Abstract
It has long been known that injury, infections, and other critical illnesses are often associated with hyperglycemia and hyperinsulinemia. Mortality of critically ill patients is greatly reduced by intensive insulin therapy, suggesting the significance of reversing or compensating for the development of acute insulin resistance. However, the development of acute injury/infection-induced insulin resistance is poorly studied, much less than the chronic diseases associated with insulin resistance, such as type 2 diabetes and obesity. We previously found that insulin resistance develops acutely in the liver after trauma and hemorrhage. The present study was designed to begin to understand the first steps in the development of trauma and hemorrhage-induced acute hepatic insulin resistance in an animal model of injury and blood loss similar to traumatic or surgical injury and hemorrhage. We present novel data that indicate that hepatic insulin resistance increased dramatically with an increasing extent of hemorrhage. With increasing extent of blood loss, there were increases in serum TNF-α levels, phosphorylation of liver insulin receptor substrate-1 on serine 307, and liver c-Jun N-terminal kinase activation/phosphorylation. Exogenous TNF-α infusion increased c-Jun N-terminal kinase phosphorylation and insulin receptor substrate-1 serine 307 phosphorylation, and inhibited insulin-induced signaling in liver. Conversely, neutralizing TNF-α antibody treatment reversed many of the hemorrhage-induced changes in hepatic insulin signaling. Our data indicate that the acute development of insulin resistance after trauma and hemorrhage may have some similarities to the insulin resistance that occurs in chronic diseases. However, because so little is known about this acute insulin-resistant state, much more needs to be done before we can attain a level of understanding similar to that of chronic states of insulin resistance.
HYPERGLYCEMIA AND hyperinsulinemia often occur after injury, including accidental and surgical trauma, burn, hemorrhage, and sepsis, and other critical illness (1,2,3,4,5,6,7), indicating the presence of acute insulin resistance. Although numerous studies have focused on the mechanisms of chronic insulin resistance in obesity and type 2 diabetes, little is known about the mechanisms underlying the acute insulin resistance after injuries and critical illness. Intensive insulin therapy, to compensate for the development of hyperglycemia and restore normoglycemia in critically ill individuals, results in 34–50% reductions in septicemia, renal failure, transfusions, polyneuropathy, and mortality (5,8). Thus, an understanding of the mechanisms of acute insulin resistance may be important for new developments to increase survival after injury and critical illness.
Although there are numerous studies on the development of insulin resistance in chronic insulin-resistant states, including type 2 diabetes, obesity, polycystic ovarian syndrome, and hypertension-related cardiovascular disease, the exact mechanisms resulting in insulin resistance have been elusive. It is likely that there are multiple possible mechanisms that are disease dependent, and the mechanisms may differ in different insulin target tissues. However, almost nothing is known about the cellular mechanisms involved in the development of insulin resistance that often occurs acutely after injury or infection. A few studies suggest the development of an acute insulin-resistant state in muscle and adipose tissue after injury (2,9,10). However, these observations do not indicate what mechanisms are involved in the development of insulin resistance and any possible causative factors. Our previous findings suggest that there is also a rapid development of hepatic insulin resistance after experimental trauma and hemorrhage, with compromised insulin-stimulated insulin receptor substrate (IRS)/phosphatidylinositol 3-kinase (PI3K)/Akt signaling and increased blood glucose and insulin levels (11), accompanied by increased TNF-α levels and IRS-1 serine (S) phosphorylation (12). Liver is the main site of gluconeogenesis, and insulin is a primary suppressor of hepatic glucose output. If liver becomes resistant to insulin, increased hepatic gluconeogenesis can result in the hyperglycemia and hyperinsulinemia that are correlated with the increased mortality of critically ill patients (5).
Insulin exerts its biological effects by binding to its specific tyrosine (Y) kinase receptor on the surface of target cells (13,14). Activation of the insulin receptor (IR) leads to its autophosphorylation and further phosphorylation of IRS, which serve as a docking molecule, favoring the generation of intracellular signals (15,16). One main pathway activated by insulin is the IRS/PI3K/Akt pathway (17,18,19). The development of insulin resistance often involves IRS proteins (15,17,20). In the chronic diseases associated with insulin resistance, there are reports of down-regulated IRS protein levels, decreased IRS tyrosine phosphorylation, defects of IRS/PI3K association, and kinase-mediated serine phosphorylation of IRS proteins, all of which can impair the ability of IRS proteins to function in insulin signaling (17,21,22).
A possible causative factor in the chronic insulin resistance in type 2 diabetes and obesity is an increase of proinflammatory cytokines, with TNF-α possibly playing a central role by inducing serine phosphorylation of IRS proteins and inhibiting insulin-stimulated tyrosine phosphorylation of IRS proteins (17,20,23,24,25,26). C-Jun N-terminal kinase (JNK) is one of the main signaling pathways activated by TNF-α (27,28). JNK1 and JNK2 are ubiquitously expressed kinases that can mediate TNF-induced serine phosphorylation of IRS-1, and JNK1 has been involved in obesity-related insulin resistance (29).
The present study was designed to begin to understand the first steps in the development of trauma and hemorrhage-induced acute hepatic insulin resistance in an animal model of injury and blood loss similar to traumatic or surgical injury and hemorrhage. We found that serum TNF-α levels, liver IRS-1 serine phosphorylation, and liver JNK activation/phosphorylation positively correlated with the extent of hemorrhage, whereas insulin-induced activation/phosphorylation of IRS-1 and Akt in the liver decreased with an increasing extent of hemorrhage. Exogenous TNF-α infusion increased JNK phosphorylation, induced IRS-1 S307 phosphorylation, and inhibited insulin’s activation of IRS-1 and Akt in rat liver. Conversely, treatment with a neutralizing TNF-α antibody after trauma and hemorrhage restored insulin sensitivity. These data suggest that within a brief time after the onset of hemorrhage, there is the rapid development of hepatic insulin resistance, that is at least in part due to a rapid increase in liver JNK activity, induced by rapidly elevated TNF-α, and resulted in serine phosphorylation of IRS-1 and decreased insulin-induced signal transduction via the PI3K/Akt pathway.
Materials and Methods
Animal model of trauma and hemorrhage
A model of trauma and hemorrhage in the rat, as previously described (11,12), was used in this study. Briefly, male Sprague Dawley rats were anesthetized, a 5-cm ventral midline laparotomy was performed representing soft tissue trauma, and the abdomen was closed. Polyethylene-50 catheters (Becton Dickinson, Franklin Lakes, NJ) were placed in the right and left femoral arteries and the right femoral vein for bleeding, monitoring of mean arterial pressure, and fluid resuscitation, respectively. The rats were bled rapidly to a mean arterial pressure of 35–40 mm Hg within 10 min. Once mean arterial pressure reached 40 mm Hg, the timing of the hemorrhage period began and was maintained for 90 min. At the end of the hemorrhage period, the rats were resuscitated with four times the withdrawn blood volume using Ringer’s lactate infused by syringe pump (KD Scientific, Helliston, MA) at a constant rate over 60 min. Trauma-only rats underwent the same surgical procedure (laparotomy and catheterization), but neither hemorrhage nor resuscitation was performed. All procedures were performed in accordance with the guidelines set forth in the Animal Welfare Act and the Guild for the Care and Use of Laboratory Animal by the National Institutes of Health, and the experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Study design
Due to the considerable trauma incurred during anesthesia and opening of the abdominal cavity to perform the insulin injections (see Tissue harvesting procedures), it was impossible to have a completely naive control group. Thus, the baseline animal was selected in these experiments to be the trauma-alone rats (T0′) that were subjected to anesthesia, laparotomy, and catheterization, and then killed immediately. Additional trauma-only groups were subjected to these same procedures and then killed at 90′ (T90′) or 210′ (T210′) after catheterization. Matched to these groups were the trauma and hemorrhage groups that were subjected to the same procedures as the trauma groups but also subjected to hemorrhage and then killed at 90 min, the end of the hemorrhage period (TH90′), or 60 min after completion of the 60-min resuscitation period (TH210′ = 90-min hemorrhage + 60-min resuscitation + 60-min recovery).
