Abstract
In the absence of sulfur (S), Chlamydomonas reinhardtii increases the abundance of several transcripts encoding proteins associated with S acquisition and assimilation, conserves S amino acids, and acclimates to suboptimal growth conditions. A positive regulator, SAC1 (for sulfur acclimation protein 1), and a negative regulator, SAC3, were shown to participate in the control of these processes. In this study, we investigated two allelic mutants (ars11 and ars44) affected in a gene encoding a SNRK2 (for SNF1-related protein kinase 2) kinase designated SNRK2.1. Like the sac1 mutant, both snrk2.1 mutants were deficient in the expression of S-responsive genes. Furthermore, the mutant cells bleached more rapidly than wild-type cells during S deprivation, although the phenotypes of ars11 and ars44 were not identical: ars11 exhibited a more severe phenotype than either ars44 or sac1. The phenotypic differences between the ars11 and ars44 mutants reflected distinct alterations of SNRK2.1 mRNA splicing caused by insertion of the marker gene. The ars11 phenotype could be rescued by complementation with SNRK2.1 cDNA. In contrast to the nonepistatic relationship between SAC3 and SAC1, characterization of the sac3 ars11 double mutant showed that SNRK2.1 is epistatic to SAC3. These data reveal the crucial regulatory role of SNRK2.1 in the signaling cascade critical for eliciting S deprivation responses in Chlamydomonas. The phylogenetic relationships and structures of the eight members of the SNRK2 family in Chlamydomonas are discussed.
Sulfur (S) is an essential element present in proteins, lipids, carbohydrates, electron carriers, various metabolites (some involved in the detoxification of heavy metals and xenobiotics), and signaling molecules (Meister and Anderson, 1983; Gupta et al., 1990; Schultze et al., 1992; Marrs, 1996; Grossman and Takahashi, 2001). The preferred S source for most organisms is sulfate (SO42−), which can be limiting in the environment; limitations for SO42− may result in reduced quality and yield of seeds and cause stunted plant growth (Mahler and Maples, 1986, 1987; Warman and Sampson, 1994). Most organisms are unable to efficiently store S; therefore, they are dependent on a continuous supply of this nutrient.
When organisms become S limited, they exhibit a suite of responses that have been described as either specific or general. The specific responses to nutrient limitation are those associated with deprivation for a single nutrient and are often involved in scavenging or conserving that specific nutrient. For example, during S deprivation, Chlamydomonas reinhardtii synthesizes periplasmic arylsulfatases (ARS) that catalyze the hydrolysis of organic SO42− esters (de Hostos et al., 1989), develops more efficient SO42− transport (Yildiz et al., 1994) and assimilation (Yildiz et al., 1996; Ravina et al., 2002), and maximizes S utilization efficiency, which could involve significant changes in cell architecture (Grossman and Takahashi, 2001; Zhang et al., 2004). General responses are those associated with deprivation for any essential nutrient and include the cessation of cell growth and division, the accumulation of storage carbohydrates, and the modulation of metabolic processes, including a decrease in photosynthetic activity. The coordination of metabolic processes in the cell during nutrient limitation is critical because an imbalance between the generation of fixed carbon and reducing equivalents with the potential for the cell to grow and divide can stimulate the production of damaging reactive oxygen species. Managing photosynthetic activities and the accumulation of fixed carbon is particularly important, and a number of investigators have characterized the ways in which photosynthesis is controlled by conditions that limit cell growth (Peltier and Schmidt, 1991; Wykoff et al., 1998).
Very little is known about regulatory mechanisms that control S nutrition in vascular plants and other photosynthetic eukaryotes. The generation of insertional mutants of Chlamydomonas has resulted in the identification of two specific regulators of S deprivation responses, SAC1 (sulfur acclimation protein 1) and SAC3. SAC1 plays a central role in controlling S deprivation responses (Davies et al., 1996; Zhang et al., 2004), and sac1 mutants are unable to synthesize ARS and to induce many genes that are normally up-regulated when the cells are starved for S (Yildiz et al., 1996; Takahashi et al., 2001; Ravina et al., 2002; Zhang et al., 2004). In addition, the general responses in sac1 mutants are impaired, and the cells rapidly bleach and die during S deprivation (Davies et al., 1996). This death is light dependent and has been linked to a failure of the cells to down-regulate photosynthetic electron flow out of PSII, suggesting that the modification of photosynthetic electron transport during S deprivation is critical for cell survival.
SAC1 has significant sequence similarity to the animal S transporters (Na+/SO42− transporters). Three additional genes that encode proteins with strong sequence similarity to mammalian Na+/SO42− transporters (SLT1–SLT3) have been identified on the Chlamydomonas genome. The physiological effects of the sac1 mutation strongly imply that SAC1 is a hierarchical regulator critical for the acclimation of cells to S deprivation and raise the possibility that transporter-like proteins might have evolved into sensor proteins that are critical for acclimation processes (Davies et al., 1996).
The SAC3 S deprivation regulator of Chlamydomonas is a putative Ser/Thr kinase in the plant-specific SNF1-related protein kinase 2 (SNRK2) family. The sac3 mutants exhibit low-level constitutive ARS activity in S-replete medium, but like wild-type cells, they accumulate high levels of ARS following exposure of the cells to S deprivation. Other S-responsive genes (in addition to ARS) are also negatively regulated by SAC3 (Ravina et al., 2002). Furthermore, the sac3 mutant does not show the dramatic decrease in chloroplast transcriptional activity that is observed in wild-type cells during S starvation, and the SAC3 kinase may be required to inactivate chloroplast RNA polymerase sigma factor SIG1 under S deprivation conditions (Irihimovitch and Stern, 2006). The relationship between SAC1 and SAC3 is nonepistatic, since the sac1 sac3 double mutant maintains the phenotype of both of the parental strains (Davies et al., 1994).
Recently, a collection of mutants that exhibit low ARS activity and that are potentially affected in S deprivation responses was generated by insertional mutagenesis (Pollock et al., 2005). Among this mutant population are two strains, ars11 and ars44, that harbor an interruption in the same gene. This gene, designated SNRK2.1, encodes a SNRK2 kinase with sequence similarity to SAC3. In this work, we demonstrate that SNRK2.1 plays a crucial role in the control of S deprivation responses. The ars11 lesion dramatically affects S deprivation-responsive gene expression, and the mutant cells rapidly bleach following transfer to medium lacking S. Furthermore, unlike sac1, ars11 is epistatic to sac3, reflecting a key position of the SNRK2.1 kinase in the control of S deprivation responses.
RESULTS
Isolation of the ars Mutants
Insertional mutants (disrupted with the AphVIII marker gene) of Chlamydomonas were screened for abnormal levels of extracellular ARS activity after transferring cells to TAP-S medium (for Tris-acetate phosphate medium without S). Two kinds of mutants were identified: those with no or low ARS activity (ars− mutants) and those that exhibited more ARS activity than the parental strain (ars+ mutants). Approximately 30,000 transformants were screened, and from those 50 strains exhibited an ars− phenotype and two exhibited an ars+ phenotype (Pollock et al., 2005).
