1 kb region upstream of the transcriptional start site of the differentially expressed genes. The minimal essential binding site for KLF4 identified by Shields and Yang (1998), namely 5′-G/AG/AGGC/TGC/T-3′, and a 5′-CACCC-3′ motif described by Garrett-Sinha and colleagues (1996) were taken into account. We found between one and ten putative KLF4 binding sites in the promoter regions of Cd99l2, Dapp1, Hrmt1l3, Hsp5, Htatip2, Nr4a1, Nr4a2, and Sec8l1 suggesting a direct effect of the lack of KLF4 protein on the transcription of these genes (Tab. 4).
A very high proportion of differentially expressed genes are located on chromosome 7
Using the filter criteria mentioned above we detected 51 differentially expressed genes. Hence, in case of equipartition, only 2 to 3 genes per chromosome should be identified as differentially expressed due to the lack of KLF4. Unexpectedly, we found that 20 genes (39.2%) of the identified 51 genes were located on chromosome 7 and again 10 of these 20 genes (including Htatip2) were located in a small region (B3) on the long arm of chromosome 7 (Fig. 6). Interestingly a similar arrangement of HMRT1L3, HTATIP2, cDNA clone IMAGE:6514950 and the HPS5 gene in the mouse was observed in a subregion of human chromosome 11, emphasizing the possibility of a cluster regulation.
Figure 6.
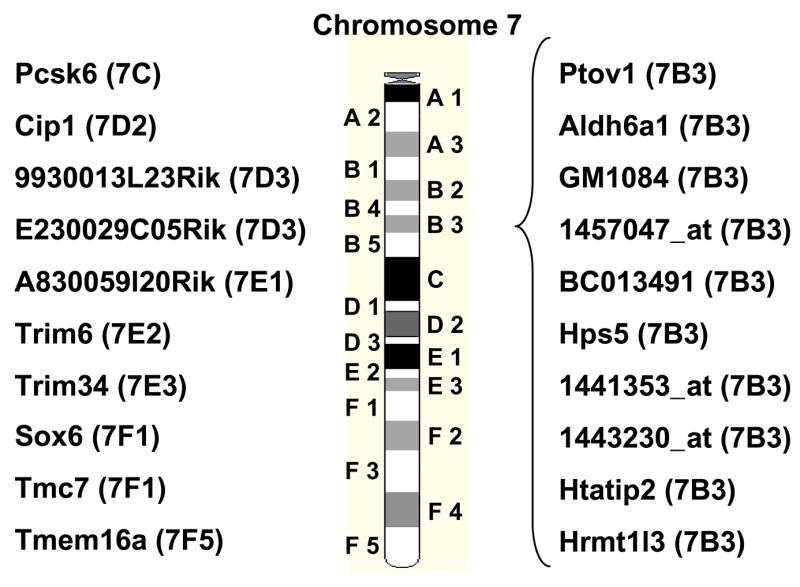
A very high proportion of regulated genes in mutant mice is localized on chromosome 7. Twenty of the 51 genes identified by microarray analysis are localized on chromosome 7 and, furthermore, 10 genes are localized within the small subregion B3. Subregions are shown in parentheses.
