Abstract
We report the characterization of a naturally occurring polymorphism in CD40 ligand (CD40L, CD154) expressed by activated T cells from a young female patient. This polymorphism encodes a nonconservative Gly → Arg substitution in amino acid 219 in the extracellular, CD40 binding domain of the molecule. Studies carried out with 293 epithelial cells ectopically expressing the polymorphic protein (CD154/G219R) revealed reduced levels of binding to different anti‐CD154 monoclonal antibodies (mAb) and CD40‐immunoglobulin (CD40‐Ig). However, recognition of the polymorphic and wild‐type CD154 molecules by a polyclonal antiserum was comparable, suggesting that the polymorphism affects the ability of the protein to interact with CD40 but does not significantly alter its surface expression. To determine if reduced cross‐linking of CD40 mediated decreased functional effects, three CD40‐dependent properties were measured. We found that pathways leading to the induction of surface CD23, CD80, and Iγ transcription were activated in response to CD154/G219R signalling. However, the decrease in affinity for CD40 by the mutated CD154 affected the ability of CD40‐Ig to efficiently interfere with the binding and effectively block induced CD80 expression. In contrast, we found that the 5c8 mAb, which recognized the polymorphic molecule to a similar extent as wild‐type CD154, effectively blocked the interaction between CD154/G219R and CD40 as measured by CD80 expression. These findings suggest that naturally occurring polymorphisms in the CD154 molecule may affect the ability of CD40‐mediated functions to be blocked by soluble CD40 or anti‐CD154 mAb in the therapeutic treatment of disease and graft rejection.
Introduction
The interaction of CD154 (CD40 ligand) expressed on activated CD4+ T cells with CD40 expressed on B cells is essential for the development of a humoral immune response against thymus‐dependent (TD) antigens (reviewed in 1). The absolute requirement for CD40 signalling in humoral immunity has been demonstrated in animal models lacking either functional CD40 2 or CD154, 3 and in humans suffering from X‐linked hyper‐immunoglobulin M (IgM) (HIM) syndrome [reviewed in 4]. In these instances there is a clear absence of isotype switching in B cells, a lack of germinal centres, and deficient primary and secondary responses to TD antigens.
T‐cell activation requires both T‐cell receptor (TCR)‐mediated signals and costimulatory signals that are provided at least in part by the T‐cell‐associated CD28 molecule when bound to its counter receptors CD80 (B7‐1) or CD86 (B7‐2) on antigen‐presenting cells (APCs). 5,6 The interaction of CD40 and CD154 plays an important role in T‐cell activation by up‐regulating the costimulatory molecules CD80/CD86 on B cells and other APCs (reviewed in 7). Several groups have shown that T‐cell activation can be blocked by treatment with CTLA4‐immunoglobulin (CTLA4‐Ig), a receptor for CD80/CD86. 8–10 Additionally, in different mouse models of T‐cell‐mediated autoimmune disease, inhibiting CD154 signalling by blocking antibodies resulted in the arrest of collagen‐induced arthritis, experimental allergic encephalomyelitis (EAE), lupus nephritis, colitis, and oophoritis (reviewed in 11). The role of CD154 in regulating these diseases is believed to be primarily mediated through up‐regulating costimulatory molecules on APCs. Similarly, anti‐CD154 antibodies and CTLA4‐Ig have been used to abrogate graft rejection in mice 12,13 and primates. 14 Thus, the importance of inhibiting CD154‐dependent signalling events in B cells and other APCs cannot be underestimated in abrogating the onset of disease and in the prevention of allograft rejection.
In this report we have characterized a polymorphism in the CD154 gene isolated from a young female child, GP. This paternally inherited polymorphism results in a Gly to Arg substitution at amino acid 219 and has previously been shown to occur at a very low frequency in the Caucasian population. 15 Structural and functional properties of the CD154/G219R were examined to determine if the polymorphism altered recognition and signalling by CD40. Our data revealed a reduction in binding to the CD40 molecule by the CD154/G219R protein compared to wild type protein. However, this binding did initiate downstream functions that resulted in the induction of CD23 and CD80 as well as germline Iγ transcripts in Ramos B cells with no substantial difference in response to the wild‐type or polymorphic molecule. Finally, experiments were performed to test the ability of blocking antibodies or recombinant CD40 to block the induced CD80 response of activated Ramos B cells. In accordance with our findings of a difference in binding affinity between the polymorphic and wild‐type proteins to CD40, we observed a difference in the extent of CD80 down‐modulation by the blocking reagent CD40‐immunoglobulin (CD40‐Ig) in Ramos B cells activated with the CD154/G219R protein.