An adaptor-mediated PCR technique (Padegirnas and Reichert, 1998) was successfully used to determine the genomics regions flanking the AphVIII marker for a number of the ars mutants. Data showing ARS activities and the flanking regions of some of these mutants were reported previously (Pollock et al., 2005). Among the ars− mutants for which flanking sequences were obtained, both ars11 and ars44 had an AphVIII insertion in the same gene, but at different sites within that gene. The interrupted gene encodes a putative Ser/Thr protein kinase belonging to the SNRK2 plant kinase family (Halford and Hardie, 1998). This gene has been designated SNRK2.1.
Cosegregation of Paromomycin Resistance with the ars Phenotype
To determine whether the ars− phenotype was linked to the AphVIII insertion, the ars11 and ars44 mutants were crossed with the wild-type 21gr strain, which is sensitive to paromomycin. For both mutants, the paro+ progeny (approximately 30 of a total of 60 analyzed) cosegregated with the ars− phenotype, strongly supporting the conclusion suggested by the occurrence of two alleles with related phenotypes that the insertion was the cause of the mutant phenotype.
Physiological Characterization of ars11 and ars44 Mutants
The ars11 and ars44 mutants exhibited no or low ARS activity when the cells were deprived of S, although the phenotypes of the strains were not identical. Like sac1, ars11 had no detectable ARS activity on agar plates, while ars44 exhibited reduced ARS activity; the ars44 strain accumulated substantial levels of ARS activity if the cells were maintained on TAP-S plates for a long enough period of time (Fig. 1A). In liquid cultures, ars44 showed essentially no ARS activity after 24 h of S starvation (Fig. 1B), and even after several days of S starvation, very low-level activity was observed (data not shown).
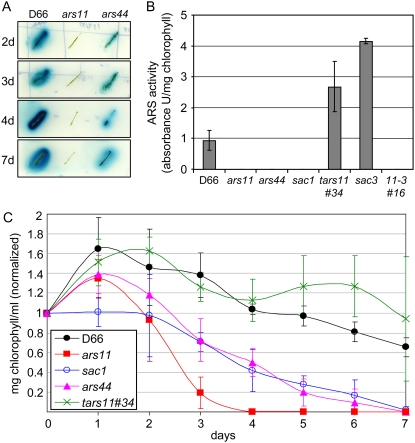
Figure 1.
ARS activity and chlorophyll in the ars mutants. A, ARS activity on solid medium. Cells were streaked onto the surface of solid TAP-S medium and grown for several days before ARS activity was assayed. ARS activity is detected as a blue halo surrounding the colonies. B, ARS activity in liquid medium. ARS activity was measured after 24 h of S starvation. The data are averages of three independent experiments. C, Loss of chlorophyll in S-starved cells. Chlorophyll a and b content was normalized to the value on day 0 for each strain. Values represent means of three independent experiments. Strains used are D66 (parental strain), ars11 and ars44 (snrk2.1−), tars11#34 (ars11 complemented with SNRK2.1), sac1 (sac1 mutant), sac3 (sac3 mutant), and 11-3#16 (ars11 sac3 double mutant).
Davies et al. (1996) showed that the sac1 strain is defective for the specific and general S deprivation responses. One feature of sac1 is its rapid loss of chlorophyll and death, relative to wild-type cells, following the imposition of S deprivation; this death response may be a consequence of the inability of the mutant to decrease the rate of photosynthetic electron flow through PSII (Davies et al., 1996). The ars11 and ars44 mutants also bleached (Fig. 1C) and died much more rapidly than wild-type cells when transferred to TAP-S medium, suggesting that, like sac1, these strains are aberrant for both the specific and general S deprivation responses. Interestingly, the ars11 strain bleached and died significantly more rapidly than either ars44 or sac1.
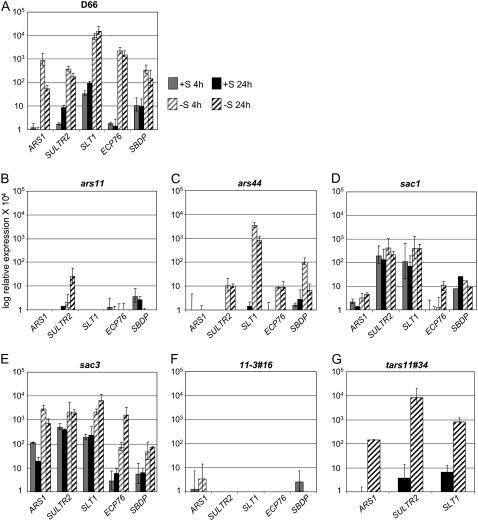
To further analyze the responses of the ars11 and ars44 strains to S deprivation, the levels of transcripts from several genes that were already known to be controlled by the S status of the medium were analyzed; these genes included ARS1 for the arylsulfatase (de Hostos et al., 1989), SLT1 for the Na+/SO42−-like transporter (Zhang et al., 2004), SULTR2 for the H+/SO42−-like transporter (W. Pootakham and A.R. Grossman, unpublished data), ECP76 for an extracellular protein that is probably associated with the cell wall (Takahashi et al., 2001), and SBDP for a putative selenium-binding protein (Zhang et al., 2004; Fig. 2, A–C). Both the ars11 and ars44 mutants had lower basal levels for most of these transcripts, with little or no increase (relative to wild-type cells) following the imposition of S deprivation (with some exceptions for ars44). However, both the ARS activity assay and the kinetics of the bleaching response (Fig. 1) demonstrated that the phenotype of ars44 is significantly less severe than that of ars11. This difference in severity is apparent based on the analysis of transcript levels from most of the genes examined and exemplified by the changes in SLT1 transcript abundance following S deprivation; while no increase in the transcript level was observed in ars11, a strong increase (somewhat lower than that of the parental D66 strain) was observed for ars44. The pattern of expression for these same genes was also analyzed in sac1 (Fig. 2D). As in ars11, the sac1 mutant showed no or only a small increase in the levels of known S deprivation-responsive transcripts when the cells were starved for S, although the basal transcript levels in TAP medium in the sac1 strain were generally higher than in either ars11 or ars44. In contrast, the sac3 mutant showed relatively high levels of transcripts for a number of the genes tested, but especially for ARS1, under S-replete conditions, with increased accumulation when the cells were starved for S (Fig. 2E). These results demonstrate that the SNRK2.1 protein is critical for the accumulation of transcripts from the S deprivation-responsive genes when cells are starved for S. The inability of the mutant cells to respond to S deprivation at the level of transcript accumulation (and likely gene activation) may also lead to the rapid bleaching and loss of viability following the transfer of the mutant strains to TAP-S medium.