Discussion
Krüppel-like factor 4 is essential for normal development and function of several tissues and cell types. However, the role of KLF4 in testes is unknown, although this transcription factor is strongly expressed in the testis (Behr and Kaestner, 2002; Shields et al., 1996). KLF4 has been implicated in cell cycle control because of (i) its high expression in terminally differentiated epithelial cells and male postmeiotic germ cells, (ii) cell culture experiments and (iii) findings in Klf4 mutant and transgenic mice (Behr and Kaestner, 2002; Foster et al., 2005; Ghaleb et al., 2005; Katz et al., 2005; Rowland et al., 2005; Segre et al., 1999; Shields et al., 1996). Moreover, microarray analyses revealed that induced KLF4 expression is associated with up-regulation of many genes involved in cell cycle arrest and down-regulation of genes promoting the cell cycle (Chen et al., 2003). Interestingly, using expression profiling, we found almost no differentially expressed genes that are associated with cell cycle control indicating that KLF4 is not involved in G0 arrest in Sertoli cells. This indication is corroborated by the unchanged testicular weights and PCNA stainings. In contrast, we found several genes like Htatip2 (Ito et al., 2003; Lindfors et al., 2001), Sec8l1 (Rosse et al., 2006; Yeaman et al., 2004; Yeaman et al., 2001), Dapp1 (synonym Bam32) (Anderson et al., 2000), and Hps5 (Gautam et al., 2004; Wei, 2006), which are involved in the maintenance of the differentiated function and/or directed vesicle transport in polarized epithelial cells. We have shown that Klf4 mutant Sertoli cells exhibit extensive vacuolization (Fig. 3). It is conceivable that these Sertoli cells fail to accomplish normal exocytosis as a direct consequence of the strongly reduced levels of Sec8l1, Dapp1 and/or Hps5. The Sec6/8 complex is required for several steps in exocytotic transport of vesicles in other mammalian cells (Yeaman et al., 2001). Moreover, reduced apical secretion (due to reduced exocytosis) of the mutant Sertoli cells could explain the delayed lumen formation of the seminiferous tubules (Fig. 2). A related finding is the reduced expression of Dapp1, which is involved in actin remodeling (Allam et al., 2004). It is well established that actin remodeling plays an important role during exocytosis (Bader et al., 2004). The Hermansky-Pudlak-Syndrome (HPS) is a genetic disease characterized by impaired vesicle transport in e.g. melanocytes, thrombocytes, and pneumocytes. One of the genes that can be affected in Hermansky-Pudlak-Syndrome is Hps5, which is more than 4-fold down-regulated in the mutant Sertoli cells. Altogether, we have identified three genes, which are significantly down-regulated and have been described to be important for vesicle transport and exocytosis. Interestingly, strong vacuolization in colonic goblet cells lacking KLF4 was also observed, since these cells accumulated aberrantly formed secretory vacuoles in the apical portion (Katz et al., 2002). Also in the cornea the basal epithelial cells lacking KLF4 exhibited strong vacuolization (Swamynathan et al., 2007). It was also suggested that KLF4-deficient epidermis showed impaired secretion of lipids (Segre et al., 1999). Thus, it is likely that KLF4 in general is a regulator of genes involved in vesicle formation and transport in epithelial cells.
The expression of Htatip (synonyms TIP30 and CC3) is also associated with the differentiated state of epithelial cells. Htatip2 mutant mice exhibit high susceptibility to carcinogenesis, i.e. differentiated epithelial cells de-differentiate and become cancer cells (Ito et al., 2003). Moreover, Htatip2 was also identified in an in vitro screen for genes up-regulated during epithelial differentiation of an intestinal epithelial cell line (Lindfors et al., 2001). Taken together, our findings regarding gene expression changes in the mutant Sertoli cells support the view that KLF4 is mainly involved in achieving the fully differentiated, mature state of the polarized epithelial Sertoli cell and not in cell cycle control of these cells. Since it has been shown that mice lacking either, FSH (Kumar et al., 1997) or the FSH receptor (Dierich et al., 1998), exhibit reduced numbers of Sertoli cells, it is proven that FSH influences postnatal Sertoli cell proliferation. Therefore, it was first puzzling that FSH promotes both, expression of Klf4, a factor generally thought to induce G0 arrest, and Sertoli cell proliferation. However, after this study it is clear that the role of KLF4 in Sertoli cells is different from its role in gastric epithelial cells, which exhibit an increased proliferation rate in Klf4-mutant mice (Katz et al., 2005). In Sertoli cells the lack of KLF4 appears to exclusively affect differentiation of the epithelial Sertoli cell, but not proliferation.