Materials and methods
Cell lines and antibodies
The human B‐cell lymphoma clone Ramos 2G6·4CN3F10 (Ramos 2G6) is an interleukin‐4 (IL‐4) responsive subclone of RA‐1 16 and has been previously described. 17 293 cells are available from American Type Culture Collection (ATCC, Rockville, MD). OKT8 (ATCC) was used as a fluorosceinated conjugate in fluorescence‐activated cell sorting (FACS) analyses. Anti‐TRAP antibodies (both mouse monoclonal and rabbit polyclonal sera) and the CD40‐Ig fusion protein CD40 have been described previously. 18 The anti‐T cell/B cell activating molecule (T‐BAM) monoclonal antibody (mAb) 5c8, has been previously shown to be a blocking antibody for CD154 functions. 19 The fluoroscein isothiocyanate (FITC)‐labelled and unlabelled anti‐CD154 mAb (24‐31), the FITC‐labelled anti‐CD23 mAb, the biotinylated mouse anti‐human CD20, and streptavidin R–phycoerythrin (PE) were all purchased from Ancell (Bayport, MN). FITC‐labeled F(ab′)2 goat anti‐mouse IgG + IgM (Jackson Immunoresearch Lab, PA), FITC‐labelled goat anti‐rabbit IgG F(ab′)2 (Sigma Chemical Co., St Louis, MO), and FITC‐labelled goat anti‐human IgG F(ab′)2 (Sigma) were used as secondary antibodies with mAb anti‐TRAP, polyclonal anti‐TRAP, and CD40‐Ig, respectively.
Isolation of GP’s cDNA and identification of the Gly219 → Arg219 polymorphism
Peripheral blood mononuclear cells (PBMC) were obtained from patient and control blood using Ficoll–Hypaque (Sigma). Cells (106) were activated with phorbol 12‐myristate 13‐acetate (PMA; 20 ng/ml) and ionomycin (1 mg/ml) (Sigma) in 3 ml of culture media for 5 hr. T cells were isolated by sheep red blood cell (SRBC) rosetting and CD4+ T cells were purified by treatment of T cells with OKT8 (ATCC), followed by negative depletion using magnetic beads coated with goat anti‐mouse immunoglobulin (Advanced Magnetics, Cambridge, MA). CD4+ T cells were activated with PMA and ionomycin as described above.
Poly A+ RNA was isolated from activated CD4+ T cells using the FastTract kit of Invitrogen (San Diego, CA). cDNA was prepared as described previously. 20 To isolate the CD154/G219R coding region polymerase chain reaction (PCR) was carried out using primers specific for the 5′ and 3′ regions of the coding region. Primers used were (sense oligo) CD40L40‐60: 5′‐CACAGCATGATCGAAACATAC‐3′ and (antisense oligo) CD40L807–830: 5′‐CAGAGAGTTTGAGTAAGCCAAAGGAC‐3′ (all numbering according to 21). Amplification was as previously described. 20 The PCR products were subcloned and sequenced according to standard protocols. For direct sequencing of DNA, high molecular weight genomic DNA was isolated from GP using standard methods. A region spanning the identified mutation was amplified by PCR using oligonucleotide primers hCD40L.563‐583 (5′‐CCCAAGTCACCTTCTGTTCCA‐3′) and hCD40L.834 805 (5′‐aaggTGTTCAGAGTTTGAGTA AGCCAAAGGACGT‐3′). Reaction conditions were as described previously 20 with cycling parameters of 1 min at 94°, 1·5 min at 58°, 2 min at 72° for 30 cycles. Asymmetric PCR was carried out on the eluted fragment using 1 µl of the first‐round PCR product, 30 pmol of primer 834,1 pmol of primer 563, following the above cycling parameters. Following purification, the single‐stranded product was sequenced using standard protocols.
Transfection of 293 cells
Wild‐type and G219R CD154 cDNAs containing the coding region and 300 bp of 3′ untranslated region (3′UTR) were cloned into the pcDNA3 expression vector (Invitrogen). 293 cells (5 × 105) were cotransfected with 20 µg of each CD154 plasmid and 5 µg of pCDM8 (containing the human CD8 cDNA) using calcium phosphate. Media was replaced after 14 hr and cells analysed by FACS for CD154 and CD8 expression 48 hr later.
