Abstract
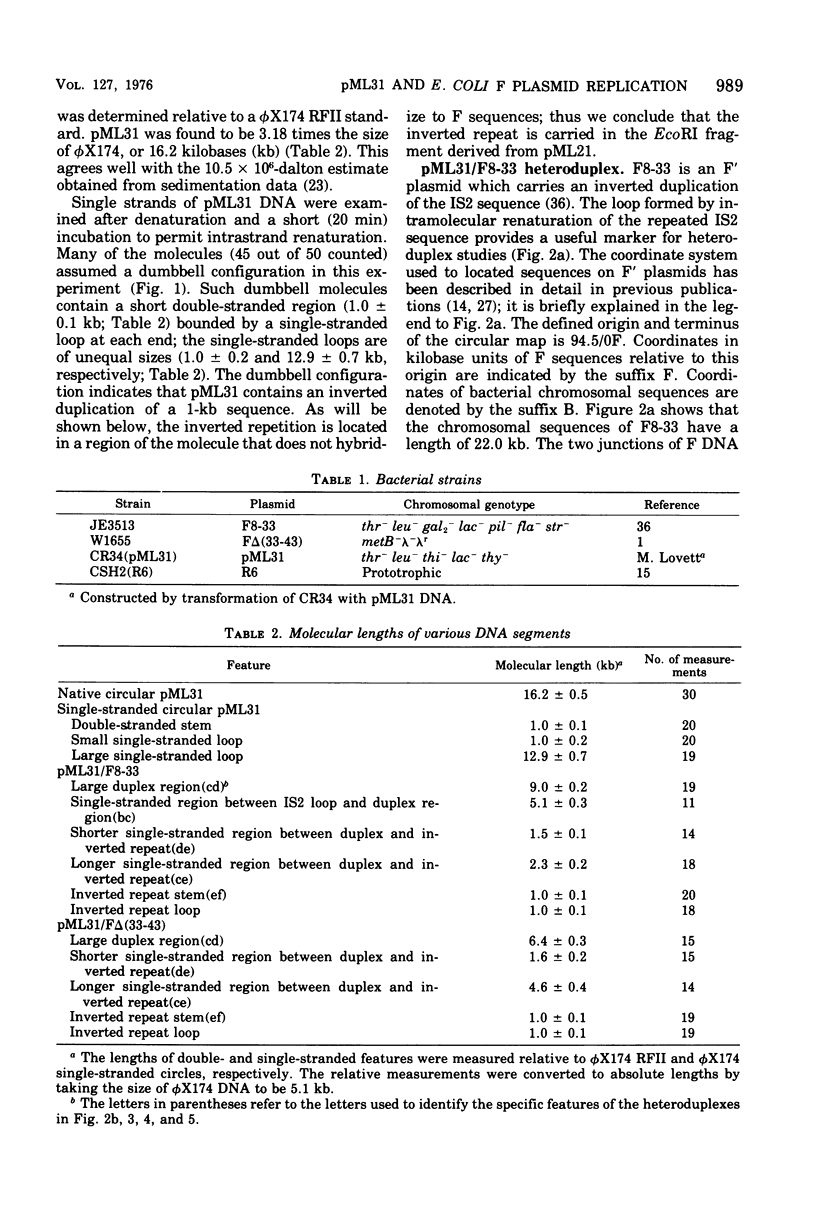
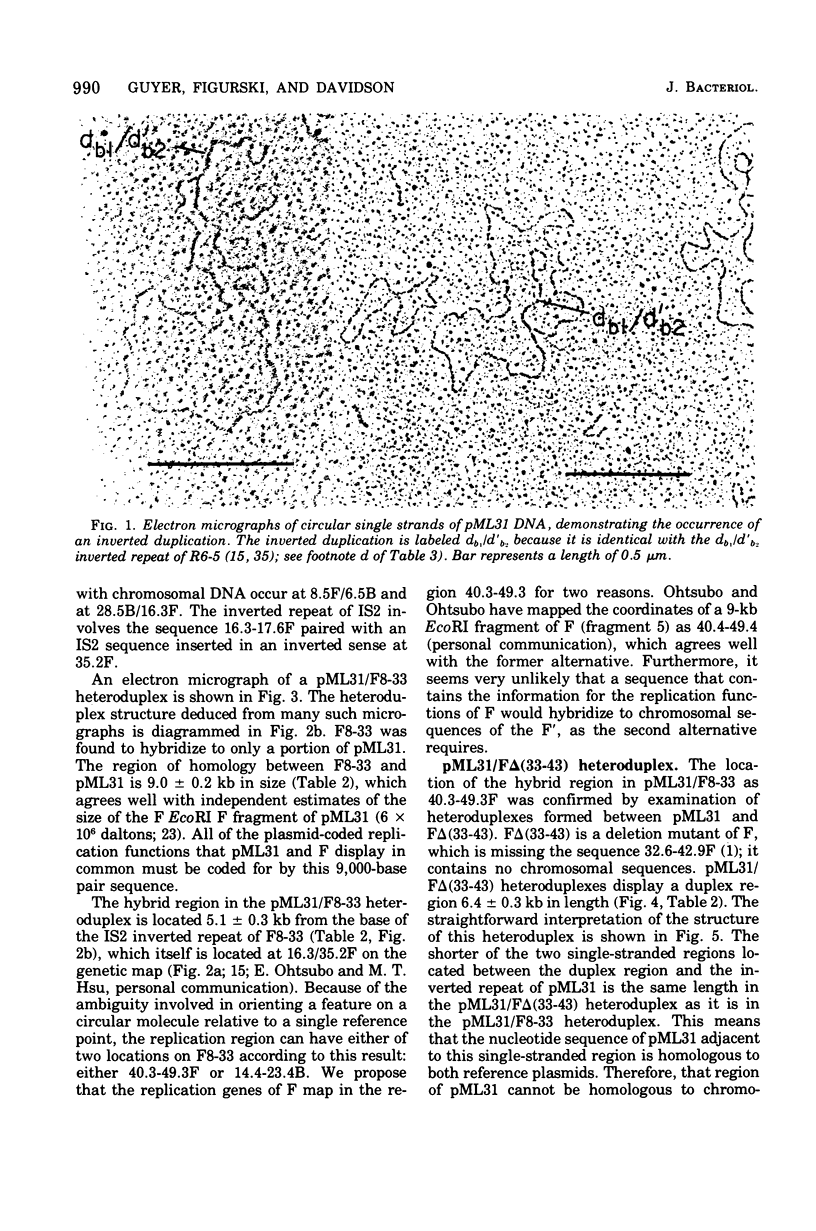
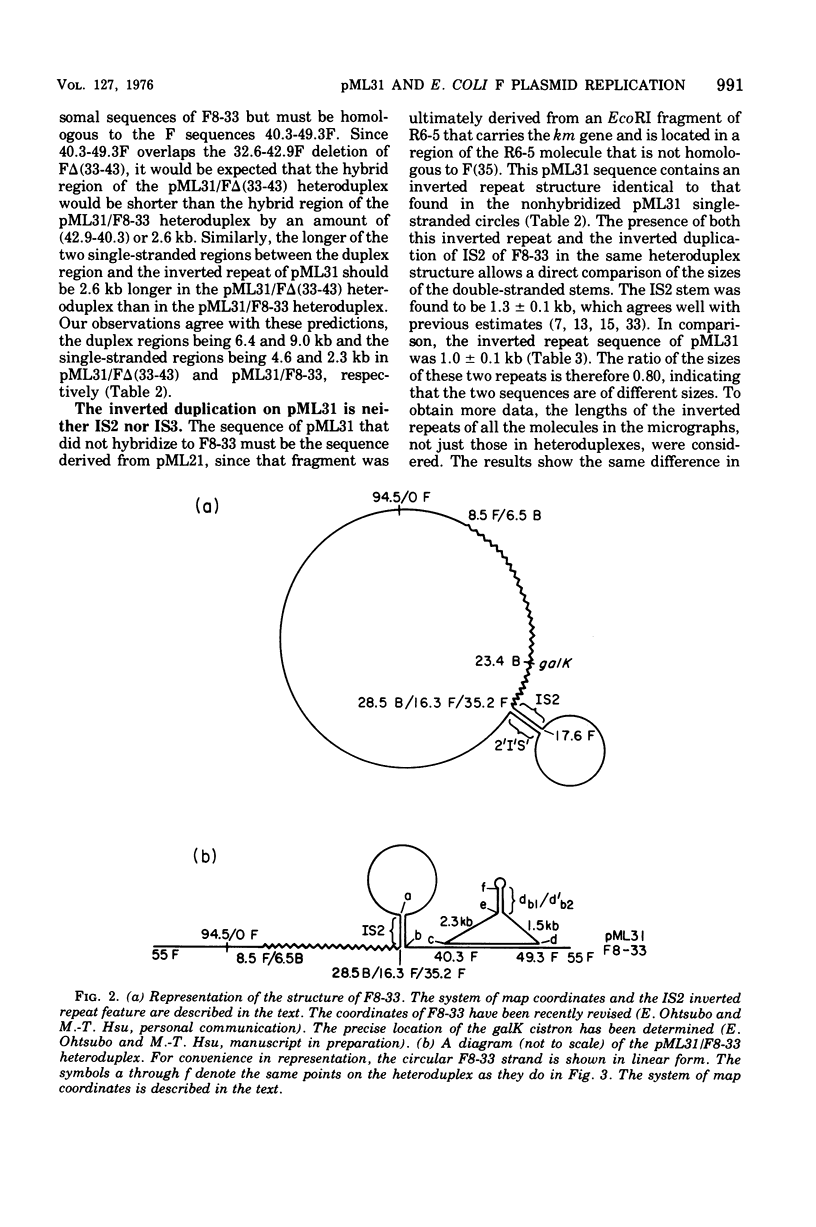
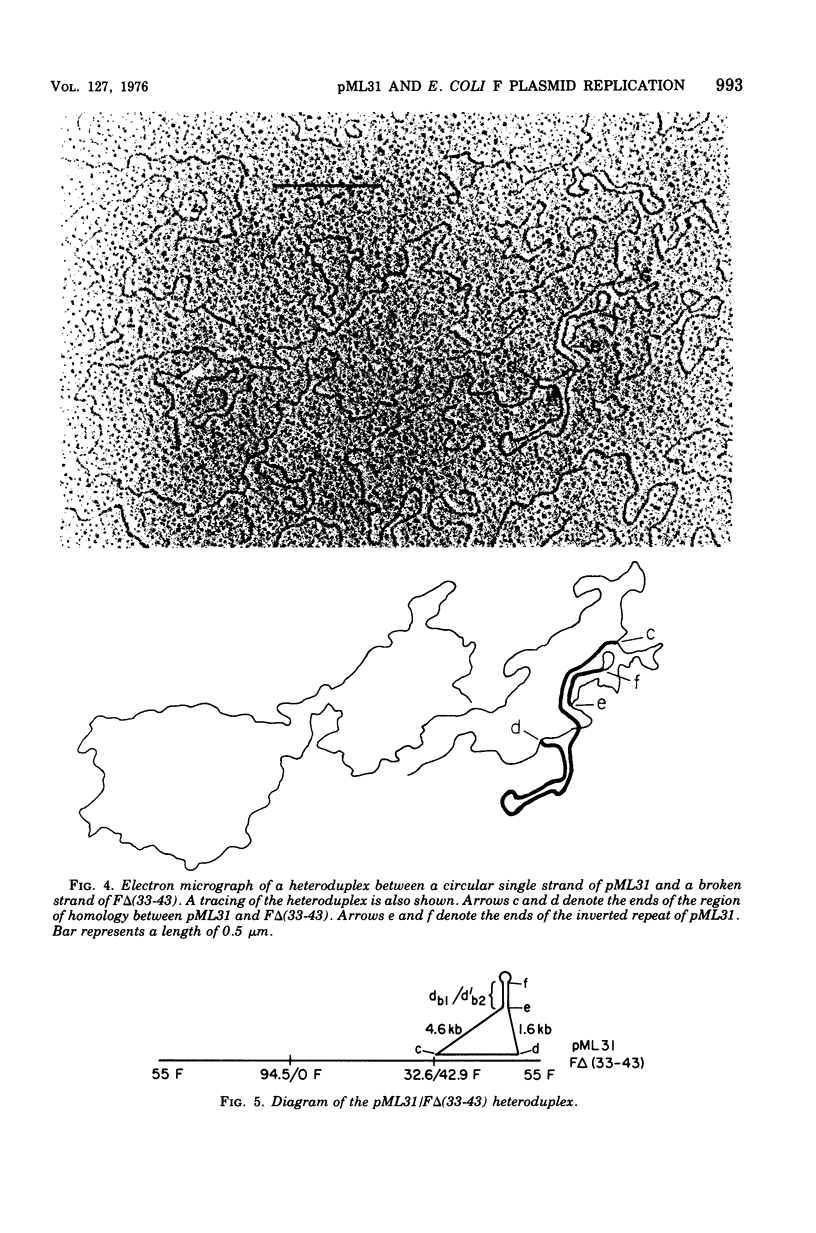
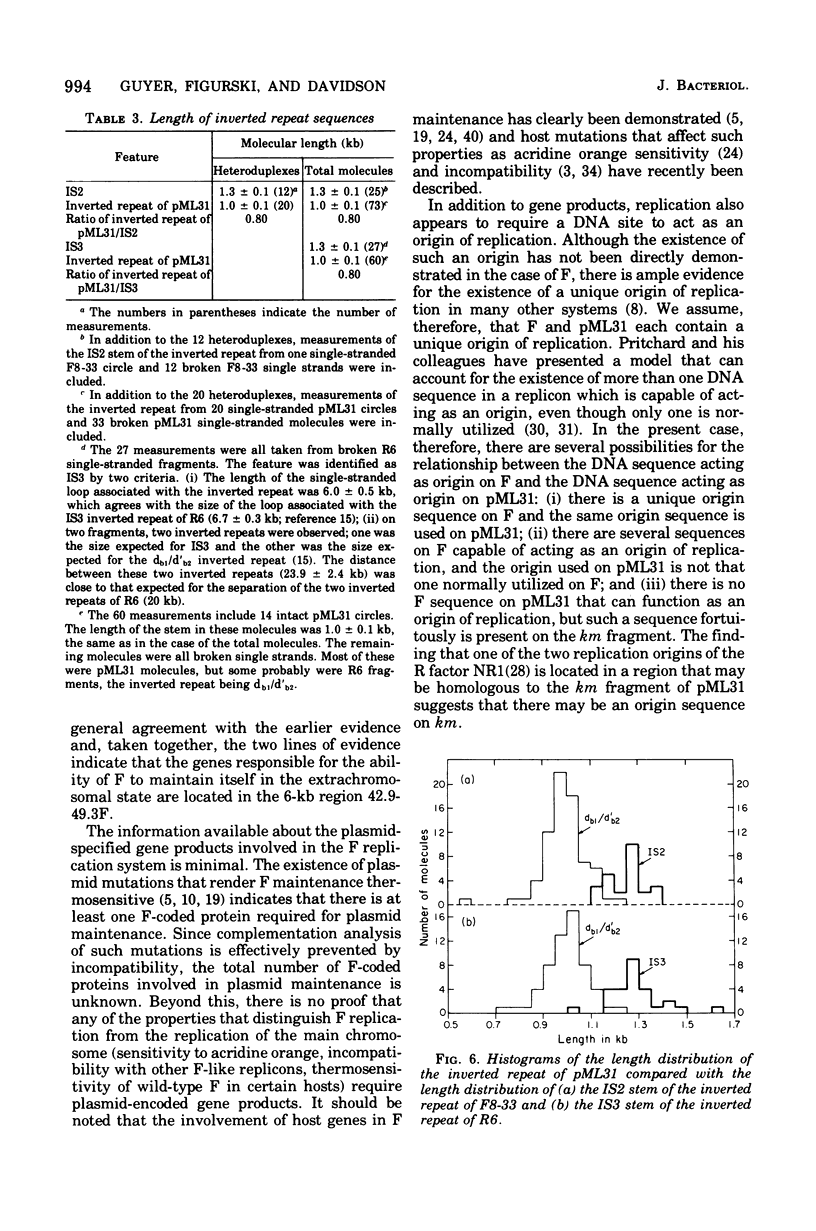
pML31, a plasmid chimera constructed to contain the replication genes of an Flac plasmid, has been studied by electron microscope methods. Heteroduplex analysis shows that the only F sequence present in pML31 is that with corrdinates 40.3-49.3F. This region has previously been identified as essential for plasmid maintenance. The sequence of pML31, which was derived originally from R6-5, carries the km gene(s) and an inverted duplication of a 1.0-kilobase sequence. On the basis of length measurements, the repeated sequence is different from IS1, IS2, IS3, and an inverted repeat associated with the km gene(s) of plasmid JR67.