We used unselected total testicular RNA samples to identify differentially expressed genes by micro array analyses. Therefore, we can not definitively exclude the possibility that the genes identified in this study were not expressed in Sertoli cells and that the differential abundance of the mRNAs was, at least in part, due to the lack of a specific germ cell population during the first spermatogenic wave. However, germ cells do not have - in contrast to Sertoli cells - a distinct secretory machinery. Hence, it is unlikely that the differentially expressed genes involved in secretion are expressed in germ cells. Again, using the whole testis as RNA source we can exclude obtaining differential mRNA levels due to differences in isolation and/or propagation of individual cell cultures. Using our approach we assured that the results obtained are indeed based on biological effects and not on cell culture artefacts.
FSH-R knockout (FORKO) mice exhibit a phenotype (Grover et al., 2004) which is in part similar to that seen in Klf4 mutant Sertoli cells. FORKO mice also have big dilations and vacuoles in the Sertoli cell cytoplasm delimited by plasma membranes. However, all organelles appeared normal on the electron microscopic level. Since Klf4 is FSH-inducible and because of the high grade of vacuolization also seen in Klf4-deficient Sertoli cells it is possible that insufficient KLF4 levels in the FORKO mice can, at least in part, contribute to the phenotype of these mice.
While Klf4 is essential for normal development and function of several cell types, it is dispensable for basal Sertoli cell function as Sertoli cells lacking Klf4 are still able to support spermatogenesis, and the mice are fertile. This might reflect a certain back up mechanism of the germ line-supporting Sertoli cells. Indeed, there are several papers reporting that the lack of proteins, which are highly or even specifically (e.g. the FSH receptor) expressed in Sertoli cells, does not prevent spermatogenesis and that these mice were still fertile (Bailey et al., 2002; Dierich et al., 1998; Lindeboom et al., 2003; Lu et al., 1999; Matzuk et al., 1992; Pitman et al., 1998; Sylvester and Griswold, 1994). So far, there are according to our knowledge only few “Sertoli cell genes” which are essential for adult Sertoli cell function and fertility: the androgen receptor (Chang et al., 2004; De Gendt et al., 2004; Holdcraft and Braun, 2004), ERM (Chen et al., 2005), Peroxisomal multifunctional protein 2 (Huyghe et al., 2006), and Connexin43 (Brehm et al., 2007; Sridharan et al., 2007; Winterhager et al., 2007). All other genes investigated so far appear not to be absolutely essential for basal Sertoli cell function. Compensatory up-regulation (Hummler et al., 1994) and/or functional equivalence (Lee et al., 2005) of genes are well-known mechanisms to overcome the problems of the lack of a single gene. We suspect that significant up-regulation of the FSH-inducible nuclear orphan receptors Nr4a1 and Nr4a2 (McLean et al., 2002) (supporting tables therein) might compensate the loss of Klf4. It is striking that (i) Klf4 as well as Nr4a1 and Nr4a2 are strongly FSH-inducible exhibiting comparable induction kinetics, i.e. 20–40-fold induction after 2 hours followed by a quick decline, (ii) all factors contain zinc finger DNA-binding domains and (iii) that Nr4a1 as well as Nr4a2 are up-regulated in response to the lack of KLF4.
The interstitial Leydig cells are the main site of testosterone production in the male. There are many different interactions between the different types of somatic cells within the testis to maintain a normal intratesticular milieu. Interestingly, the lack of Klf4 in Sertoli cells reduces serum testosterone levels in adult males (older than 56 days) to less than 50% of the control value. This may indicate that in the adult SC-Klf4-ko mice the direct intratesticular communication between the Sertoli cells and the Leydig cells might be affected, although adult testicular function appears to be normal in mutant mice with respect to histology and fertility. Detailed future studies on the adult SC-Klf4-ko testis might reveal whether these mice exhibit impaired paracrine communication between the Sertoli cells and Leydig cells or whether the reduced serum testosterone levels are due to an as yet unidentified endocrinological imbalance in SC-Klf4-ko mice. Serum T levels in younger mice could not be measured due to the large volume of serum needed to perform a reliable determination.