Cytofluorographic analysis of CD154 expression in 293 transfectants
Mock‐transfected 293 cells (1 × 105) or 293 transfectants were incubated with saturating concentrations of either monoclonal or polyclonal anti‐CD154 antibodies according to previously published protocols. 22
Ramos B‐cell cocultures: up‐regulation of surface CD23 and CD80
Mock transfected 293 cells (5 × 104) or 293 cells transiently transfected with the CD154 plasmids were harvested 24 hr post‐transfection and replated with 2 × 105 Ramos B cells. Cocultures were established in 1·0 ml RPMI/10% fetal bovine serum (FBS) in 24‐well plates at 37°, 5% CO2. In blocking experiments, 5 µg/ml of CD40‐Ig, 5c8 mAb, 24‐31 mAb, or isotype‐control antibodies were added to transfected or mock‐transfected cells in 96‐well plates for 1 hr at 37° prior to addition of Ramos B cells. After 24 hr, cells were harvested and assayed for CD80 up‐regulation by incubating with saturating amounts of biotin‐conjugated mouse anti‐human CD20 mAb and FITC‐labelled mouse anti‐human CD80 mAb or matching isotype controls followed by streptavidin–PE. Cells were analysed for two‐colour expression by FACS.
Identification of Iγ transcripts
Ramos B cells (2 × 105) were cultured for 2 days with either 0·5 × 106 transfected (293/G219R or 293/wt) 293 cells or 293 cells alone in the presence or absence of 200 U/ml IL‐4 (Peprotech, Rocky Hill, NJ). RNA isolation, PCR amplification, and identification of transcripts have been previously described. 22
Results
Identification of a naturally occurring polymorphism in one allele of the CD154 gene
In analysing the T‐cell responses of a young girl displaying non‐X‐linked HIM we isolated mRNA from activated T cells and used reverse transcription (RT)–PCR to analyse CD154 expression. After amplification of the CD154 coding region, a single band of approximately 800 bp was isolated, subcloned, and independent clones sequenced. Comparison of the coding region with the published sequence for CD154 revealed both a wild‐type (CD154/wt) and polymorphic form (CD154/G219R) of the expressed sequence (Fig. 1a). The polymorphic sequence contained a single base conversion of a G → A at nucleotide position 655 resulting in a non‐conservative substitution of Arg for Gly at amino acid 219 in the extracellular domain (Fig. 1c). This nucleotide change was confirmed at the genomic level by amplifying the corresponding region using asymmetric PCR and sequencing the single‐stranded products. As can be seen in Fig. 1(b), both a G and an A at position 655 were identified in DNA from the patient. Analysis of the patient’s healthy parents revealed that the polymorphism was paternally inherited (data not shown).
Figure 1.
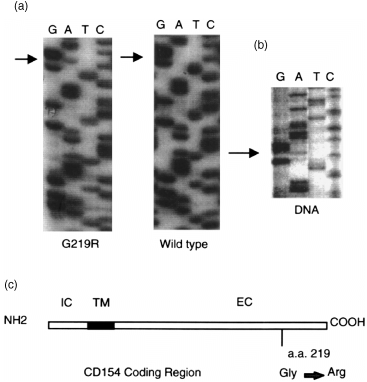
Both a wild‐type and polymorphic form of the CD154 protein are expressed in activated T cells. mRNA was isolated from activated CD4+ T cells, reverse transcribed, and analysed for CD154 expression by PCR. Sequence patterns in (a) are from different cDNA clones showing the region immediately 5′ and 3′ of the polymorphism at nucleotide position 655. (b) Total DNA from GP was amplified by asymmetric PCR and sequenced. The sequence reveals both a G and A at position 655. (c) Schematic representation of the location of the polymorphism at aa 219 in the extracellular domain of CD154. IC = intracellular domain; TM = transmembrane domain; EC = extracellular domain. Numbering of amino acid residues is according to Spriggs et al. 21
Binding of the CD154/G219R to CD40 and anti‐CD154 mAb is reduced compared with CD154/wt
To examine if the G219R change affected binding of CD154 to CD40, the polymorphic and wild‐type sequences were subcloned into the expression vector pCDNA.3. Transfection constructs containing either the CD154/G219R cDNA or the CD154/wt cDNA were used in transient transfection assays of 293 cells and assayed for CD154 surface expression by binding to a CD40‐Ig fusion protein. 23 To control for differences in transfection efficiency within a particular experiment, we cotransfected a plasmid containing a human CD8 cDNA (pCDM8) and normalized the CD154 expression by dividing percentage of CD154+ cells by the percentage of CD8+ cells. Our results of multiple independent transfections revealed a 25% decrease in binding of the G219R protein to CD40‐Ig compared to wild‐type binding that was statistically significant (Fig. 2a, panel 1, P < 0·02). To establish whether this difference reflected a change in the CD40 binding pocket of the CD154/G219R, or a defect in surface expression of this protein, we evaluated the transfectants for binding to both polyclonal and monoclonal antibodies.