Figure 2.
qPCR analysis of S-regulated gene expression. The parental strain (A) and mutants (B–E) were grown on TAP medium, washed with TAP-S medium, and then resuspended in either TAP or TAP-S. Samples were taken for qPCR analysis at 4 and 24 h following the transfer of exponentially growing cells to fresh TAP or TAP-S. Levels of individual transcripts are given as relative fold abundance with respect to the housekeeping control gene (CBLP). None of the values obtained was much below 1, and those values that were below 1 are represented as 0 on the graphs. Experiments were performed in triplicate. For the 11-3#16 double mutant, the transcript levels were analyzed only at 4 h (F), and for the SNRK2.1 complemented strain, tars11#34, the ARS1, SULTR2, and SLT1 transcript levels were analyzed at 24 h (G). The strains used for these analyses were D66, ars11 and ars44 (snrk2.1−), tars11#34 (complemented with SNRK2.1), sac1 (sac1 mutant), sac3 (sac3 mutant), and 11-3#16 (ars11 sac3 double mutant).
The cDNAs and Sites of Insertion in ars11 and ars44
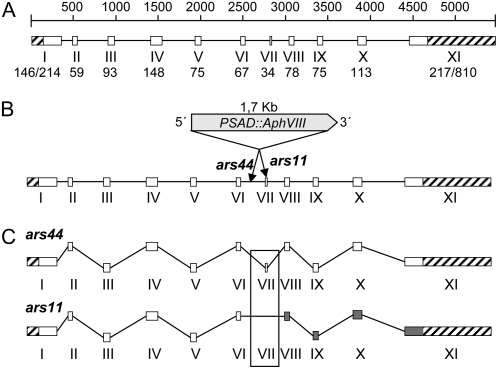
There were no EST sequences to help identify the complete coding sequence (CDS) and intron-exon boundaries of the SNRK2.1 genomic sequence. Therefore, a 2,129-bp cDNA was amplified by reverse transcription (RT)-PCR from the parental strain RNA. Based on the sequence of the cDNA, the gene contains a 1,173-bp CDS with an 810-bp 3′ untranslated region (UTR) and a 146-bp 5′ UTR. Other cDNA sequences that could represent alternative splice forms of the SNRK2.1 transcript were also amplified (see below). The cDNA sequence was different from the predicted sequence of the ab initio model for the gene that was generated by the Joint Genome Institute (JGI; http://genome.jgi-psf.org/Chlre3/Chlre3.home.html). We recently updated this model to take into account cDNA sequence information. The genomic sequence corresponding to the cDNA was 5.46 kb in length, consisting of 11 exons and 10 introns (Fig. 3A), encoding a predicted polypeptide of 390 amino acids.
Figure 3.
A, Genomic structure of SNRK2.1. The white blocks and lines represent exons and introns, respectively. Exons are numbered with roman numerals, and the corresponding sizes (bp) are given below. For exons I and XI, the two sizes given represent the 5′ UTR/CDS and CDS/3′ UTR, respectively. 5′ and 3′ UTRs are represented with striped blocks. The diagram is drawn to scale. B, Marker insertion in SNRK2.1 of ars11 and ars44. The PSAD∷AphVIII marker gene is inserted within exon VII in the ars11 mutant and within intron 6 in the ars44 mutant. In both cases, the marker gene has the same orientation as SNRK2.1. C, mRNA maturation in the ars11 and ars44 mutants. In the ars44 mutant, all of the introns appear to be properly spliced and a normal protein is synthesized. The ars11 mRNA lacks exon VII and an aberrant protein is synthesized. The gray blocks represent exons for which the reading frame has changed.
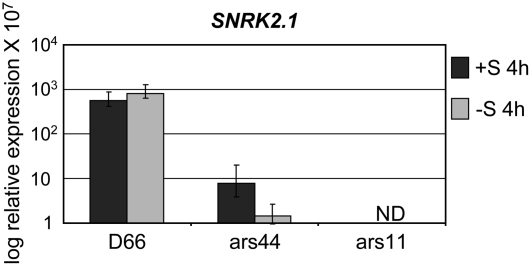
Adaptor-mediated PCR was used to identify one of the genomic regions flanking the AphVIII marker gene for both ars11 and ars44 (the border close to the 3′ end of AphVIII; Pollock et al., 2005). In ars11, the insertion was positioned within exon VII, whereas in ars44, the insertion was within intron 6 (Fig. 3B). To identify the flanking regions on both sides of the insertions, specific primer pairs were designed to anneal to the SNRK2.1 genomic sequence close to the site of insertion and to the marker gene. Amplifications using these primers and analysis of the flanking sequences showed that neither of the insertion events was accompanied by deletions or by a reorganization of the genome around the site of the insertion (Kindle, 1990). Using RT-PCR, we demonstrated that neither ars11 nor ars44 was completely lacking SNRK2.1 transcripts; however, the mRNA from SNRK2.1 in ars11 was missing the sequence encoded by exon VII, while ars44 retained a full-length SNRK2.1 transcript that was indistinguishable from that of the parental strain (Fig. 3C). In ars11, splicing resulted in a fusion of exon VI to exon VIII, which eliminated exon VII (the site of the insertion) from the processed transcript (Fig. 3C). Furthermore, the translated protein of the ars11 mutant would have a shift in the reading frame generated as a consequence of the fusion of exons VI to VIII. When SNRK2.1 transcript accumulation was determined using primers specific for the 3′ UTR, no significant difference between the mutant and wild-type transcript levels was observed (data not shown). However, when SNRK2.1 transcript accumulation in ars11 was determined using primers specific for exons VI and VII, no transcript was detected. In contrast, the same primers did reveal the presence of a transcript with the proper fusion of exon VI and VII in ars44, although the transcript was reduced by approximately two orders of magnitude in this mutant strain (with the greatest reduction observed in cells exposed to 4 h of −S conditions) relative to parental cells (Fig. 4). Thus, the ars44 strain is likely able to synthesize normal SNRK2.1 protein, but the level of this protein may be significantly lower than in parental cells because of the low-level accumulation of the mRNA. The molecular differences associated with mRNA processing are likely responsible for the different mutant phenotypes. Furthermore, the data demonstrate that splicing of intron 6 of SNRK2.1 can occur even if the intron is interrupted with the 1.7-kb PSAD∷AphVIII marker gene.
Figure 4.
SNRK2.1 expression as determined by qPCR. Primers used were specifically designed to detect the joining of exons VI and VII (see Materials and Methods for details). RNA sample preparation was as described for Figure 2. Levels of individual transcripts are given as relative fold abundance with respect to the housekeeping control gene (CBLP). ND, No transcript detected. Experiments were performed in triplicate.