Tissue harvesting procedures
At the 0′, 90′, or 210′ time points, the abdominal cavity was opened, the portal vein was exposed, and insulin (5 U in 0.5 ml saline) or saline alone was injected into the portal vein. Unless otherwise noted, 1 min after the injection, livers were removed and snap frozen in liquid nitrogen.
Exogenous TNF-α infusion
TNF-α (Genentech, San Francisco, CA) at a dose of 125 μg/kg body weight (in 1 ml normal saline containing 0.2% BSA) or 1 ml vehicle (i.e. normal saline with 0.2% BSA) was infused via the portal vein over a period of 30 min at a constant infusion rate (30). At 60 min after completion of TNF-α infusion, the abdominal cavity was opened, and insulin or saline was injected into the portal vein. Livers were removed at 1 min after injection and snap frozen in liquid nitrogen.
Systemic neutralization of TNF-α or IL-6
Neutralizing goat antirat-TNF-α antibody (200 μg; Biosource, Camarillo, CA) or normal goat IgG (200 μg; R&D Systems, Minneapolis, MN) was infused via the right femoral vein catheter after the onset of resuscitation over a period of 10 min at a constant infusion rate. Neutralizing goat antirat-IL-6 antibody (5 μg; R&D Systems) was administered ip after the onset of resuscitation as previously described (31).
Measurement of serum TNF-α and IL-6 levels
Just before insulin or saline injection, blood was withdrawn from the inferior vena cava, placed at room temperature, allowed to coagulate, and then centrifuged at 5000 × g for 15 min. The serum was stored at −80 C. TNF-α levels were measured by Linco “multi-plex” cytokine kit (Luminex Corp., Austin, TX). IL-6 levels were measured by a rat IL-6 ELISA kit (R&D Systems).
Immunoprecipitation and Western immunoblot analysis
Liver tissue from each animal (∼0.2 g) was homogenized in 1 ml lysis buffer containing 20 mm HEPES (pH 7.9), 1.5 mm MgCl2, 20 mm KCl, 20% glycerol, 0.2 mm EDTA, 2 mm Na3Vo4, 10 mm NaF, 1% Triton X-100, 0.2 mm phenylmethylsulfonylfluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Tissue lysates were centrifuged at 10,000 × g for 10 min, and the supernatants were stored at −80 C until use. Tissue lysate protein concentrations were assayed (Bio-Rad Laboratories, Hercules, CA).
For immunoprecipitation, 1 mg protein from each liver sample in lysis buffer was incubated with antibody against JNK (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or IRS-1 (Upstate Biotechnology, Lake Placid, NY) overnight at 4 C. Protein A or G-agarose (fast flow; Pharmacia Biotech, Providence, RI) was then added, and incubations continued for 4 h at 4 C. Immunoprecipitated proteins were resolved by sodium dodecyl sulfate, 7.5% PAGE, and transferred to nitrocellulose paper. The Western transfers were immunoblotted with anti-JNK, anti-IRS-1 antibodies (Santa Cruz Biotechnology), anti-phospho-tyrosine, or anti-p85 subunit of PI3K antibodies (Upstate Biotechnology). For immunoprecipitation or Western blotting, 30 μg protein per lane was resolved by 7.5% SDS-PAGE and transferred to nitrocellulose paper. The Western transfers were immunoblotted with primary antibodies: anti-phospho-JNK, anti-phospho-S307-IRS-1, and anti-phospho-Y612-IRS-1 (Invitrogen’s Biosource International, Carlsbad, CA), anti-phospho-S473-Akt (Cell Signaling Technology, Danvers, MA), anti-JNK, and anti-Akt (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated secondary antibody was then added for detection of bound antibody by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) or Supersignal Femto Maximum Sensitivity Substrate Reagent (Pierce Biotechnology, Rockford, IL). Each blot was stripped 15 min using Reblot (CHEMICON International, Inc., Temecula, CA) and then reprobed with a different antibody.
JNK activity immunoassay
JNK activity immunoassay was performed according to the manufacturer’s protocol (Calbiochem; EMD Chemicals Inc., San Diego, CA). In brief, JNK was immunoprecipitated from liver protein lysates, immunoprecipitates were washed three times, the kinase reaction was started by adding c-Jun protein and an ATP mixture, and samples were incubated for 25 min at 30 C. Proteins were subjected to Western blot analysis as described previously using a phospho-c-Jun antibody.
Northern blot hybridization analysis
Total hepatic RNA was isolated by the Ultraspec RNA isolation system (Biotecx Laboratories, Houston, TX). Total RNA (10 μg) from each animal was subjected to formaldehyde gel electrophoresis and transferred to nylon membrane (Ambion, Austin, TX) as described previously (32,33). IGF binding protein (IGFBP)-1 cDNA probe was radiolabeled with [32P]deoxycytidine triphosphate using Prime-It II random primer labeling kit (Stratagene, La Jolla, CA), and blots were incubated with this probe overnight, washed, and autoradiographed.
Densitometric and statistical analysis
Enhanced chemiluminescence images of immunoblots and autoradiographs of Northern blots were scanned and quantified using Zero d-Scan (Scanalytics Corp., Fairfax, VA). All data were analyzed by ANOVA using the InStat statistical program by GraphPad Software, Inc. (San Diego, CA).
Results
Correlation between serum TNF-α levels, inhibition of hepatic insulin signaling, and the extent of hemorrhage after trauma and hemorrhage
Previously, we found that trauma and hemorrhage rapidly induces hyperinsulinemia and hyperglycemia, in part due to the compromised hepatic insulin signaling via the IRS/PI3K/Akt pathway. This acute hepatic insulin resistance is associated with an increase of TNF-α levels and S307 phosphorylation of IRS-1 in the liver (11,12). The main purpose of the present study was to study the role of TNF-α in trauma and hemorrhage-induced acute hepatic insulin resistance.
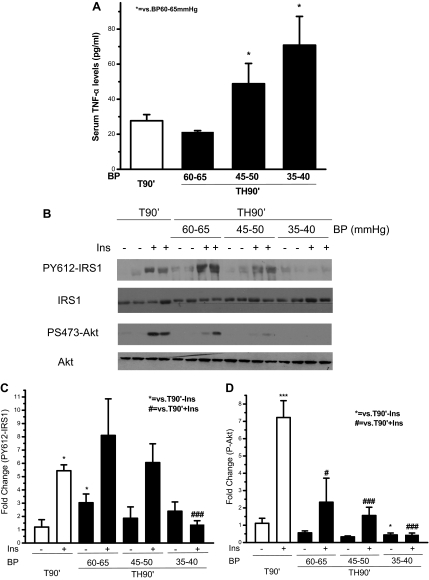
It was first asked whether serum TNF-α levels correlated with the extent of hemorrhage, i.e. the amount of blood loss, indicated by blood pressure. The extent of hemorrhage was divided into three groups with blood pressure at 60–65, 45–50, and 35–40 mm Hg, respectively. Circulating TNF-α levels in trauma-only (T90′) animals were just above the detection limit (24.4 pg/ml), due to a couple of animals with slightly elevated TNF-α. After trauma followed by hemorrhage for 90 min (TH90′), serum TNF-α levels were lower than the detection limit if blood pressure was maintained at 60–65 mm Hg. However, more severe hemorrhage (blood pressure at 45–50 mm Hg) resulted in a significant increase in serum TNF-α levels to an average of 50 pg/ml and a further increase to an average of 70 pg/ml if blood pressure was only 35–40 mm Hg (Fig. 1A). Therefore, there was a correlation between the serum TNF-α levels and the extent of hemorrhage, even at this very early time point.