Figure 2.
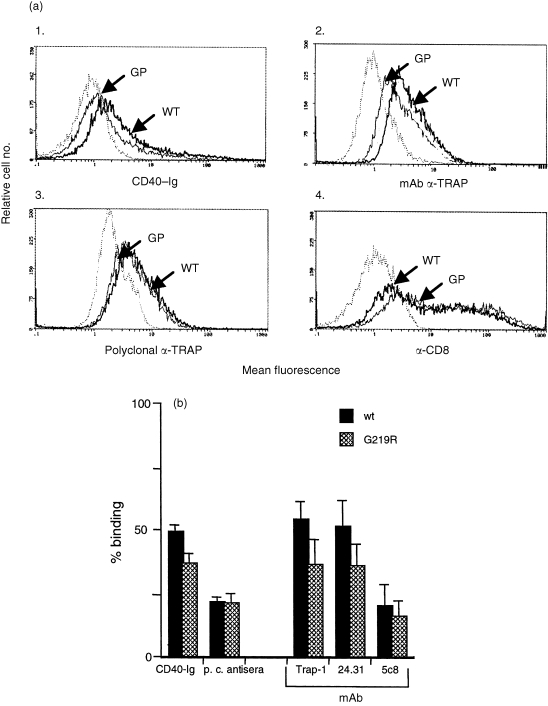
Binding profiles of CD154/wt and CD154/G219R proteins to different reagents. (a). 293 cells were transiently transfected with 20 µg CD154/wt or CD154/G219R plus 5 µg pMCD8. Equal numbers of cells were analysed 48 hr later for expression of CD154 by staining with CD40‐Ig (panel 1), anti‐TRAP mAb (panel 2), and a rabbit polyclonal anti‐TRAP antiserum (panel 3). Cells were also stained with FITC‐labelled OKT8 to control for transfection efficiency (panel 4). Dotted peaks in each panel represent background staining with either isotype control mAb (panels 2 and 4) or secondary antibodies alone (panels 1 and 3). (b). Data was analysed from multiple (> 3) independent transfections to establish the level of recognition of the CD154/G219R protein by the different reagents. The transfection efficiency within an experiment was normalized to the expression of CD8. Error bars represent SEM. Differences in recognition of CD154/G219R and CD154/wt by CD40‐Ig, mAb anti‐TRAP, and mAb 24‐31, were determined to be statistically significant to a 95% confidence level by a standard paired t‐test (P < 0·02). The difference in binding for polyclonal anti‐TRAP and mAb 5c8 to either CD154/G219R or CD154/wt was determined not to be significant (P < 0·8 and P < 0·33, respectively).
Using a rabbit polyclonal anti‐CD154 antisera we observed no significant difference in binding over several experiments. In some individual experiments we did detect a slight reduction in binding to the G219R/CD154 molecule but in others we detected no difference at all (Fig. 2a, panel 3). We also analysed the binding to three different mAbs, TRAP‐1 (which has previously been shown to bind outside of CD40 binding pocket; 4 5c8 mAb 24 and a commercially available mAb, 24‐31. With all mAbs tested we observed reduced binding to the CD154/G219R compared to wild‐type CD154 (Fig. 2a, b). However, this difference was particularly obvious with the anti‐TRAP‐1 and 24‐31 mAbs that revealed a significant and reproducible decrease in binding of 34 and 30%, respectively. In contrast, the 4% difference in the recognition of the two proteins by 5c8 mAb did not approach statistical significance.