Potential Alternative Splicing of SNRK2.1
As mentioned above, SNRK2.1 cDNA generation led to multiple cDNAs of different lengths. At least five different SNRK2.1 cDNAs were identified using the same set of specific primer pairs for RT-PCR (the RNA template was from D66 cells grown in TAP and TAP-S); these cDNAs were designated SNRK2.1 v1, v2, v3, v4, and v5, and the differences among them are shown in Supplemental Figure S1. SNRK2.1 v1 was the longest cDNA, encoding a polypeptide of 390 amino acids, and is the isoform discussed above (referred to as SNRK2.1). All of these potential transcripts encoded putative polypeptides that used the same start codon, with SNRK2.1 v1, v2, and v3 maintaining the same coding frame and stop codon. In contrast, the SNRK2.1 v4 and v5 reading frames changed as a result of the alternative splicing, which also generated a different stop codon. For SNRK2.1 v2 to v4, the potential alternative 5′ splice sites were positioned inside a 54-bp GC-rich region of the transcript. The deduced SNRK2.1 v2 protein maintained the kinase catalytic domain and the end of the C-terminal domain, whereas v3 lacked 20 amino acids from the kinase domain and part of the C-terminal region. The SNRK2.1 v4 and v5 predicted polypeptides lacked the kinase domain and the entire C terminus. An alignment of all of the potential splice variants is shown in Supplemental Figure S2. None of the potential splice variants was associated with a specific culture condition (e.g. TAP compared with TAP-S), and the only variant reproducibly observed was SNRK2.1 v1; the others were less abundant and in some reactions were not detected. Finally, only in SNRK2.1 v1 did all of the splice junctions match the consensus junctions associated with Chlamydomonas genes (Silflow, 1998), which raised the possibility that the variants were a consequence of artifacts associated with cDNA synthesis and amplification. In addition, two alternative 3′ polyadenylation sites were identified by 3′ RACE from the SNRK2.1 cDNA sequences (Supplemental Fig. S3); these sites were not associated with specific recombinant libraries or splice variants.
To help establish if the small SNRK2.1 transcripts were potentially artifacts, we used specific primers to generate PCR products from a pGEM-T clone harboring the SNRK2.1 v1 cDNA. A multiband amplification pattern was observed, and at least one of the products had a gap in the sequence starting within the same GC-rich region that was identified as the 5′ splice junction for putative splice variants. However, the products generated by PCR from the plasmid did not perfectly match any of the putative SNRK2.1 splice versions (data not shown). These observations raise the possibility that putative alternative splice forms can arise from aberrant cDNA synthesis and amplification in vitro, and not from in vivo splicing of precursor mRNA.
Complementation of the snrk2.1 Mutation
Four of the SNRK2.1 cDNA potential splice variants (v1, v2, v3, and v4) were introduced into the ars11 mutant strain. Each cDNA version was inserted between the PSAD promoter and the 3′ UTR terminator and cloned into a vector containing the ble gene as a selectable marker. Fifty ble-resistant colonies for each splice variant were tested for ARS activity following exposure to TAP-S. Four colonies transformed with the SNRK2.1 v1 cDNA restored the wild-type ARS phenotype, based on ARS plate assays. An analysis of ARS activity in liquid for one of the potentially complemented mutant strains (tars11#34) showed that the strain was rescued for the ars− phenotype; it produced high levels of ARS when deprived of S (Fig. 1B), with no ARS activity detectable when grown in TAP (data not shown). No complemented colonies were obtained that harbored v2, v3, or v4 cDNA variants. Analysis of chlorosis of the tars11#34 strain in TAP-S also revealed restoration of the wild-type phenotype (Fig. 1C); transformants expressing ARS survived S deprivation for an extended period of time, like the D66 parental strain. Finally, the levels of ARS1, SLT1, and SULTR2 mRNAs measured in tars11#34 were similar to those of the parental strain (Fig. 2G). These results clearly demonstrated that the mutant phenotype can be complemented by the full-length SNRK2.1 gene.
ars11 sac3 Double Mutant Analysis
SNRK2.1 is required for the activation of genes associated with S deprivation responses, while SAC3 acts as a negative regulator of at least some of these same genes (Davies et al., 1999). To help define the role of the SAC3 and SNRK2.1 kinases in the regulation of S deprivation responses, the epistatic relationships between the sac3 and ars11 lesions were examined by constructing strains harboring both lesions. Four double mutants were identified, and none exhibited detectable ARS activity when grown on TAP or following transfer of the cells to TAP-S (data not shown), indicating that the sac3 lesion is hypostatic to the snrk2.1 lesion; SNRK2.1 is needed in a sac3 mutant to enable the constitutive expression of ARS. Expression of ARS1, SLT1, SULTR2, ECP76, and SBDP was analyzed in the randomly selected double mutant 11-3#16 (Fig. 2F). The double mutant, like the ars11 single mutant, exhibited extremely low levels of transcript accumulation for all tested transcripts in both TAP and TAP-S (Fig. 2, compare B with F).
The SNRK2 Family
SNRK2.1 belongs to the plant-specific Ser/Thr protein kinase family SNRK2. Like other SNRK2 family members, SNRK2.1 has an N-terminal conserved catalytic domain similar to those of SNF1/AMP kinases and a short C-terminal regulatory domain that is not highly conserved. SAC3 (Davies et al., 1999; Irihimovitch and Stern, 2006) is also a member of the SNRK2 family, with 43% sequence identity with SNRK2.1. In addition to SNRK2.1 and SAC3, there are six other SNRK2 family members encoded on the Chlamydomonas genome. The complete eight-member family, SNRK2.1 to SNRK2.8, with SAC3 now being designated SNRK2.2, is presented in Table I. The deduced proteins for SNRK2.4, SNRK2.5, SNRK2.6, and SNRK2.8 should be taken as provisional, since the corresponding JGI gene models are impossible to accurately predict because of gaps in the genomic sequences and incomplete EST sequence coverage. Although we have improved on these gene models based on EST and genomic sequences and similarities to other SNRK2 kinases, they are still incomplete, mainly in their C-terminal regions. SNRK2.1 and SNRK2.3 are clustered in the same genomic region (scaffold 7, linkage group II) in a tail-to-head orientation and separated by less than 500 bp. SNRK2.3 and SNRK2.4 are the most closely related to SNRK2.1. These three family members form a Chlamydomonas SNRK2 subgroup that is most different from the plant SNRK2 homologs. Members of this subgroup also have unique sequence features that are not present in other SNRK2 proteins; one major feature is the presence of an extra loop of 17 to 22 amino acids immediately preceding the SNF1 kinase activation domain (Hardie and Carling, 1997), which is shown in the alignment in Figure 5 (broken line). Although this extra loop is not conserved at the sequence level among the subfamily members, it does contain a NLH motif (asterisks above the broken line in Fig. 5) and several residues that might serve as phosphorylation sites. An alignment of the entire Chlamydomonas SNRK2 family of proteins is shown as Supplemental Figure S4.
Table I.