Figure 1.
Correlation between serum TNF-α levels, the inhibition of hepatic insulin (Ins) signaling, and the extent of hemorrhage after trauma alone or trauma and hemorrhage. Rats were subjected to T or trauma and hemorrhage. In the trauma and hemorrhage group, rats were bled to a mean arterial pressure of 60–65, 45–50, or 35–40 mm Hg within 10 min and then maintained for 90 min. A, Serum TNF-α levels. Data are presented as the mean + sem of samples from four to eight rats in each group. B, Saline (−) or 5 U insulin (+) was injected into the portal vein, and 1 min later the liver was removed, and protein extracts were prepared, resolved by SDS-PAGE, and subjected to Western blot analysis using specific anti-PY612-IRS1, anti-IRS1, anti-PS473-Akt, and anti-Akt antibodies. Representative Western blots are presented. The original blots were cut and rearranged for the sake of clarity. C and D, Autoradiographs were quantified by scanning densitometry. Data are presented as the mean ± sem of samples from four to eight rats in each group. In this and all the following figures: * or #, P < 0.05; ** or ##, P < 0.01; and *** or ###, P < 0.001. BP, Blood pressure.
If TNF-α causes hepatic insulin resistance, the increased TNF-α levels after the more severe hemorrhage should result in an inhibition of insulin-induced signaling in rat liver. Therefore, insulin-induced tyrosine phosphorylation of IRS-1 and S473 phosphorylation of Akt were examined. In the trauma alone group (T90′), there was a 5.5-fold induction of PY612 of IRS-1 and 7-fold induction of PS473-Akt. In the 60–65 mm Hg blood pressure group, the basal PY612-IRS-1 was elevated to almost 3-fold of that in T90′ animals, but the fold induction of PY612-IRS-1 after insulin injection was only approximately 2.5-fold. In response to hemorrhage, compensatory mechanisms are activated to increase blood pressure, and many hormones are involved in this process. It is possible that some of these hormones increase basal levels of PY612 IRS-1 when hemorrhage is relatively mild (60–65 mm Hg).
In the trauma and hemorrhage groups (TH90′), there was a gradual decrease of insulin-stimulated PY612-IRS-1 (Fig. 1, B and C) and PS473-Akt (Fig. 1, B and D) when the blood pressure during the hemorrhage period was decreased to 60–65 or 45–50 mm Hg, and a total loss of insulin-induction of PY612-IRS-1 and PS473-Akt when the blood pressure was decreased to 35–40 mm Hg. The amount of total IRS-1 and Akt protein was not changed after hemorrhage (Fig. 1B). Therefore, as the severity of the hemorrhage increased and the target blood pressure decreased, insulin’s activation of the IRS/Akt signaling pathway was progressively inhibited, and this decrease in insulin signaling correlated with increased serum TNF-α levels.
S307 phosphorylation of IRS-1 and phosphorylation of JNK correlated with the extent of hemorrhage
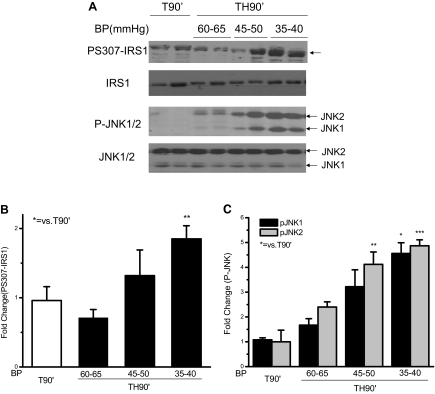
Recent evidence suggests that TNF-α may inhibit insulin signaling by increasing phosphorylation of specific serine sites, most often measured at S307, but also other serine residues of the IRS-1 protein (20,29,34). In the present study, S307 phosphorylation of IRS-1 in the liver correlated with the amount of hemorrhage, and there was a significant increase in IRS-1 S307 phosphorylation after trauma and hemorrhage (Fig. 2, A and B) when the blood pressure was reduced to 35–40 mm Hg, consistent with our previous findings (12).
Figure 2.
Serine 307 phosphorylation of IRS1 and phosphorylation of JNK1/2 were correlated with the extent of hemorrhage in rat liver after trauma and hemorrhage. At the same time points and treatment regimens described in Fig. 1, the liver was removed, and protein extracts were subjected to Western blot analysis with specific anti-PS307-IRS1, anti-IRS1, anti-P-JNK1/2, and anti-JNK1/2 antibodies. A, Representative Western blots are presented. B and C, Data are presented as the mean ± sem of samples from four to eight rats in each group. BP, Blood pressure.
TNF-α can activate the JNK signaling pathway (35,36,37), and JNK has been suggested as a kinase that can serine phosphorylate IRS-1 (25,38). We hypothesized that TNF-α induces the acute hepatic insulin resistance after trauma and hemorrhage by activating JNK and thereby promoting serine phosphorylation of IRS-1. Therefore, phosphorylation of JNK was also measured. Comparing the three groups of animals maintained at different blood pressures for 90 min after the initial hemorrhage, JNK phosphorylation increased with increasing hemorrhage severity. JNK1 and JNK2 phosphorylation in animals with blood pressure of 35–40 mm Hg was significantly increased to 5-fold of that in T90′ animals (Fig. 2, A and C), indicating a positive correlation between JNK phosphorylation, S307 phosphorylation, hemorrhage severity, and decreased insulin-induced signaling.
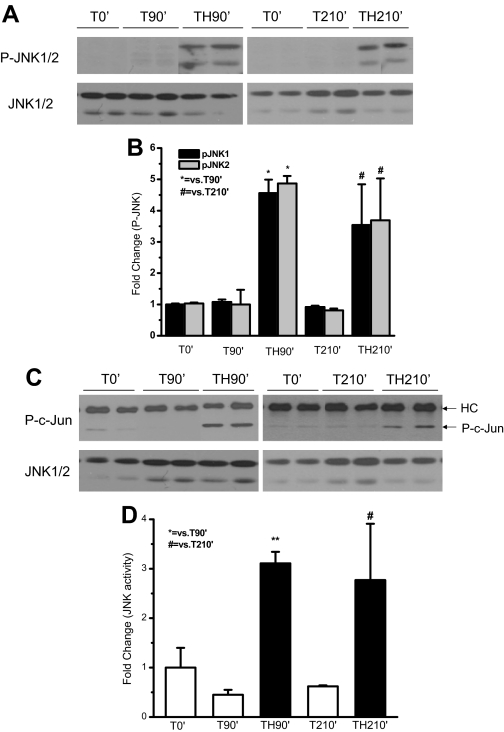
Phosphorylation and activity of JNK was increased after trauma and hemorrhage
As shown in Fig. 2, JNK phosphorylation was induced after TH90′. It was then determined whether JNK phosphorylation was also increased after TH210′ (90 min hemorrhage, followed by 60 min resuscitation and 60 min recovery). There was no significant change in phosphorylation of JNK after trauma alone. After trauma and hemorrhage, a 5-fold (TH90′) and 3.5-fold (TH210′) increase in JNK1 and JNK2 phosphorylation was detected, compared with trauma only (T90′ and T210′) (Fig. 3, A and B). In vitro kinase assays further confirmed that the kinase activity of the highly phosphorylated JNK after trauma and hemorrhage was increased (Fig. 3, C and D) at both 90′ and 210′, compared with trauma alone.
Figure 3.