Functional studies with CD154/G219R
To establish if the change in CD40 binding by the CD154/G219R affected downstream functions we analysed the up‐regulation of CD23 and CD80 on Ramos B cells stimulated with 293 cells transiently transfected with either constructs containing the wild type or G219R CD154 cDNAs. Using transfectants in which the expression level was very similar, we found no qualitative difference in the expression of CD23 or CD80 on Ramos B cells after stimulation for 24 hr with either CD154/G219R or CD154/wt (a representative experiment is shown in Fig. 3a). However, we did observe a slight, but reproducible, reduction in expression of both CD23 and CD80 in cells stimulated with the CD154/G219R compared to CD154/wt (compare panels 2 and 3 and panels 5 and 6).
Figure 3.
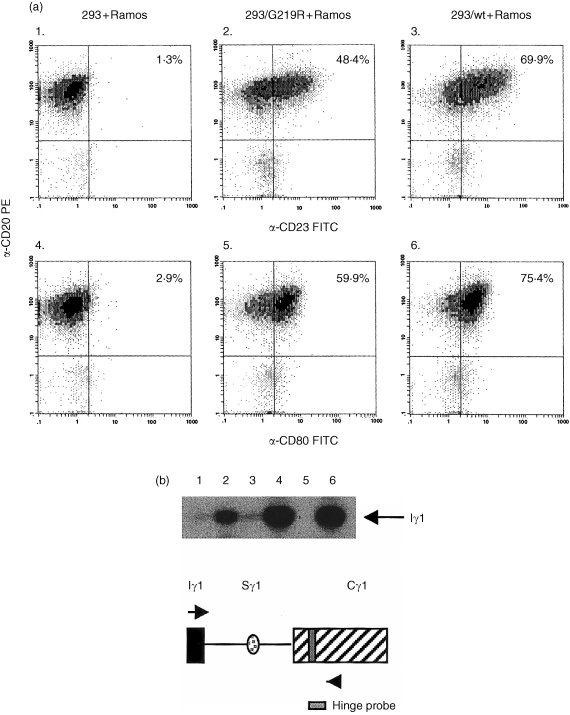
(a) Up‐regulation of CD23 and CD80 as a consequence of CD154/wt or CD154/G219R signalling. Shown are two‐colour histograms of Ramos B cells stimulated with mock transfected or 293 cells transiently transfected with either CD154/G219R or CD154/wt. Cocultures were established with a B cell to stimulator cell ratio of 4 : 1 for 24 hr prior to staining with biotin‐conjugated anti‐CD20 mAb, followed by streptavidin–PE and FITC‐conjugated anti‐CD23 mAb or FITC‐conjugated anti‐CD80 mAb. Numbers in the upper right hand quadrant of each histogram refer to the percentage of CD20 cells that are also positive for CD23 or CD80 expression. (b). Effects of CD154/wt and CD154/G219R signalling on Iγ transcription. Ramos B cells (5 × 105) were incubated for 2 days with an equal number of 293 cells transiently transfected, 293/G219R, or 293/wt in the presence or absence of IL‐4. B cells were assayed for Iγ expression by RT–PCR using Iγ‐ and Cγ‐specific primers that recognize Iγ transcripts of all subclasses and hybridization to a γ1‐specific hinge probe (arrows in schematic drawing indicate approximate location of PCR primers). Shown is the expression of Iγ1 transcripts isolated from Ramos B cells incubated under the following conditions: Lane 1, 293 cells; Lane 2, 293 + IL‐4; Lane 3, 293/G219R cells; Lane 4, 293/G219R + IL‐4; Lane 5, 293/wt; Lane 6, 293/wt + IL‐4.
To measure the effect of the CD154/G219R polymorphism on an early signal for switch recombination, Ramos B cells were assayed for the expression of Iγ transcripts in response to CD154 signalling in the presence or absence of IL‐4 for 2 days (Fig. 3b). Total RNA from cocultures established with transiently transfected 293 cells and Ramos B cells was subjected to RT–PCR to amplify germline Iγ transcripts using primers homologous to the Iγ and CH2Cγ regions of all four γ subclasses. Resulting products were hybridized to a Cγ1 hinge region‐specific probe to identify Iγ1‐specific transcription (Fig. 3b). Under these conditions of stimulation we observed clear induction of germline transcripts in cultures stimulated with IL‐4 alone (Fig. 3b, lane 2) and an induction over this level in IL‐4 cultures stimulated with either CD154/G219R or CD154/wt (lanes 4 and 6, respectively). These experiments were repeated with CD19+/IgM+/IgG– B cells and again we observed no difference in the induction of Iγ with either CD154/G219R or CD154/wt (data not shown). Therefore, CD154/G219R is fully capable of inducing signalling pathways that result in the expression of germline transcripts in B cells.