The Chlamydomonas SNRK2 family
Protein identifiers, scaffold localization, and the presence/absence of associate ESTs are given and can be found on the Chlamydomonas JGI Web server. When ESTs are present, they were used to validate the genomic sequences and gene models. The amino acid identities of the deduced N-terminal catalytic sequences with those of Arabidopsis and rice are given as a range of identities (the analysis was performed using MatGAT 2.0; Montclair State University).
| Name | Mutant | Identifier | Size | Identity | ESTs | Scaffold | Exons |
|---|---|---|---|---|---|---|---|
| % | |||||||
| SNRK2.1 (ARS11) | ars11/ars44 | 206379 | 390 | 34–40 | No | 7 | 11 |
| SNRK2.2 (SAC3) | sac3 | 185806 | 357 | 47–56 | Yes, full | 78 | 11 |
| SNRK2.3 | 53567 | 408 | 34–41 | Yes, full | 7 | 11 | |
| SNRK2.4a | 131569 | 315 | 35–41 | Yes, partial | 33 | 10 | |
| SNRK2.5a | 132038 | 312 | 37–41 | Yes, partial | 47 | 6 | |
| SNRK2.6a | 131583 | 289 | 40–46 | Yes, partial | 33 | 7 | |
| SNRK2.7 | 153921 | 372 | 43–50 | Yes, full | 69 | 10 | |
| SNRK2.8a | 113331 | 330 | 60–69 | No | 4 | 9 |
Gene models that are likely inaccurate.
Figure 5.
Amino acid sequence alignment. The predicted SNRK2.1, SNRK2.2 (SAC3), SNRK2.3, and SNRK2.7 proteins were aligned with representative Arabidopsis and rice SNRK2 kinases (accession nos. as follows: AtSNRK2.1, NP_196476; AtSNRK2.2, NP_190619; AtSNRK2.3, NP_201489; SAPK1, NP_001050274; SAPK2, NP_001060312; SAPK4, NP_001044930) using BioEdit 7.0.5.3 software. The black and gray boxes indicate identical and similar amino acids, respectively. The C-terminal subdomains are highlighted with heavy black lines above the sequences and roman numerals above the line (Yoshida et al., 2006). The extra loop segment present in CrSNRK2.1 and CrSNRK2.3 (dotted line), the conserved NLH motif (***), and the conserved phosphorylated Ser of the activation domain (#) are noted.
DISCUSSION
In this study, we identified SNRK2.1, a member of the plant-specific SNRK2 kinase family and the larger SNF1 superfamily, as a key regulator of the pathway that governs S deprivation responses in Chlamydomonas. Two independent mutant strains in which the SNRK2.1 gene was interrupted, ars11 and ars44, were identified. The insertions did not cause reorganization or deletions of the genomic region at the insertion site, and the mutant phenotypes were linked to the insertions. Even though the mutants had a sequence of 1.7 kb (AphVIII under the control of the PSAD promoter and the 3′ UTR) integrated into the SNRK2.1 gene, the splicing machinery of Chlamydomonas was able to excise the inserted sequence and generate a mature mRNA; this mRNA was aberrant in the case of ars11. Insertion of the AphVIII marker gene into exon VII of SNRK2.1 in ars11 caused the loss of this exon in the final splice product and a change in the reading frame of the C-terminal region of the protein. These modifications of the SNRK2.1 protein are likely the reason for the severe mutant phenotype (the lesion probably represents a null mutation). In contrast, in ars44, the marker gene is integrated into intron 6. This interrupted intron (with the inserted marker DNA) was spliced out of the nascent transcript, generating a mature transcript that appeared to be identical to the transcript in the parental strain, although the level of accumulation of this mature transcript was more than 10 times lower than in the parental strain (Fig. 4). Therefore, the leaky phenotype of ars44 is probably a consequence of inefficient splicing of intron 6, which in turn could generate less protein.
Interestingly, in spite of a clear difference in ARS activity between ars11 and ars44 mutants based on agar plate assays (Fig. 1A), both strains showed no ARS activity when they were transferred to liquid TAP-S medium for 24 h (Fig. 1B); there was little or no ARS activity measured in either of the mutants, even after several days of S starvation (data not shown). Several hypotheses might explain these findings. (1) There may be a difference in some crucial condition (e.g. oxygen availability) that results in elevated induction/activation of ARS activity on solid medium. This idea is supported by the finding that cells growing under the agar, at lower oxygen levels, exhibit higher ARS activity than cells growing on top of the agar (data not shown). (2) The splicing machinery may be somewhat different under different growth conditions, which might change the efficiency at which some transcripts are spliced. (3) ARS stability could be lower in cells growing in a liquid environment. Further experimentation should distinguish these possibilities.
The analysis of multiple potential splice variants of the SNRK2.1 transcript has raised critical issues concerning the in vivo synthesis of these variants and whether the procedure for cDNA synthesis could lead to their artifactual generation. The likelihood of an artifact generated during the synthesis of the cDNA is supported by the finding that the sequences of the splice junction sites for the potential variants identified differ markedly from the Chlamydomonas consensus splice junction sequences (Silflow, 1998). Also, the levels of splice variants observed following RT-PCR were highly variable, and truncated transcripts were also observed if the full-length SNRK2.1 cDNA (as plasmid) was used for PCR amplification. In addition, no complementation of the ars11 mutant was observed when mutant cells were transformed with the SNRK2.1 v2 to v4 cDNAs. Others recent works have demonstrated that PCR synthesis of cDNAs could generate artifactual alternative splice forms (Hampl et al., 1998; Oh et al., 2005; Cocquet et al., 2006). In most cases, the shortened transcripts were deleted for part of the internal coding sequence, and it was suggested that these transcripts formed as a consequence of sequence identity between the 3′ and 5′ splice sites among putative introns, allowing for the formation of heteroduplexes. All of the putative splice variants of SNRK2.1 have sequence identity between the 3′ and 5′ splice sites (Supplemental Fig. S1C). During PCR, full-length and partially elongated products would be generated. If the partial products have 3′ sequences that can pair with multiple sites within the complementary strand, they can anneal to the complementary full-length strands and serve as primers for elongation. This would lead to the generation of a product that contains internal deletions. Furthermore, since these products would contain the primer sites for both the 5′ and 3′ PCR primers, they would be efficiently amplified in subsequent PCR cycles. The GC-rich region at which all 5′ splice sites are localized could elicit the formation of partial elongation products able to form heteroduplexes with the complementary cDNA strand. Although the existence of multiple splice variants cannot be completely excluded based on this work, our results do suggest that caution should be used when considering the generation of splice variants. Additionally, the existence of variability in the placement of the poly(A) tail (Supplemental Fig. S3) suggests some complexity in the regulation of SNRK2.1 transcripts.