Phosphorylation and activity of JNK1/2 increased in rat liver after trauma and hemorrhage. At 0′ (after T, but before any further treatment), 90′ (without or with hemorrhage), or 210′ (without hemorrhage or with hemorrhage and 60 min recovery after 60 min resuscitation), the liver was removed. A, Protein extracts were subjected to Western blot analysis with specific anti-P-JNK1/2 and anti-JNK1/2 antibodies. Representative Western blots are presented. C, Protein extracts were subjected to in vitro JNK kinase activity assay using c-Jun as the substrate. Representative Western blots are presented. HC indicates the heavy chain of immunoprecipitated anti-JNK antibody. B and D, Data are presented as the mean ± sem of samples from four to eight rats in each group.
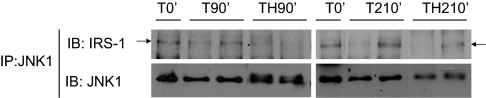
JNK was associated with IRS1 in rat liver
In vitro studies suggested that JNK and IRS-1 interact with each other, and activated JNK can induce S307 phosphorylation of IRS-1 (25,38), but it is unknown whether JNK interacts with IRS-1 in vivo. Because JNK1 is thought to be the main isoform of JNK that is involved in insulin resistance (29), the interaction between JNK1 and IRS-1 was determined. After immunoprecipitation of liver extracts with an anti-JNK1 antibody, hepatic association between JNK1 and IRS-1 was detected in trauma alone (T0′, T90′, and T210′) and in trauma and hemorrhage (TH90′ and TH210′) animals (Fig. 4). There was no significant change in JNK1/IRS-1 association among these groups, suggesting that JNK1 was constitutively associated with IRS-1. The constitutive interaction between JNK2 and IRS-1 was also detected in the liver of trauma alone and in trauma and hemorrhage animals (data not shown). Therefore, both JNK1 and JNK2 were associated with IRS-1 in the liver.
Figure 4.
The interaction between JNK and IRS1 in rat liver. At the same time points and treatment regimens described in Fig. 3, the liver was removed, and protein extracts were immunoprecipitated with specific anti-JNK1 antibody and then subjected to Western blot analysis by specific anti-IRS-1 and anti-JNK antibodies. Representative Western blots are presented. IP, immunoprecipitation; IB, immunoblot.
TNF-α induced phosphorylation of JNK and S307 phosphorylation of IRS-1 in rat liver
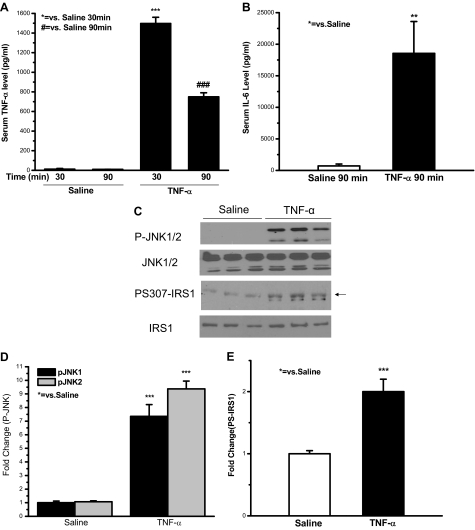
The data in Figs. 1–3 suggested that increased TNF-α levels after trauma and hemorrhage might cause JNK activation and thereby induce phosphorylation of S307 on IRS-1. It was then asked whether TNF-α was able to do this in rat liver. Our previous studies suggest that the increased level of TNF-α is probably produced in the liver after trauma and hemorrhage (12). Therefore, to mimic the elevation of hepatic TNF-α levels after trauma and hemorrhage, exogenous TNF-α was infused into healthy rats via the portal vein for 30 min, and rats were killed after a 60-min waiting period (90 min total) to equal the T90′/TH90′ time point. Our previous findings suggested that locally produced TNF-α may be more important than systemic TNF-α in inducing the hepatic insulin resistance after trauma and hemorrhage. If so, local levels of TNF-α, produced in the liver, may be higher than systemic levels of TNF-α. Thus, in the TNF-α-infused animals, a high concentration of TNF-α was infused to ensure that high systemic levels were obtained so that there would be sufficient local liver concentrations of TNF-α.
Serum TNF-α levels in rats infused with TNF-α or saline were determined. In rats infused with saline for 30 or at 90 min, after an additional 60-min waiting period, serum TNF-α levels were approximately 10 pg/ml (Fig. 5A). In contrast, 30-min TNF-α infusion caused an almost 140-fold increase of circulating TNF-α levels (1500 pg/ml). Serum TNF-α levels in rats infused with TNF-α after the 60-min waiting period (90-min time point) remained as high as 80-fold of that in rats infused with saline (Fig. 5A). Because IL-6 expression can be induced by TNF-α and serum IL-6 levels increase after trauma and hemorrhage (unpublished data) (39,40), serum IL-6 levels were also measured after TNF-α infusion. Serum IL-6 levels increased by about 25-fold in animals infused with TNF-α at the 90-min time point, compared with that of animals infused with saline (Fig. 5B). This suggests that TNF-α infusion via the portal vein increased circulating TNF-α and IL-6 levels, higher than what have been observed in trauma and hemorrhage animals (12) (unpublished data). Although the circulating TNF-α levels in infused animals were five times higher than the systemic levels after trauma and hemorrhage, there may be little difference in the actual local TNF-α levels, to which liver cells were exposed after TNF-α infusion vs. after trauma and hemorrhage.
Figure 5.
Exogenous TNF-α infusion induced JNK phosphorylation and IRS-1 S307 phosphorylation in rat liver. TNF-α at a dose of 125 μg/kg body weight (in 1 ml saline containing 0.2% BSA) or 1 ml saline containing 0.2% BSA was infused via the portal vein over a period of 30 min at a constant infusion rate. A and B, Blood was collected at 30 min of TNF-α or saline infusion, or at 60 min after a 30-min infusion. Serum TNF-α and IL-6 levels were examined. C, Sixty minutes after 30 min of TNF-α or saline infusion, either saline or insulin was injected into the portal vein, and 1 min later the liver was removed. Liver protein was subjected to Western blot analysis with specific anti-P-JNK, anti-JNK, anti-PS307-IRS-1, and anti-IRS-1 antibodies. Representative Western blots are presented. D and E, Data are presented as the mean ± sem of samples from three rats in each group.
In healthy rats, exogenous infusion of TNF-α significantly induced phosphorylation of JNK in the liver by approximately 8- to 9-fold, compared with the animals infused with saline (Fig. 5, C and D). S307 phosphorylation of IRS-1 was also increased in the livers of animals infused with TNF-α, reaching 2-fold of that in animals infused with saline (Fig. 5, C and E). The amount of total JNK protein or total IRS-1 protein was not affected by TNF-α infusion at the time points measured (Fig. 5C). This indicates that exogenous TNF-α infusion was able to induce JNK phosphorylation and IRS-1 S307 phosphorylation in the liver.
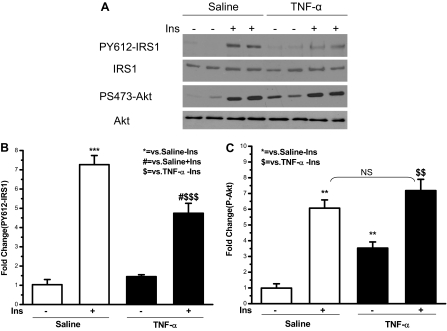
TNF-α inhibited insulin-induced phosphorylation/ activation of IRS1 and Akt in rat liver
Because TNF-α induced S307 phosphorylation of IRS1 in rat liver (Fig. 5) and phosphorylation of this site has inhibited tyrosine phosphorylation of IRS-1 (17,21,22), the effects of TNF-α infusion on hepatic insulin signaling were next determined. In Fig. 6, A and B, insulin was observed to induce tyrosine phosphorylation of IRS-1 7-fold in the liver of saline-treated animals. However, in the animals infused with TNF-α, insulin induced tyrosine phosphorylation of IRS-1 was less, approximately 5-fold, suggesting an inhibition of tyrosine phosphorylation of IRS-1 by TNF-α. Insulin induced S473 phosphorylation of Akt by 6-fold in the saline group (Fig. 6, A and C). After TNF-α infusion, basal phosphorylation of Akt was significantly elevated to 3.5-fold of that in animals infused with saline. Compared with this increased basal phosphorylated Akt, there was only a 2-fold induction of Akt phosphorylation by insulin in animals infused with TNF-α. There was no change of Akt protein levels when animals were treated with TNF-α (Fig. 6A). Therefore, similar to tyrosine phosphorylation of IRS-1, TNF-α infusion resulted in a lesser-fold induction of Akt phosphorylation by insulin.