The mouse chromosome 7 contains 1408 known genes (6.3% of all identified mouse genes). Interestingly, 39% of all genes (20 out of 51) identified as differentially expressed in Klf4 mutant Sertoli cells were located on chromosome 7 indicating a disproportionally high subset of KLF4-affected genes on this chromosome. Ten of these 20 affected genes on chromosome 7 were located in the small subregion B3. This could suggest that a whole cluster of genes in this B3 region of chromosome 7 is regulated by KLF4, further strengthening the idea that KLFs may function generally as switches for gene clusters within activated regions of chromosomes. Cluster regulation of a number of genes by KLF4 has previously been described by Segre and colleagues (Patel et al., 2003; Segre et al., 1999) and Chen et al. (Chen et al., 2003). Moreover, several human homologs of the KLF4-regulated genes on mouse chromosome 7 can be found highly conserved in their arrangement on human chromosome 11.
In conclusion, we showed that Klf4 plays an important role during functional maturation of testicular Sertoli cells. Expression profiling revealed plausible molecular links between the lack of KLF4 and the observed phenotype in Sertoli cells thereby contributing to our understanding of postnatal development of the germinal epithelium in mice. In general, KLF4 appears to regulate genes involved in vesicle transport and exocytosis. However, it remains to be elucidated why KLF4 regulates cell cycle genes in many experimental settings but not in Sertoli cells.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Be 2296/4) to RB and NIH grant DK053839 to KHK.
We appreciate the support of Dr. R. Renkawitz-Pohl, University of Marburg, and Dr. H.-W. Denker, Institute of Anatomy, University of Duisburg-Essen. We thank Dr. Ludger Klein-Hitpass, BioChip Laboratory, University of Duisburg-Essen Medical School for performing microarray hybridization and his excellent assistance in statistical analyses, Dr. M. Bergmann and Dr. K. Militzer for helpful discussions, D. Schünke for assistance with electron microscopy and R. Sandhowe-Klaverkamp for performing the testosterone and FSH assays. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Be 2296/4) to RB and NIH grant DK053839 to KHK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allam A, Niiro H, Clark EA, Marshall AJ. The adaptor protein Bam32 regulates Rac1 activation and actin remodeling through a phosphorylation-dependent mechanism. J Biol Chem. 2004;279:39775–82. doi: 10.1074/jbc.M403367200. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Lipp P, Bootman M, Ridley SH, Coadwell J, Ronnstrand L, Lennartsson J, Holmes AB, Painter GF, Thuring J, Lim Z, Erdjument-Bromage H, Grewal A, Tempst P, Stephens LR, Hawkins PT. DAPP1 undergoes a PI 3-kinase-dependent cycle of plasma-membrane recruitment and endocytosis upon cell stimulation. Curr Biol. 2000;10:1403–12. doi: 10.1016/s0960-9822(00)00794-6. [DOI] [PubMed] [Google Scholar]
- Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S. Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta. 2004;1742:37–49. doi: 10.1016/j.bbamcr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–53. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- Bartlett JM, Weinbauer GF, Nieschlag E. Differential effects of FSH and testosterone on the maintenance of spermatogenesis in the adult hypophysectomized rat. J Endocrinol. 1989;121:49–58. doi: 10.1677/joe.0.1210049. [DOI] [PubMed] [Google Scholar]
- Behr R, Kaestner KH. Developmental and cell type-specific expression of the zinc finger transcription factor Kruppel-like factor 4 (Klf4) in postnatal mouse testis. Mech Dev. 2002;115:167–9. doi: 10.1016/s0925-4773(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lecureuil C, Steger K, Konrad L, Biermann K, Failing K, Bergmann M. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–81. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Wreford NG, Morrison JR. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat sertoli cells. Endocrinology. 2003;144:3722–31. doi: 10.1210/en.2003-0379. [DOI] [PubMed] [Google Scholar]