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony W. M., Deonier R. C., Lee H. J., Hu S., Otsubo E., Davidson N., Broda P. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. IX. Note on the deletion mutant of F, F delta(33-43). J Mol Biol. 1974 Nov 15;89(4):647–650. doi: 10.1016/0022-2836(74)90041-2. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanson G. S., Iyer V. N. dnaB gene of Escherichia coli K-12 affects superinfection inhibition between F' plasmids. J Bacteriol. 1975 Jul;123(1):137–146. doi: 10.1128/jb.123.1.137-146.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Rosner J. L. Acquisition of a determinant for chloramphenicol resistance by coliphage lambda. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5041–5045. doi: 10.1073/pnas.72.12.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway B. G., Bergquist P. L. Temperature-sensitive mutations affecting the replication of F-prime factors in Escherichia coli K 12. Mol Gen Genet. 1973 Dec 31;127(4):297–306. doi: 10.1007/BF00267100. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Hirsch H. J., Starlinger P., Brachet P. Two kinds of insertions in bacterial genes. Mol Gen Genet. 1972;119(3):191–206. doi: 10.1007/BF00333858. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J., Oka A. Discontinuous replication of colicin E1 plasmid deoxyribonucleic acid. J Bacteriol. 1975 Aug;123(2):739–742. doi: 10.1128/jb.123.2.739-742.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli. I. Isolation and specificity of host and plasmid mutations. Genetics. 1973 May;74(1):17–31. doi: 10.1093/genetics/74.