Figure 6.
Insulin (Ins)-induced hepatic tyrosine phosphorylation of IRS-1 and phosphorylation of Akt were inhibited by exogenous TNF-α infusion. At the same time points and treatment regimens described in Fig. 5, the liver was removed, and protein extracts were subjected to Western blot analysis by specific anti-PY612-IRS-1, anti-IRS-1, anti-PS473-Akt, and anti-Akt antibodies. A, Representative Western blots are presented. B and C, Data are presented as the mean ± sem of samples from three rats in each group. $, P < 0.05. $$, P < 0.01. $$$, P < 0.001. NS, Not significant.
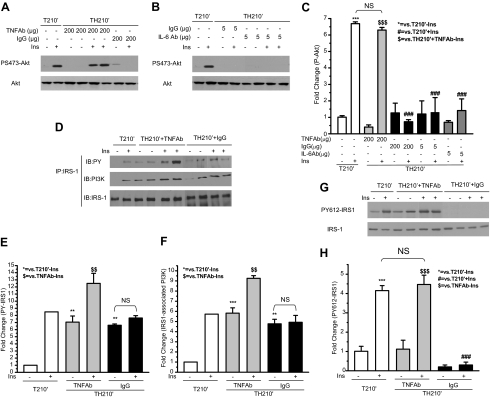
Neutralizing TNF-α antibody restored insulin-induced S473 phosphorylation of Akt, tyrosine phosphorylation of IRS-1, and IRS-1/PI3K association in rat liver after trauma hemorrhage
To investigate further the role of TNF-α in inducing hepatic insulin resistance after trauma and hemorrhage, a neutralizing antibody specific for TNF-α was used to test whether the hepatic insulin resistance after trauma and hemorrhage could be reversed. In T210′ animals, there was a 6.5-fold induction of P Akt in the liver (Fig. 7, A and C). In TH210′ animals treated with IgG, insulin failed to induce phosphorylation of Akt in the liver, similar to what was previously observed in TH210′ animals without IgG treatment (11,12). However, in TH210′ animals treated with anti-TNF-α, the fold induction of Akt phosphorylation by insulin was close to that in T210′ animals (Fig. 7, A and C), suggesting a recovery of insulin’s ability to activate Akt. IL-6 is another cytokine whose expression is also increased after trauma and hemorrhage (unpublished data) (39,40). In contrast to the TNF-α antibody, the neutralizing antibody specific for IL-6 did not restore insulin’s ability to activate Akt, similar to the lack of effect of IgG (Fig. 7, B and C). The expression of total Akt protein was not changed, whether the animal was treated with TNF-α antibody, IL-6 antibody, or IgG.
Figure 7.
Neutralizing TNF-α antibody restored insulin (Ins)-induced phosphorylation of Akt, tyrosine phosphorylation of IRS-1, and IRS-1/PI3K association after trauma and hemorrhage. Neutralizing TNF-α antibody (200 μg), IL-6 antibody (5 μg), or IgG (200 or 5 μg) was administered as described in Materials and Methods. After the completion of infusion, either saline or insulin was injected into the portal vein, and 1 min later the liver was removed. A and B, Liver protein was subjected to Western blot analysis with specific anti-P-Akt, and anti-Akt antibodies. Representative Western blots are presented. D, Liver protein extracts were immunoprecipitated with specific anti-IRS-1 antibody and then subjected to Western blot analysis by specific anti-phospho-tyrosine (PY), anti-PI3K, and anti-IRS-1 antibodies. G, Liver protein was subjected to Western blot analysis with specific anti-PY612-IRS-1 and anti-IRS-1 antibodies. Representative Western blots are presented. C, E, F, and H, Data are presented as the mean ± sem of samples from three to four rats in each group. NS, Not significant; IP, immunoprecipitation; IB, immunoblot.
Our previous findings suggest that after trauma and hemorrhage, the inability of insulin to activate Akt is due to the loss of tyrosine phosphorylation of IRS-1 and consequent loss of IRS-1/PI3K association (12). Because TNF-α antibody administration restored insulin’s activation of Akt, it was then asked if the activation of proteins upstream of Akt, such as IRS-1 tyrosine phosphorylation and IRS-1/PI3K association, also recovered after administration of TNF-α antibody. Total tyrosine phosphorylation of IRS-1 was determined by immunoprecipitation with anti-IRS-1 antibody, followed by immunoblotting with an anti-phospho-tyrosine antibody. In the T210′ group, insulin induced an 8.5-fold of tyrosine phosphorylation of IRS-1 (Fig. 7, D and E). Treatment with both of TNF-α antibody and IgG resulted in an elevated basal tyrosine phosphorylation of IRS-1, suggesting that antibody treatment may increase basal tyrosine phosphorylation of IRS-1. Even with the highly elevated basal levels of tyrosine phosphorylation of IRS-1, anti-TNF-α treatment enabled insulin to induce an almost 2-fold induction of IRS-1 tyrosine phosphorylation (Fig. 7, D and E). Insulin still did not induce tyrosine phosphorylation of IRS-1 in TH210′ animals treated with IgG.
Tyrosine phosphorylation of IRS-1 leads to the association of IRS-1 protein with PI3K. The effects of TNF-α antibody on insulin-stimulated IRS-1/PI3K association were also examined. In the trauma alone group (T210′), there was a 5.5-fold increase in association of IRS-1 and PI3K after insulin injection (Fig. 7, D and F). Like basal tyrosine phosphorylation of IRS-1, basal level of IRS-1/PI3K association was also increased by both the TNF-α antibody and by IgG, suggesting nonspecific effects of antibody treatment. However, in addition to the elevated basal levels of IRS-1/PI3K association, insulin further induced an increased IRS-1/PI3K association in the anti-TNF-α treatment group (by 1.5-fold), but not in the IgG treatment group (Fig. 7, D and F).
Our previous findings showed that trauma and hemorrhage did not alter the basal level of total tyrosine phosphorylation of IRS-1 and IRS-1/PI3K association in the absence of insulin but inhibited insulin-induced total tyrosine phosphorylation of IRS-1 and IRS-1/PI3K association (12). In the current study, the increased basal levels of total tyrosine phosphorylation of IRS-1 and IRS-1/PI3K association were due to antibody injection, suggesting that antibody injection has caused some biological response. The antibodies (produced from goats) may cause an immune response or activate signaling pathways in rats. It is also possible that the injected antibodies were contaminated by endotoxin in the process of purification, which increased basal levels of IRS-1/PI3K association.