- Chandolia RK, Weinbauer GF, Simoni M, Behre HM, Nieschlag E. Comparative effects of chronic administration of the non-steroidal antiandrogens flutamide and Casodex on the reproductive system of the adult male rat. Acta Endocrinol (Copenh) 1991;125:547–55. doi: 10.1530/acta.0.1250547. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–81. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary J, Skinner MS. Transcription Factors in Sertoli Cells. Sertoli Cell Biology. 2005:251–280. [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–77. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Zhao YD, Bunick D. Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod. 1994;51:1000–5. doi: 10.1095/biolreprod51.5.1000. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Skinner MK. Embryonic Sertoli Cell Differentiation. Sertoli Cell Biology. 2005:43–70. [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–32. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–7. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durig J, Nuckel H, Huttmann A, Kruse E, Holter T, Halfmeyer K, Fuhrer A, Rudolph R, Kalhori N, Nusch A, Deaglio S, Malavasi F, Moroy T, Klein-Hitpass L, Duhrsen U. Expression of ribosomal and translation-associated genes is correlated with a favorable clinical course in chronic lymphocytic leukemia. Blood. 2003;101:2748–55. doi: 10.1182/blood-2002-09-2683. [DOI] [PubMed] [Google Scholar]
- Ellis PJ, Furlong RA, Wilson A, Morris S, Carter D, Oliver G, Print C, Burgoyne PS, Loveland KL, Affara NA. Modulation of the mouse testis transcriptome during postnatal development and in selected models of male infertility. Mol Hum Reprod. 2004;10:271–81. doi: 10.1093/molehr/gah043. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. Embo J. 2007;26:4138–48. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, Ruppert JM. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–95. [PubMed] [Google Scholar]
- Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK, Frost AR, Louro ID, Townes TM, Paterson AJ, Kudlow JE, Lobo-Ruppert SM, Ruppert JM. Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia. Oncogene. 2005;24:1491–500. doi: 10.1038/sj.onc.1208307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–90. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- Gautam R, Chintala S, Li W, Zhang Q, Tan J, Novak EK, Di Pietro SM, Dell’Angelica EC, Swank RT. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) J Biol Chem. 2004;279:12935–42. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92–6. doi: 10.1038/sj.cr.7290271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godmann M, Kromberg I, Mayer J, Behr R. The mouse Kruppel-like Factor 4 (Klf4) gene: four functional polyadenylation sites which are used in a cell-specific manner as revealed by testicular transcript analysis and multiple processed pseudogenes. Gene. 2005;361:149–56. doi: 10.1016/j.gene.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Grover A, Sairam MR, Smith CE, Hermo L. Structural and functional modifications of sertoli cells in the testis of adult follicle-stimulating hormone receptor knockout mice. Biol Reprod. 2004;71:117–29. doi: 10.1095/biolreprod.103.027003. [DOI] [PubMed] [Google Scholar]
- Hamil KG, Hall SH. Cloning of rat Sertoli cell follicle-stimulating hormone primary response complementary deoxyribonucleic acid: regulation of TSC-22 gene expression. Endocrinology. 1994;134:1205–12. doi: 10.1210/endo.134.3.8161377. [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–67. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O’Brien DA, Hess RA, Franca LR, Kiyokawa H, Cooke PS. Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod. 2005;72:1429–36. doi: 10.1095/biolreprod.105.040386. [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Jirawatnotai S, Kiyokawa H, Cooke PS. Thyroid hormone regulates the cell cycle inhibitor p27Kip1 in postnatal murine Sertoli cells. Endocrinology. 2003;144:3732–8. doi: 10.1210/en.2003-0389. [DOI] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci U S A. 1994;91:5647–51. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe S, Schmalbruch H, De Gendt K, Verhoeven G, Guillou F, Van Veldhoven PP, Baes M. Peroxisomal multifunctional protein 2 is essential for lipid homeostasis in Sertoli cells and male fertility in mice. Endocrinology. 2006;147:2228–36. doi: 10.1210/en.2005-1571. [DOI] [PubMed] [Google Scholar]
- Ito M, Jiang C, Krumm K, Zhang X, Pecha J, Zhao J, Guo Y, Roeder RG, Xiao H. TIP30 deficiency increases susceptibility to tumorigenesis. Cancer Res. 2003;63:8763–7. [PubMed] [Google Scholar]