Blocking CD40‐mediated CD80 expression is less efficient in cultures established with CD154/G219R transfectants
To establish whether signalling through CD154/G219R could be inhibited by either soluble CD40 or blocking antibodies to CD154, cocultures were established with Ramos B cells and CD154 transfectants that had been incubated in the presence of CD40‐immunoglobulin, 5c8 mAb, 24‐31 mAb, or an isotype‐control antibody. We found that the 24‐31 mAb failed to significantly block CD154 : CD40 binding at the same concentration that we observed inhibition with both CD40‐Ig and 5c8 mAb. However, there was a twofold decrease in CD40‐mediated inhibition of CD80 expression when Ramos B cells were incubated with 293 cells expressing CD154/G219R versus 293 cells expressing wild‐type CD154 (Fig. 4a, b). In three independent experiments we observed an increased level of CD80 repression on B cells blocked with CD40‐Ig and stimulated with CD154/wt, compared to Ramos B cells first incubated with CD40‐Ig then stimulated with CD154/G219R transfectants. In contrast, we observed a similar level of repression of CD80 in B cells stimulated with either CD154/G219R or CD154/wt after pretreatment with the 5c8 mAb. These results are consistent with the data showing that the G219R polymorphism had a very minor affect on the binding of the 5c8 mAb to CD154 (Fig. 2b).
Figure 4.
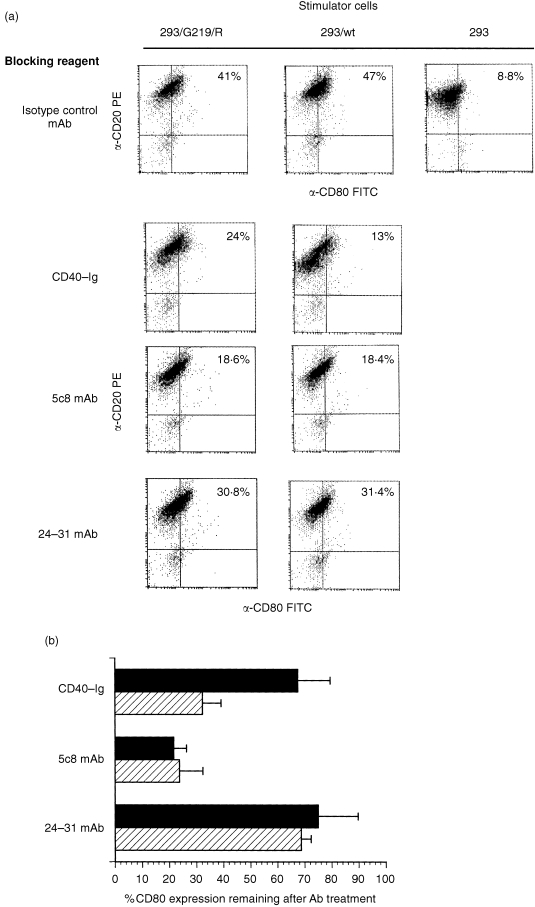
Suppression of CD154‐directed responses in Ramos B cells. (a). Histogram analysis of a representative experiment showing inhibition of CD80 expression by CD40‐Ig, 5c8 mAb, and 24‐31 mAb. 293 transfectants expressing CD154/wt or CD154G219R were incubated with 5 µg/ml CD40‐Ig, 5c8 mAb, 24‐31 mAb, or isotype‐control antibody for 1 hr at 37° prior to the addition of 5 × 105 Ramos B cells. Twenty‐four hours later cells were analysed for CD80 expression by staining with mAbs directed against CD20 and CD80. Results indicate percentage of CD20 positive cells that also stain positive for CD80 surface expression after incubation. Ramos B cells stimulated with mock‐transfected 293 cells alone and stained for CD20 and CD80 expression gave a background expression of 8% for CD80+/CD20+ cells (data not shown). Ramos B cells cocultured with mock‐transfected 293 cells that had been pretreated with different antibodies, showed no expression of CD80 over background (data not shown). (b). Compiled results of three experiments demonstrating the suppression of CD40‐induced CD80 expression. Error bars indicate SEM. P‐values are < 0·01, < 0·67 and < 0·72 for CD40‐Ig, 5c8 mAb and 24‐31 mAb, respectively.