Because anti-TNF-α and IgG treatment increased the basal levels of total tyrosine phosphorylation of IRS-1 (Fig. 7, D and E), the effects of anti-TNF-α on the phosphorylation of a specific tyrosine site (612) on IRS-1 were next examined. Tyrosine 612 of IRS-1 plays a key role in recruiting PI3K to IRS-1 (41). In trauma-only animals (T210′), phosphorylation of Y612 was increased by 4-fold after insulin injection in the liver (Fig. 7, G and H). Unlike the total tyrosine phosphorylation of IRS-1, treatment with TNF-α antibody did not increase basal phosphorylation of Y612. More importantly, treatment with the TNF-α antibody completely restored the ability of insulin to activate Y612 of IRS-1 after trauma and hemorrhage. In the trauma and hemorrhage animals treated with IgG, the basal level of IRS-1 Y612 phosphorylation was slightly reduced, and no induction of PY612 by insulin was observed (Fig. 7, G and H). Therefore, neutralization of TNF-α restored insulin signaling via the IRS-1/PI3K/Akt signaling pathway in the liver.
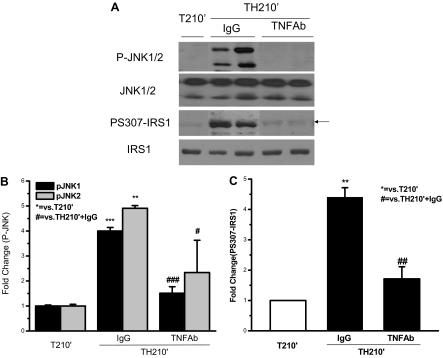
Hemorrhage-induced phosphorylation of JNK and S307 phosphorylation of IRS-1 was inhibited by neutralizing TNF-α antibody
The data in Fig. 7 suggested that administration of TNF-α neutralizing antibody restored insulin-induced tyrosine phosphorylation of IRS-1. It was next asked whether this effect of TNF-α antibody treatment was through preventing phosphorylation of JNK and S307 on IRS-1. As indicated in Fig. 3, trauma and hemorrhage (TH210′) induced an almost 4-fold increase of JNK phosphorylation when compared with the T210′ group. In trauma and hemorrhage (TH210′) animals treated with IgG, JNK phosphorylation remained as high, 4- to 5-fold, as that of trauma and hemorrhage animals (TH210′; Fig. 8, A and B, vs. Fig. 3, A and B). However, TNF-α antibody administration significantly reduced JNK phosphorylation to the level of trauma-only animals (Fig. 8, A and B). The total amount of JNK protein was not affected by anti-TNF-α or IgG treatment (Fig. 8A). This indicates that increased JNK phosphorylation after trauma and hemorrhage can be reversed by TNF-α neutralization.
Figure 8.
Trauma and hemorrhage-induced phosphorylation of JNK and S307 phosphorylation of IRS-1 was inhibited by neutralizing TNF-α antibody. At the same time points and treatment regimens described in Fig. 7, the liver was removed, and protein extracts were subjected to Western blot analysis by specific anti-P-JNK, anti-JNK, anti-PS307-IRS-1, and anti-IRS-1 antibodies. A, Representative Western blots are presented. B and C, Data are presented as the mean ± sem of samples from three rats in each group.
We previously found that phosphorylation of S307 of IRS-1 is increased in trauma and hemorrhage animals (12). In the present study, the liver of trauma and hemorrhage animals treated with IgG still exhibited significantly elevated S307 phosphorylation of IRS-1 (Fig. 8, A and C). However, the level of S307 phosphorylation of IRS-1 in trauma and hemorrhage animals (TH210′) was significantly decreased after TNF-α treatment, suggesting that TNF-α mediated trauma and hemorrhage-induced S307 phosphorylation of IRS-1.
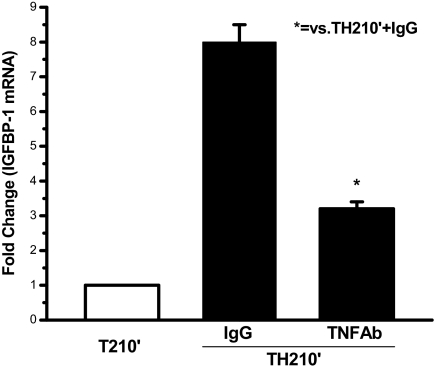
Neutralizing TNF-α antibody restored insulin’s inhibition of IGFBP-1 mRNA expression
Insulin normally inhibits the expression of IGFBP-1 mRNA (42,43) and requires the PI3K/Akt pathway for this effect. It was previously found that after trauma and hemorrhage, the loss of insulin-induced PI3K signaling, probably in combination with increased proinflammatory cytokine levels, resulted in markedly increased IGFBP-1 mRNA expression (11). Thus, it was next asked whether TNF-α antibody treatment, after trauma and hemorrhage, would reduce the increase in IGFBP-1 mRNA. As presented in Fig. 9, IGFBP-1 mRNA expression levels were highly elevated in the liver of trauma and hemorrhage animals treated with IgG, and were significantly reduced by anti-TNF-α treatment, suggesting that TNF-α neutralizing antibody could also restore insulin’s action on further downstream of PI3K/Akt pathway.
Figure 9.
Neutralizing TNF-α antibody restored insulin’s inhibition of IGFBP-1 mRNA expression after trauma and hemorrhage. In the T210′ group and the TH210′ group pretreated with TNF-α antibody or IgG, the liver was removed, and total RNA was isolated and subjected to Northern analysis using an IGFBP-1 probe. The data are presented as the mean + sem of samples from three rats in each group.
Discussion
Our previous findings suggest that acute hepatic insulin resistance after trauma and hemorrhage is correlated with increased levels of TNF-α and serine phosphorylation of IRS-1 (12). In the present study, we found that the extent of hemorrhage was directly correlated with the levels of serum TNF-α, the phosphorylation of IRS-1 S307, and the inhibition of insulin-induced activation of IRS-1 and Akt. This supports a role for TNF-α in the rapid development of hepatic insulin resistance after trauma and hemorrhage. The study of TNF-α in cultured cells and in animals, and the study of TNF-α receptor deficient mice indicate that TNF-α can be an important mediator of the chronic insulin-resistant state in white and brown adipose tissue and skeletal muscle (23,24,44,45,46,47). Whether TNF-α can induce insulin resistance in liver, a major target tissue of insulin, is much less studied.
In the present work, infusion of TNF-α via the portal vein rapidly inhibited insulin-induced hepatic IRS-1 tyrosine phosphorylation and Akt phosphorylation, suggesting that TNF-α can inhibit insulin signaling in the liver. Consistent with the present finding that TNF-α infusion enhanced basal P-Akt in liver, TNF-α has been reported to phosphorylate Akt in primary cultures of mouse hepatocytes (48). Although TNF-α infusion resulted in phosphorylated Akt in liver, the elevated levels of TNF-α inhibited the ability of insulin to induce P-Akt. TNF-α does not require active IRS-1 for its signaling. Thus, TNF-α can induce P-Akt even when it inhibits the ability of insulin to do so. Because TNF-α infusion does not completely mimic trauma and hemorrhage, there are additional factors or mechanisms that occur in hemorrhaged animals, in addition to the increased TNF-α levels.
Serine phosphorylation of IRS-1 on residue 307 is increased in chronic insulin-resistant states, including in rodent models (29) and human skeletal muscle (rat IRS-1 S307 corresponds to S312 of human IRS-1) (20). S307 phosphorylation inhibits insulin-mediated activation of PI3K by disturbing IR/IRS-1 interaction, resulting in decreased insulin action (20,24,25,38,45,49,50,51), and much of this work has been performed in cultured cells. Consistent with these in vitro studies, the current data indicate that 90-min TNF-α infusion also induced S307 phosphorylation of IRS-1 in liver and inhibited insulin-induced IRS-1 tyrosine phosphorylation. Although TNF-α levels remained higher in TNF-α-infused in animals than the levels of TNF-α achieved after trauma and hemorrhage, these higher levels of TNF-α only partially inhibited insulin’s activation of IRS-1 and Akt. This suggests that other factors, in addition to, or in combination with TNF-α, contribute to the impaired hepatic insulin signaling after trauma and hemorrhage. It is unknown whether neutralization of TNF-α is as effective in reversing insulin resistance in other tissues as it is in liver after trauma and hemorrhage.