The SNRK2, SNRK1, and SNRK3 kinase families are plant specific and belong to the SNF1 superfamily. SNRK2 kinases constitute a large protein family in plants with an N-terminal conserved catalytic domain and a short regulatory C-terminal region. The regulatory region is not highly conserved but usually has a characteristic stretch of the acidic amino acids Asp and Glu, which define subfamilies SNRK2a and SNRK2b, respectively (Halford and Hardie, 1998). Furthermore, SNRK2 proteins have multiple phosphorylation states that are critical for activity, but in most cases the specific phosphorylated residues have not been identified (Kobayashi et al., 2004; Boudsocq et al., 2007). Members of the SNRK2 protein family play a role in the acclimation of plants to environmental stresses; a number of the plant enzymes are involved in controlling osmotic stress responses and are activated by abscisic acid (ABA; Li and Assmann, 1996; Mikolajczyk et al., 2000; Boudsocq et al., 2004; Kobayashi et al., 2004). Some downstream target genes that are activated by plant SNRK2 kinases are SSHLP, a phosphatidylinositol transfer-like protein (Monks et al., 2001), AKIP1, a protein regulating RNA stability (Li et al., 2002), and a basic Leu zipper transcription factor required for ABA gene regulation.
Interestingly, some members of the Arabidopsis (Arabidopsis thaliana) SNRK2 kinase family may function in controlling S limitation responses. Arabidopsis mutants defective for SNRK2.3 exhibit a slight decrease in the level of SULTR2;2 mRNA (encoding the low-affinity SO42− transporter) and elevated O-acetyl-Ser (precursor to Cys) accumulation (Kimura et al., 2006). However, snrk2.3 mutant plants did not show alterations in the expression of other S-responsive genes (APR and SAT), the accumulation of SO42−, or differences in their growth phenotype relative to the wild-type strain when placed in medium devoid of S. These findings suggest a much more moderate role of AtSNRK2.3 than CrSNRK2.1 in controlling S starvation responses. Curiously, five of the 10 AtSNRK2 genes showed increased expression following S starvation of plants (Kimura et al., 2006), which raises the possibility that the activity of other SNRK2 proteins from Arabidopsis may affect S starvation responses. Overall, these results suggest that the SNRK2 family in plants and algae may have some conserved functionalities with respect to controlling nutrient deprivation responses.
The Arabidopsis kinase SNRK2.6 can be activated by two independent mechanisms, one that is ABA dependent and another that is ABA independent and stress dependent (Yoshida et al., 2006). The C-terminal domain, required for both mechanisms, can be functionally divided into domains I and II (Fig. 5). Domain I functions in ABA-independent activation, while domain II, which contains the acidic residues, functions in ABA-dependent activation. Chlamydomonas SNRK2 members have the conserved C-terminal domain I and have retained an acidic stretch of amino acids that characterizes domain II, although the number of acidic amino acids in this region is fewer than in most SNRK2 proteins of plants.
Domain II of Arabidopsis SNRK2.6 is needed to elicit full stomatal closure and for the interaction with the PP2C-type phosphatase ABI1, and deletion of the rice (Oryza sativa) OSRK1 C terminus destroys the functionality of the protein (Chae et al., 2007). Similarly, the full-length SNRK2.1 v1 cDNA can fully complement the ars11 mutant, while the SNRK2.1 cDNA v2, which lacks 45 amino acids of the C terminus (part of both domains I and II), appears to be unable to rescue the mutant phenotype.
Some of the Chlamydomonas SNRK2 proteins (SNRK2.1, SNRK2.3, and SNRK2.4) have a specific feature that is not present in plant SNRK2 proteins: an inserted, nonconserved loop of 17 to 22 amino acids located immediately to the N-terminal side of the activation domain (Fig. 5). The activation domain contains the conserved Ser phosphorylation site (Johnson et al., 1996) that is crucial for kinase activity and/or activation of the enzyme (Kobayashi et al., 2004) upon imposition of a stimulus (Boudsocq et al., 2007). The extra loop present in a subset of the Chlamydomonas SNRK2 proteins (1, 3, and 4) has several potential phosphorylation sites that may participate in regulation/activation (Supplemental Fig. S4).
SNRK2.1, SAC1, and SAC3 are the three genes that have been shown to have a regulatory role in the S deprivation responses of Chlamydomonas. While other mutants have been isolated that exhibit aberrant S deprivation responses, in most cases the gene responsible for the mutant phenotype has not been identified (Davies et al., 1994; Pollock et al., 2005). It is also intriguing that the regulators encoded by SNRK2.1 and SAC3 (SNRK2.2) both belong to the plant-specific SNRK2 family. This strongly indicates the importance of phosphorelays in the control of S deprivation-triggered responses. Curiously, SNRK2.1 and SAC3 have opposite regulatory effects, with the former required for activation of the S-responsive genes, including those encoding ARS and the sulfate transporters, and SAC3 required for full suppression of the same genes during growth under S-replete conditions.
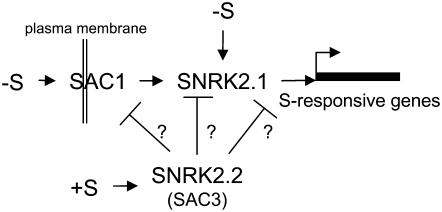
Based on the phenotypes of the sac1 and ars11 mutants, SAC1 and SNRK2.1 have some similar regulatory features. However, SAC1 resembles animal Na+/SO4− transporters, such as SLT1 and SLT2 in Chlamydomonas (Davies and Grossman, 1998). Both SAC1 and SNRK2.1 are required for the appearance of ARS activity and the accumulation of transcripts that normally become abundant during S deprivation (Fig. 2, B and D). However, the regulatory aberrations are more extreme in ars11 than in the sac1 mutant; this is most evident in a comparison of the levels of transcripts for the sulfate transporters SULTR2 and SLT1 in the two strains and in the finding that ars11 bleaches significantly more rapidly than sac1 following the imposition of S deprivation. Furthermore, while the sac1 mutant shows no up-regulation of S deprivation-regulated genes, the levels of transcripts from these genes during S-replete growth are similar to those of the parental D66 strain. In contrast, ars11 has extremely low levels of the SLT1 and SULTR2 transcripts, even under nutrient-replete conditions, relative to the parental strain. As deduced from the mixed phenotype of the sac1 sac3 double mutant, there is no epistatic relationship between SAC1 and SAC3 (the double mutant exhibits low constitutive ARS activity in TAP and TAP-S media; Davies et al., 1994). In contrast, SNRK2.1 is epistatic to SAC3; the ars11 sac3 double mutant does not have any detectable ARS activity in TAP-S (Fig. 1B) or TAP (data not shown), and the patterns of ARS1, SLT1, SULTR2, ECP76, and SDBP transcript accumulation are similar to those of the ars11 single mutant (Fig. 2F). Hence, SAC3 and SAC1 appear to be dependent on SNRK2.1 for their phenotypic features. These genetic and phenotypic results suggest that SNRK2.1 is central to the pathway regulating S-responsive gene expression in Chlamydomonas. SAC1 could be a membrane-bound regulator that senses the S status of the environment and that initiates a signaling cascade that enhances SNRK2.1 activation, which is downstream in this pathway and more directly involved in the activation of a specific transcriptional regulator(s). A possible model that accounts for many of the responses observed in Chlamydomonas during S deprivation, and the ways in which the various mutant strains are affected in these responses, is depicted in Figure 6.