Discussion
In this study we have characterized the binding and functional properties of a CD154 protein containing a polymorphism in the tumour necrosis factor‐α (TNF‐α) homology domain. The observation that normal CD40‐mediated signalling occurs within the context of attenuated CD154/G219R : CD40 binding suggests that contact between receptor and ligand can be reduced to a limited extent with minimal quantitative, and no qualitative effects on a subset of induced functions. Differences in binding of distinct mAb by CD154/G219R, together with our finding that there is relative little difference in recognition of either wild‐type or polymorphic proteins by polyclonal antiserum, suggest that the G219R change does not significantly affect the ability of the protein to be expressed on the cell surface.
G219 is a solvent‐accessible residue that resides within the F strand of the second β pleated sheet of the CD154 monomer. 25 Recent remodelling of the CD40 : CD154 binding surface identified 23 residues in CD154 that are predicted contact points with CD40. 26 In accordance with this model, G219 is centered between two contact residues but does not make direct contact with CD40. One possibility to explain the decreased CD40 binding in CD154/G219R is that the replacement of the Gly with an Arg residue reduces the flexibility of the region affecting the local environment of the CD40 binding residues, C218 and Q220. 26 This model is consistent with the binding pocket being perceptibly altered in the polymorphic protein, as revealed by the failure of CD40‐Ig to effectively block the interaction between CD154/G219R and CD40.
The G219R change appears to be the first naturally occurring polymorphism in CD154 extensively analysed. Multiple single‐site mutations have been identified in HIM patients that result in defective CD154 function. Of the mis‐sense mutations analysed, few appear to directly affect the CD40 binding pocket. 27 Many of these mutations occur in residues buried within the hydrophobic core and are thought to disrupt the tertiary and/or quaternary structure of the CD154 molecule. In contrast, mutations of surface‐accessible residues are believed to directly affect critical CD154–CD40 contacts or the formation of the CD154 trimer. 28
Using different mAb and CD40 as binding reagents we were able to assess whether the G219R polymorphism overlapped with specific recognition epitopes. Determination that anti‐TRAP‐1 binds outside the CD40 recognition domain was previously demonstrated by the failure of CD40‐Ig to block the binding of TRAP‐1 to CD154. 4 However, we found that the G219R polymorphism diminished binding of CD154 to both CD40 and TRAP‐1. Again, this suggests that the G219R polymorphism effects changes in the secondary structure of the molecule that encompasses multiple determinants. The 24‐31 mAb was the least effective reagent at blocking CD40–CD154 interactions suggesting that the 24–31 epitope does not overlap the CD40 binding domain to a very large extent. In contrast, the 5c8 determinant appears to be highly overlapping with the binding site for CD40· 29 Surprisingly, we found that the binding of 5c8 mAb and CD40‐Ig to CD154/G219R was not entirely concordant, which may reflect the fact that 5c8 is recognizing a determinant in, or close to, the CD40 binding pocket that is relatively unaffected by the polymorphism.
Our observations that the CD40‐mediated responses of B cells stimulated with CD154/G219R are less effectively blocked by CD40‐Ig than responses induced with wild‐type CD154 have implications with respect to the use of this recombinant reagent in therapeutic treatment. It has previously been shown that antibodies directed against murine CD154 are effective at blocking the onset and progression of collagen‐induced arthritis (CIA). 30 Likewise, blocking antibodies or reagents against CD154 or CD40 have been successful in mouse models at inhibiting the onset of numerous autoimmune disorders (reviewed in 11) and in conjunction with blocking reagents to CTLA4, inhibiting graft rejection. 12–14 The presence of polymorphic CD154 molecules, which are normal for eliciting downstream functions but have a lower avidity of binding to CD40, could substantially effect the ability to suppress CD154 expression under pathological conditions.
Acknowledgments
We gratefully acknowledge Dr Richard Krozcek (Max Planck Institute) for the gifts of anti‐TRAP polyclonal and monoclonal antisera and the CD40‐Ig fusion protein and Dr Seth Lederman (Columbia University) for the 5c8 mAb. Dr Lederman and Dr Leonard Chess are thanked for their help in the initial stages of this project. The antihuman CD8 mAb was a gift from Dr Yacov Ron (UMDNJ). This work was supported by National Institutes of Health Grant AI37081 to L.R.C.