JNK is one of the signaling pathways activated by TNF-α (35). We observed a significant increase of JNK phosphorylation and kinase activity in vivo after trauma and hemorrhage, which may lead to the inhibition of insulin signaling in the liver after trauma and hemorrhage. Activation of the JNK1 pathway interferes with insulin action, JNK1 activity is abnormally elevated in various tissues under diabetic conditions, and insulin resistance is substantially reduced if JNK1 activation is inhibited or JNK1 is reduced (29,38,52,53). Novel to the present study are the data indicating that activation of JNK occurs extremely rapidly in vivo after trauma and hemorrhage and that this activation may play a role in inducing acute hepatic insulin resistance.
In vitro studies suggest the direct involvement of JNK in phosphorylation of IRS-1 at S307 (25,29,34,38). Among the kinases that may phosphorylate IRS1, JNK is especially interesting because it directly associates with IRS1 and phosphorylates IRS-1 mainly at S307 in vitro (25,38,54). However, it is unknown whether IRS-1 and JNK directly associate in vivo. Here, we report that both JNK1 and JNK2 coimmunoprecipitated with IRS-1 in the liver. This IRS-1 associated JNK was highly activated after trauma and hemorrhage, but not after trauma only. We have not distinguished whether it is JNK1 or JNK2 that is most important in the current study. Both JNK1 and JNK2 bound to IRS-1, and which of the two is most important in the development of acute vs. chronic forms of insulin resistance will need to be answered in the future work.
In the present study, neutralizing TNF-α antibody restored insulin’s ability to activate the IRS-1/PI3K/Akt pathway in the liver after trauma and hemorrhage, and prevented JNK phosphorylation and IRS-1 S307 phosphorylation. This demonstrates that TNF-α was a causative factor for JNK phosphorylation and IRS-1 S307 phosphorylation after trauma and hemorrhage, and confirms a role of TNF-α in the rapid development of hemorrhage-induced hepatic insulin resistance.
In addition to TNF-α, the level of other cytokines, such as IL-6, also increases after trauma and hemorrhage (unpublished data) (39,40). However, treatment with neutralizing IL-6 antibody after trauma and hemorrhage was ineffective in recovering insulin-induced signaling. This is also an additional control for the specific neutralization of TNF-α. The lack of an effect suggests that IL-6 may not play a role in the acute development of hepatic insulin resistance, at least at early time points after trauma and hemorrhage. Intraperitoneal application of IL-6 neutralizing antibody, as used in the present study, is effective in blocking IL-6-induced hepatic injury (31) and IL-6-induced cardiac dysfunction (55,56). Therefore, the lack of an effect of IL-6 antibody treatment to restore insulin sensitivity after trauma and hemorrhage cannot be explained by the mode of administration and the dose of IL-6 antibody. Although IL-6 has played a role in chronic forms of insulin resistance, the lack of a role in the present study is not too surprising because blood levels of IL-6 increase later than TNF-α after injury (unpublished data) (57,58) and are, therefore, unlikely to play a role in the rapid development of insulin resistance after trauma and hemorrhage at the time points studied in the present work. In animal models of chronic insulin resistance, neutralizing TNF-α results in a marked increase in insulin-stimulated phosphorylation of IR and IRS-1 in muscle and fat tissues, but not in liver (59), different than the results presented in the current manuscript. This suggests that muscle and fat, rather than liver, are the tissues responsible for the TNF-α-related chronic insulin resistance in type 2 diabetes and obesity but that in the acute insulin-resistant state in response to hemorrhage, TNF-α action on the liver plays a significant role. Therefore, TNF-α may be important in acute insulin resistance via actions on the liver after trauma and hemorrhage, distinct from its actions on muscle and fat in type 2 diabetes and obesity.
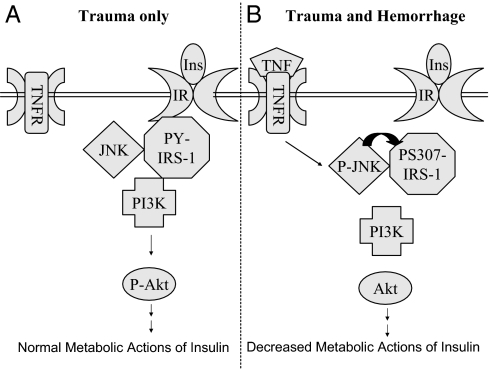
The present study allows us to propose a mechanistic model for the acute hepatic insulin resistance that develops soon after trauma and hemorrhage (Fig. 10). In the liver of trauma-only animals, insulin activates the IRS/PI3K/Akt pathway. Inactive JNK binds to IRS but does not interfere with insulin signaling. After trauma and hemorrhage, acutely increased levels of TNF-α activate JNK in the liver, which in turn induces serine phosphorylation of IRS-1 and inhibits insulin-induced activation of the IRS-1/PI3K/Akt pathway. There are numerous differences between the acute insulin-resistant state that develops after this hemorrhage and the studies on chronic insulin resistance with type 2 diabetes and obesity. The present findings provide both mechanistic insights and potential targets for treatment of patients that develop this acute form of hepatic insulin resistance after trauma and hemorrhage.
Figure 10.
Proposed model for TNF-α-mediated acute hepatic insulin (Ins) resistance after trauma and hemorrhage. A, In the liver of trauma-only animals, insulin binds to IR on the cell membrane, resulting in tyrosine phosphorylation of IRS-1, association of PI3K with IRS-1, and Akt phosphorylation. JNK is associated with IRS-1, but JNK is not phosphorylated or activated. B, In the liver of trauma and hemorrhage animals, local TNF-α levels rapidly increase and bind to the TNF receptor (TNFR) on the cell membrane, leading to phosphorylation and activation of JNK. Activated JNK in turn induces S307 phosphorylation of IRS-1 and inhibits tyrosine phosphorylation of IRS-1. This leads to a loss of association of PI3K with IRS-1 and impaired Akt phosphorylation.
Acknowledgments
We thank Dr. I. H. Chaudry and Mr. Z. F. Ba for assistance with the technical aspects of the animal model. We also thank the Metabolism Core Laboratory of the Clinical Nutrition Research Center at The University of Alabama at Birmingham (P30-DK56336) for measurements of serum TNF-α and IL-6 levels. We thank Dr. M. G. Schwacha for his helpful and insightful discussions and suggestions.
Footnotes
This work was supported by a National Institute of Health Grant (DK 62071) and a grant from the Department of Defense (PRMRP-PR042212) (to J.L.M.).
Disclosure Statement: The authors have nothing to declare.
First Published Online January 10, 2008
Abbreviations: IGFBP, IGF binding protein; IR, insulin receptor; IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinase; PI3K, phosphatidylinositol 3-kinase; T0′, trauma-alone rats; T90′, trauma-alone rats killed at 90′; T210′, trauma-alone rats killed at 210′.