- Jiang C, Xuan Z, Zhao F, Zhang MQ. TRED: a transcriptional regulatory element database, new entries and other development. Nucleic Acids Res. 2007;35:D137–40. doi: 10.1093/nar/gkl1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, Furth EE, Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–45. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–28. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A. 2007;104:16558–63. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaewsongkram J, Yang Y, Golech S, Katz J, Kaestner KH, Weng NP. Kruppel-like factor 4 regulates B cell number and activation-induced B cell proliferation. J Immunol. 2007;179:4679–84. doi: 10.4049/jimmunol.179.7.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–4. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Lecureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis. 2002;33:114–8. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–7. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Miyashiro L, Skinner MK. Role of transforming growth factor-alpha and the epidermal growth factor receptor in embryonic rat testis development. Biol Reprod. 2000;62:477–90. doi: 10.1095/biolreprod62.3.477. [DOI] [PubMed] [Google Scholar]
- Lindeboom F, Gillemans N, Karis A, Jaegle M, Meijer D, Grosveld F, Philipsen S. A tissue-specific knockout reveals that Gata1 is not essential for Sertoli cell function in the mouse. Nucleic Acids Res. 2003;31:5405–12. doi: 10.1093/nar/gkg723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindfors K, Halttunen T, Kainulainen H, Maki M. Differentially expressed CC3/TIP30 and rab11 along in vivo and in vitro intestinal epithelial cell crypt-villus axis. Life Sci. 2001;69:1363–72. doi: 10.1016/s0024-3205(01)01216-4. [DOI] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–8. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–9. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Friel PJ, Pouchnik D, Griswold MD. Oligonucleotide microarray analysis of gene expression in follicle-stimulating hormone-treated rat Sertoli cells. Mol Endocrinol. 2002;16:2780–92. doi: 10.1210/me.2002-0059. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Ohnami S, Laub F, Aoki K, Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F, Yoshida T. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–6. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Orth JM, Jester WF, Li LH, Laslett AL. Gonocyte-Sertoli cell interactions during development of the neonatal rodent testis. Curr Top Dev Biol. 2000;50:103–24. doi: 10.1016/s0070-2153(00)50006-4. [DOI] [PubMed] [Google Scholar]
- Patel S, Kartasova T, Segre JA. Mouse Sprr locus: a tandem array of coordinately regulated genes. Mamm Genome. 2003;14:140–8. doi: 10.1007/s00335-002-2205-4. [DOI] [PubMed] [Google Scholar]
- Pitman JL, Lin TP, Kleeman JE, Erickson GF, MacLeod CL. Normal reproductive and macrophage function in Pem homeobox gene-deficient mice. Dev Biol. 1998;202:196–214. doi: 10.1006/dbio.1998.8978. [DOI] [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol. 2006;26:727–34. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–82. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Follicle-stimulating hormone induced changes in gene expression of murine testis. Mol Endocrinol. 2004;18:2805–16. doi: 10.1210/me.2003-0203. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res. 2001;32:601–8. doi: 10.1016/s0188-4409(01)00328-9. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–60. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–17. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Yang VW. Identification of the DNA sequence that interacts with the gut-enriched Kruppel-like factor. Nucleic Acids Res. 1998;26:796–802. doi: 10.1093/nar/26.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76:804–12. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–94. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester SR, Griswold MD. The testicular iron shuttle: a “nurse” function of the Sertoli cells. J Androl. 1994;15:381–5. [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, Davids JA, de Rooij DG. Postnatal development of testicular cell populations in mice. J Reprod Fertil. 1993;99:479–85. doi: 10.1530/jrf.0.0990479. [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–43. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Winterhager E, Pielensticker N, Freyer J, Ghanem A, Schrickel JW, Kim JS, Behr R, Grummer R, Maass K, Urschel S, Lewalter T, Tiemann K, Simoni M, Willecke K. Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev Biol. 2007;7:26. doi: 10.1186/1471-213X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–70. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Xuan Z, Liu L, Zhang MQ. TRED: a Transcriptional Regulatory Element Database and a platform for in silico gene regulation studies. Nucleic Acids Res. 2005;33:D103–7. doi: 10.1093/nar/gki004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.