References
- 1.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Ann Rev Immunol. 1994;12:881. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 2.Kawabe T, Naka T, Yishida K, et al. The immune response in CD40‐deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 4.Kroczek RA, Graf D, Brugnoni D, et al. Defective expression of CD40 ligand on T cells causes X‐linked immunodeficiency with hyper‐IgM (HIGM1) Immunol Rev. 1994;138:39. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins MK. The ups and downs of T cell co‐stimulation. Immunity. 1994;1:443. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 7.Grewal IS, Flavell RA. CD40–CD40L interactions in T cell activation. Immunol Rev. 1996;153:85. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40‐dependent signal. J Exp Med. 1993;177:925. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yellin MJ, Sippel K, Inghirami G, et al. T lymphocyte T cell‐B cell‐activating molecule/CD40‐L molecules induce normal B cells or chronic lymphocytic leukemia B cells to express CD80 (B7/BB‐1) and enhance their costimulatory activity. J Immunol. 1994;153:666. [PubMed] [Google Scholar]
- 10.Roy M, Aruffo A, Ledbetter JA, Linsley P, Kehry M, Noelle R. Studies on the independence of gp39 and B7 expression and function during antigen‐specific immune responses. Eur J Immunol. 1994;25:596. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- 11.Grewal IS, Flavell RA. CD40 and CD154 in cell‐mediated immunity. Ann Rev Immunol. 1998;16:111. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Larsen CP, Elwood ET, Alexander DZ, et al. Long‐term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 13.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13 967. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4‐Ig and anti‐CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q, Rohrer J, Allen RC, et al. A single strand conformation polymorphism study of CD40 ligand. J Clin Invest. 1996;97:196. doi: 10.1172/JCI118389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein G, Giovanella B, Westman A, Stehlin JS, Mumford D. An EBV‐genome‐negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV‐positive sublines by in vitro infection. Intervirology. 1975;5:319. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JP, Mostowski HS. A bioassay for the measurement of human interleukin‐4. J Immunol Methods. 1990;132:287. doi: 10.1016/0022-1759(90)90040-3. [DOI] [PubMed] [Google Scholar]
- 18.Korthauer U, Graf D, Mages HW, et al. Defective expression of T‐cell CD40 ligand causes X‐linked immunodeficiency with hyper‐IgM. Nature. 1993;361:539. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 19.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact‐dependent B cell differentiation (Help) J Exp Med. 1992;175:1092. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covey LR, Cleary AM, Yellin MJ, et al. Isolation of cDNAs encoding T‐BAM, a surface glycoprotein on CD4+ T cells mediating contact‐dependent helper function for B cells: identity with the CD40‐ligand. Mol Immunol. 1994;31:471. doi: 10.1016/0161-5890(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 21.Spriggs MK, Armitage RJ, Strockbine L, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford GS, Yin CH, Barnhart B, Sztam K, Covey LR. CD40 ligand exerts differential effects on the expression of Iγ transcripts in subclones of an IgM+ human B cell lymphoma line. J Immunol. 1998;160:595. [PubMed] [Google Scholar]
- 23.Lane P, Traunecker A, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell‐associated antigen CD40 which participates in T cell‐dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 24.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T–B lymphocyte collaboration in human lymphoid follicles. J Immunol. 1992;149:3817. [PubMed] [Google Scholar]
- 25.Karpusas M, Hsu Y‐M, Wang J‐H, et al. 2A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995;3:1031. doi: 10.1016/s0969-2126(01)00239-8. [DOI] [PubMed] [Google Scholar]
- 26.Singh J, Garber E, Van Vlijmen H, et al. The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci. 1998;7:1124. doi: 10.1002/pro.5560070506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajorath J, Marken JS, Chalupny NJ, et al. Analysis of gp39/CD40 interactions using molecular models and site‐directed mutagenesis. Biochemistry. 1995;34:9884. doi: 10.1021/bi00031a009. [DOI] [PubMed] [Google Scholar]
- 28.Bajorath J, Chalupny NJ, Marken JS, et al. Identification of residues on CD40 and its ligand which are critical for the receptor–ligand interaction. Biochemistry. 1995;34:1833. doi: 10.1021/bi00006a003. [DOI] [PubMed] [Google Scholar]
- 29.Callard RE, Smith SH, Herbert J, et al. CD40 ligand (CD40L) expression and B cell function in agammaglobulinemia with normal or elevated levels of IgM (HIM) J Immunol. 1994;153:3295. [PubMed] [Google Scholar]
- 30.Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen‐induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]