References
- Del Aguila LF, Krishnan RK, Ulbrecht JS, Farrell PA, Correll PH, Lang, CH, Zierath JR, Kirwan JP 2000 Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab 279:E206–E212 [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Sayeed MM, Baue AE 1974 Insulin resistance in experimental shock. Arch Surg 109:412–415 [DOI] [PubMed] [Google Scholar]
- Ikezu T, Okamoto T, Yonezawa K, Tompkins RG, Martyn JA 1997 Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem 272:25289–25295 [DOI] [PubMed] [Google Scholar]
- Carter EA 1998 Insulin resistance in burns and trauma. Nutr Rev 56(1 Pt 2):S170–S176 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R 2001 Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- Yendamuri S, Fulda GJ, Tinkoff GH 2003 Admission hyperglycemia as a prognostic indicator in trauma. J Trauma 55:33–38 [DOI] [PubMed] [Google Scholar]
- McCowen KC, Malhotra A, Bistrian BR 2001 Stress-induced hyperglycemia. Crit Care Clin 17:107–124 [DOI] [PubMed] [Google Scholar]
- Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G 2003 Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab 88:1082–1088 [DOI] [PubMed] [Google Scholar]
- Nordenstrom J, Sonnenfeld T, Arner P 1989 Characterization of insulin resistance after surgery. Surgery 105:28–35 [PubMed] [Google Scholar]
- Rakinic J, Takimoto G, Barrett JA, Lange DA, Robin AP 1987 Adipose tissue response to insulin following injury. JPEN J Parenter Enteral Nutr 11:513–520 [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL 2003 Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 284:G107–G115 [DOI] [PubMed] [Google Scholar]
- Ma Y, Toth B, Keeton AB, Holland LT, Chaudry IH, Messina JL 2004 Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-α. Endocrinology 145:5168–5176 [DOI] [PubMed] [Google Scholar]
- White MF 1997 The insulin signalling system and the IRS proteins. Diabetologia 40:(Suppl 2)S2–S17 [DOI] [PubMed] [Google Scholar]
- Kahn CR, White MF, Shoelson SE, Backer JM, Araki E, Cheatham B, Csermely P, Folli F, Goldstein BJ, Huertas P 1993 The insulin receptor and its substrate: molecular determinants of early events in insulin action. Recent Prog Horm Res 48:291–339 [DOI] [PubMed] [Google Scholar]
- Le Roith D, Zick Y 2001 Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care 24:588–597 [DOI] [PubMed] [Google Scholar]
- White MF 2002 IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422 [DOI] [PubMed] [Google Scholar]
- Zick Y 2001 Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol 11:437–441 [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Clark SF, Urso B, James DE 2000 Signalling through the insulin receptor. Curr Opin Cell Biol 12:222–228 [DOI] [PubMed] [Google Scholar]
- Nakae J, Accili D 1999 The mechanism of insulin action. J Pediatr Endocrinol Metab 12(Suppl 3):721–731 [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF 2001 Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fea K, Roth RA 1997 Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36:12939–12947 [DOI] [PubMed] [Google Scholar]
- Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, Ohba M, Kuroki T, Leroith D, Zick Y 2001 Insulin stimulates PKCζ-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem 276:14459–14465 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS 1999 The role of TNFα and TNF receptors in obesity and insulin resistance. J Intern Med 245:621–625 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM 1994 Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA 91:4854–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF 2002 Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277:1531–1537 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J 2001 Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–869 [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M 2001 Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11:372–377 [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS 2002 A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- Wang P, Ayala A, Ba ZF, Zhou M, Perrin MM, Chaudry IH 1993 Tumor necrosis factor-α produces hepatocellular dysfunction despite normal cardiac output and hepatic microcirculation. Am J Physiol 265(1 Pt 1):G126–G132 [DOI] [PubMed] [Google Scholar]
- Toth B, Yokoyama Y, Schwacha MG, George RL, Rue III LW, Bland KI, Chaudry IH 2004 Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol 97:2184–2189 [DOI] [PubMed] [Google Scholar]
- Messina JL 1989 Insulin and dexamethasone regulation of a rat hepatoma messenger ribonucleic acid: insulin has a transcriptional and a posttranscriptional effect. Endocrinology 124:754–761 [DOI] [PubMed] [Google Scholar]
- Bennett WL, Ji S, Messina JL 2007 Insulin regulation of growth hormone receptor gene expression. Evidence for a transcriptional mechanism of down-regulation in rat hepatoma cells. Mol Cell Endocrinol 274:53–59 [DOI] [PubMed] [Google Scholar]
- Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE 2004 Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem 279:35298–35305 [DOI] [PubMed] [Google Scholar]
- Yuasa T, Ohno S, Kehrl JH, Kyriakis JM 1998 Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem 273:22681–22692 [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA 1999 The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667–676 [DOI] [PubMed] [Google Scholar]
- Rincon M, Whitmarsh A, Yang DD, Weiss L, Derijard B, Jayaraj P, Davis RJ, Flavell RA 1998 The JNK pathway regulates the in vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med 188:1817–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre V, Uchida T, Yenush L, Davis R, White MF 2000 The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275:9047–9054 [DOI] [PubMed] [Google Scholar]
- Claridge JA, Schulman AM, Young JS 2002 Improved resuscitation minimizes respiratory dysfunction and blunts interleukin-6 and nuclear factor-κ B activation after traumatic hemorrhage. Crit Care Med 30:1815–1819 [DOI] [PubMed] [Google Scholar]
- Meng ZH, Dyer K, Billiar TR, Tweardy DJ 2001 Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol 280:C343–C351 [DOI] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Cama A, Quon MJ 2001 Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 142:2833–2840 [DOI] [PubMed] [Google Scholar]
- Rechler MM 1993 Insulin-like growth factor binding proteins. Vitam Horm 47:1–114 [DOI] [PubMed] [Google Scholar]
- Rechler MM, Ooi GT, Suh D, Tseng L 1993 Rapid regulation of insulin-like growth factor binding protein-1 transcription by insulin in vivo and in vitro. Adv Exp Med Biol 343:227–236 [DOI] [PubMed] [Google Scholar]
- Rosenzweig T, Braiman L, Bak A, Alt A, Kuroki T, Sampson SR 2002 Differential effects of tumor necrosis factor-α on protein kinase C isoforms α and δ mediate inhibition of insulin receptor signaling. Diabetes 51:1921–1930 [DOI] [PubMed] [Google Scholar]
- Teruel T, Hernandez R, Lorenzo M 2001 Ceramide mediates insulin resistance by tumor necrosis factor-α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50:2563–2571 [DOI] [PubMed] [Google Scholar]
- del Aguila LF, Claffey KP, Kirwan JP 1999 TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol 276(5 Pt 1):E849–E855 [DOI] [PubMed] [Google Scholar]
- Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF 2002 Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-α: implications for insulin resistance. Diabetes 51:3176–3188 [DOI] [PubMed] [Google Scholar]
- Hatano E, Brenner DA 2001 Akt protects mouse hepatocytes from TNF-α- and Fas-mediated apoptosis through NK-κ B activation. Am J Physiol Gastrointest Liver Physiol 281:G1357–G1368 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- de Alvaro C, Teruel T, Hernandez R, Lorenzo M 2004 Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J Biol Chem 279:17070–17078 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM 1996 IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271:665–668 [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ 2003 Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2:554–565 [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M 2004 Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 10:1128–1132 [DOI] [PubMed] [Google Scholar]
- Lee YH, Giraud J, Davis RJ, White MF 2003 c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278:2896–2902 [DOI] [PubMed] [Google Scholar]
- Yang S, Hu S, Hsieh YC, Choudhry MA, Rue III LW, Bland KI, Chaudry IH 2006 Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J Mol Cell Cardiol 40:570–579 [DOI] [PubMed] [Google Scholar]
- Yang S, Hu S, Choudhry MA, Rue III LW, Bland KI, Chaudry IH 2007 Anti-rat soluble IL-6 receptor antibody down-regulates cardiac IL-6 and improves cardiac function following trauma-hemorrhage. J Mol Cell Cardiol 42:620–630 [DOI] [PubMed] [Google Scholar]
- Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ 1993 Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg 218:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaagenes P, Gundersen Y, Opstad PK 2003 Rapid rewarming after mild hypothermia accentuates the inflammatory response after acute volume controlled haemorrhage in spontaneously breathing rats. Resuscitation 58:103–112 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Budavari A, Murray D, Spiegelman BM 1994 Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-α. J Clin Invest 94:1543–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]