Figure 6.
Model explaining the regulation of the S-responsive genes in Chlamydomonas. SAC1 likely acts as a S sensor at the plasma membrane, and under S starvation conditions it initiates a signaling cascade that leads to the activation of SNRK2.1. Additionally, SNRK2.1 may be activated, to a certain level, by an internal S deficiency. SNRK.2.2 (SAC3) acts as a negative modulator of the regulatory pathway (at a point that has not been determined) and probably remains active in the presence of sufficient S. Other possible regulatory functions of SNRK2.2, SAC1, and SNRK2.1, such as the control of chloroplast gene expression, are not depicted in this model.
MATERIALS AND METHODS
Strains, Culture Conditions, and Mating
Chlamydomonas reinhardtii strains used in this work were D66 (nit2− cw15 mt+; Pollock et al., 2003), 21gr (nit5− mt−; Harris, 1989), sac1 (sac1− mt+), and sac3 (sac3− mt+; Davies et al., 1994). Cells were cultured under continuous light at 23°C in liquid and solid TAP medium (Harris, 1989). To impose S starvation, cells in midlogarithmic growth were washed twice with liquid TAP-S (Harris, 1989) and then split and resuspended in either TAP or TAP-S. Paromomycin and bleomycin were used at 10 and 3 μg mL−1, respectively. Genetic analyses were performed with the various strains according to a previously described protocol (Harris, 1989).
Generation of the ars11 sac3 Double Mutant
The sac3 mt+ mutant was crossed with the parental strain, 21gr (mt−), to obtain a sac3 mt− strain. The mating type of the ars constitutive progeny (sac3 phenotype) was determined by a PCR method (Werner and Mergenhagen, 1998), and one of the sac3 mt− progeny was crossed with ars11 mt+. The double mutant was identified by first selecting for paromomycin resistance (ars11 marker) and then determining which of the resistant isolates contained the pBK-arg2 chimeric sequence (sac3 marker) using the primers 5′-CGTACAAGGCCCATGCGTGAGTC-3′ and 5′-TCGCCGAAAATGACCCAGAGC-3′.
Transformation of Chlamydomonas
Cell wall-less strains D66 and ars11 were transformed by electroporation (Shimogawara et al., 1998) using a modifications of the procedure reported by Colombo et al. (2002).
Generation of Insertional Mutants in Chlamydomonas
The selectable marker gene used for mutant generation was AphVIII under the control of the PSAD promoter and terminator; this gene confers paromomycin resistance to transformants (Sizova et al., 2001). A 1.7-kb PCR fragment containing this construction was used to transform Chlamydomonas strain D66. After transformation, cells were incubated in TAP for 6 to 12 h under continuous light to allow for the accumulation of the AphVIII protein. Transformants were then selected on TAP supplemented with 5 μg mL−1 paromomycin.
ARS Activity and Chlorophyll Determinations
ARS activity was visualized directly on agar plates (Davies et al., 1994) or quantified from liquid medium (de Hostos et al., 1988) as absorbance units per cell number or per milligram of chlorophyll. The data presented correspond to mean values of at least three independent experiments. The concentrations of chlorophylls a and b, extracted from cells in methanol, were estimated using the equations of Porra (2002).
DNA and RNA Isolation
Genomic DNA and total RNA were isolated according to previously described methods (Schloss et al., 1984; Sambrook et al., 1989).
Synthesis of cDNA, RT-PCR, and 3′ RACE Amplifications
RNA samples were treated with DNase (Qiagen; catalog no. 79254) and further cleaned using RNeasy columns (Qiagen) following the manufacturer's directions. Single-stranded cDNAs were synthesized from total RNA using an N-(polyT)20mer primer according to the SuperScript III RNaseH reverse transcriptase manual (Invitrogen). This cDNA population was used as a substrate for real-time PCR with gene-specific primer pairs; the single-stranded cDNAs were diluted 5-fold prior to inclusion in the reaction mixture. The CDS of SNRK2.1 was obtained by RT-PCR using the specific primers 5′-GCCTTCTTGCGACTGCCATACG-3′ and 5′-CCTCAGTCGTTCATGCCGAA-3′. 3′ RACE for SNRK2.1 transcripts was performed using the specific 5′ primer 5′-GTTCCGTGAGGACCTACCCGAG-3′ and a previously described polyT-Qt primer that allowed semi-nested PCR amplifications (Frohman, 1990). All PCRs were performed in the presence of 2% to 5% dimethyl sulfoxide.
Complementation of the ars11 Mutant
The ble gene from pSP124S (Lumbreras et al., 1998) was introduced (at the XhoI-XhoI site) into the pJM43 plasmid (provided by J. Moseley) containing the PSAD promoter; the plasmid generated was designated pBleJM43. SNRK2.1 single-stranded cDNA was amplified using the modified primers 5′-ATCGATTAACGTTGGGGAACGATTCAATG-3′ and 5′-TGGATCCAACGTTGGGGAACGATTCAATG-3′, which introduced a ClaI and a BamHI site at the 5′ and 3′ end, respectively, of the amplified sequences. Four different amplicons were obtained: v1, 1,204 bp; v2, 1,068 bp; v3, 952 bp; and v4, 672 bp. These amplification products were introduced between the PSAD promoter and terminator in the pBleJM43 vector using the ClaI and BamHI sites. The new plasmids containing the differently sized sequences were designated pDV1, pDV2, pDV3, and pDV4. Sequencing of the insert in each of these vectors confirmed the absence of PCR-introduced sequence errors. The pDV1 to -4 plasmids were introduced into the ars11 mutant, and transformants were assayed for rescue of the mutant phenotytpe.