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Plasmid-instability in acrA mutants of Escherichia coli K12. J Gen Microbiol. 1974 Sep;84(1):85–93. doi: 10.1099/00221287-84-1-85. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Deonier R. C., Lee H. J., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. IV. The F sequences in F14. J Mol Biol. 1974 Nov 15;89(4):565–584. doi: 10.1016/0022-2836(74)90036-9. [DOI] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Two origins of replication in composite R plasmid DNA. Nature. 1976 Jan 29;259(5541):281–284. doi: 10.1038/259281a0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Twose T. M., Holland M. J., Rownd R. H. Denaturation mapping of R factor deoxyribonucleic acid. J Bacteriol. 1975 Sep;123(3):1035–1042. doi: 10.1128/jb.123.3.1035-1042.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard R. H., Chandler M. G., Collins J. Independence of F replication and chromosome replication in Escherichia coli. Mol Gen Genet. 1975;138(2):143–155. doi: 10.1007/BF02428118. [DOI] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- San Blas F., Thompson R., Broda P. An Escherichia coli K12 mutant apparently carrying two autonomous F-prime factors. Mol Gen Genet. 1974 May 21;130(2):153–163. doi: 10.1007/BF00269086. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres E. F., Nandadasa H. G., Pritchard R. H. Suppression of initiation-negative strains of Escherichia coli by integration of the sex factor F. J Bacteriol. 1975 Feb;121(2):554–561. doi: 10.1128/jb.121.2.554-561.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]