Quantitative PCR
Real-time PCR was performed using the Chromo4 thermocycler (Bio-Rad). Individual reactions had 25 μL final volume, consisting of 10 μL of DyNAMO SYBR Green quantitative PCR (qPCR) reagent (Finnzymes), 3.7 pmol of each primer, 1 to 2 μL of single-stranded cDNA (5-fold diluted from the reverse transcriptase reaction), and distilled water to 25 μL. The Chromo4 run protocol was as follows: denaturation at 95°C for 15 min, followed by 40 cycles of denaturation at 94°C for 10 s, annealing at 60°C for 30 s, and amplification at 72°C for 30 s, and fluorescence measurement after 80°C for 15 s. This last step avoids background signals that can result from the formation of primer dimers. The specificity of the PCR amplification was evaluated by a melting curve program (60°C–100°C, with a heating rate of 0.5°C s−1 and continuous fluorescence measurements) and electrophoretic analysis on 4% agarose gels. We used the CBLP gene as a housekeeping gene control (Chang et al., 2005). Threshold cycle (Ct) values were determined in three independent experiments, with three replicates for each experiment. Relative fold differences were calculated based on the relative ΔCt method [2−(Ctsample − Ctcontrol gene)] using the CBLP amplification product as an internal standard. The primer pairs used for qPCR were as follows: 5′-CTTCTCGCCCATGACCAC-3′ and 5′-CCCACCAGGTTGTTCTTCAG-3′ for CBLP, 5′-CGCGCCGTCACTTGTTTGTTG-3′ and 5′-GCCCACTTCTTTACCCAGCACCTC-3′ for ARS1, 5′-ACGACGCCATGGACAACATGTAC-3′ and 5′-ACCCAGTGCGCTCCGTTCAG-3′ for SAC3, 5′-TCGGTACTTGCAGCTGAGGTTAGG-3′ and 5′-ACACCGTCCAGCCCATGTATCTT-3′ for the SNRK2.1 3′ UTR, 5′-TATGGAGCGTGGGCGTTATCTTG-3′ (underlined sequence binds to the beginning of exon VII, whereas the remaining 5′ sequence of the primer binds to the end of exon VI) and 5′-CCTCCATGGTGATGCGCTT-3′ for SNRK2.1 exons VI and VII, 5′-ACGTGGCATGCAGCTCAT-3′ and 5′-CTTGCCACTTTGCCAGGT-3′ for SULTR2, 5′-ACGGGTCTTCGAGCGAATTGC-3′ and 5′-CGACTGCTTACGCAACAATCTTGG-3′ for SLT1, 5′-CCTCGCTCTCCTCGCTGCTG-3′ and 5′-CGGCCGACTTGGGTAATTGC-3′ for ECP76, and 5′-GGACGGCAGCATCATGGTGAGC-3′ and 5′-TCCACACGCCCTTGACCTTGAG-3′ for SBDP. The sizes of the amplification products were between 100 and 300 bp.
Analysis of Sequences
Sequences were analyzed using DNAstar software version 4.05 (Lasergene Navigator), BioEdit Sequence Alignment Editor version 5.0.9 (Department of Microbiology, North Carolina State University), the National Center for Biotechnology Information BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/), and Chlre3 (version 3) of the Chlamydomonas genome generated by the JGI (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html).
Sequence data for SNRK2.1 cDNA v1 has been deposited with the EMBL database under accession number AM900768.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Putative cDNA splice variants and proposed mechanism that could lead to the PCR generation of artifactual splice variants.
Supplemental Figure S2. Alignment of SNRK2.1 splice variants.
Supplemental Figure S3. Detail of exon XI of SNRK2.1 v1 showing the alternative 5′ splice region and the 3′ UTR.
Supplemental Figure S4. Alignment of the Chlamydomonas SNRK2 protein family.
Supplementary Material
Acknowledgments
We thank Dr. Jeffrey Moseley for critical reading of the manuscript and for providing pJM43 vector and the JGI for generating a draft Chlamydomonas genome sequence, which has been invaluable for both gene identification and mutant analyses. We would also to dedicate this manuscript to Winslow Briggs in honor of his 80th birthday; Winslow has been both an enormously supportive colleague and an inspiration to all members of the Grossman laboratory.
This work was supported by National Science Foundation Grant MCB 0235878 awarded to A.R.G. and by the Carnegie Institution, the Ministerio de Educación y Ciencia (Spain), and the Marie Curie OIF-6 (European Union).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David Gonzalez-Ballester (davidg3@stanford.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279 41758–41766 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Lauriere C (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63 491–503 [DOI] [PubMed] [Google Scholar]
- Cocquet J, Chong A, Zhang G, Veitia RA (2006) Reverse transcriptase template switching and false alternative transcripts. Genomics 88 127–131 [DOI] [PubMed] [Google Scholar]
- Colombo SL, Pollock SV, Eger KA, Godfrey AC, Adams JE, Mason CB, Moroney JV (2002) Use of the bleomycin resistance gene to generate tagged insertional mutants of Chlamydomonas reinhardtii that require elevated CO2 for optimal growth. Funct Plant Biol 29 231–241 [DOI] [PubMed] [Google Scholar]
- Chae MJ, Lee JS, Nam MH, Cho K, Hong JY, Yi SA, Suh SC, Yoon IS (2007) A rice dehydration-inducible SNF1-related protein kinase 2 phosphorylates an abscisic acid responsive element-binding factor and associates with ABA signaling. Plant Mol Biol 63 151–169 [DOI] [PubMed] [Google Scholar]
- Chang CW, Moseley JL, Wykoff D, Grossman AR (2005) The LPB1 gene is important for acclimation of Chlamydomonas reinhardtii to phosphorus and sulfur deprivation. Plant Physiol 138 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Yildiz F, Grossman AR (1996) Sac1, a putative regulator that is critical for survival of Chlamydomonas reinhardtii during sulfur deprivation. EMBO J 15 2150–2159 [PMC free article] [PubMed] [Google Scholar]
- Davies JD, Grossman AR (1998) Responses to deficiencies in macronutrients. In J-D Rochaix, M Goldschmidt-Clermont, S Merchant, eds, The Molecular Biology of Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 613–635
- Davies JP, Yildiz F, Grossman AR (1994) Mutants of Chlamydomonas reinhardtii with aberrant responses to sulfur deprivation. Plant Cell 6 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman AR (1999) Sac3, an Snf1-like serine/threonine kinase that positively and negatively regulates the responses of Chlamydomonas to sulfur limitation. Plant Cell 11 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos EL, Schilling J, Grossman AR (1989) Structure and expression of the gene encoding the periplasmic arylsulfatase of Chlamydomonas reinhardtii. Mol Gen Genet 218 229–239 [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Togasaki RK, Grossman AR (1988) Purification and biosynthesis of a derepressible periplasmic arylsulfatase from Chlamydomonas reinhardtii. J Cell Biol 106 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA (1990) Race: rapid amplification of cDNA ends. In PCR Protocols: A Guide to Methods and Applications. Academic Press, New York, pp 28–38
- Grossman A, Takahashi H (2001) Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annu Rev Plant Physiol Plant Mol Biol 52 163–210 [DOI] [PubMed] [Google Scholar]
- Gupta AS, Alscher RG, McCune DF (1990) Ozone exposure, glutathione levels and photosynthesis in hybrid poplar. In H Rennenberg, C Brunold, LJ Dekoli, I Stulen, eds, Sulfur Nutrition and Sulfur Assimilation in Higher Plants. SPB Academic Publishers, The Hague, The Netherlands, pp 195–197
- Halford NG, Hardie DG (1998) SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol 37 735–748 [DOI] [PubMed] [Google Scholar]
- Hampl M, Hampl J, Plaschke J, Fitze G, Schackert G, Saeger HD, Schackert HK (1998) Evidence that TSG101 aberrant transcripts are PCR artifacts. Biochem Biophys Res Commun 248 753–760 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D (1997) The AMP-activated protein kinase: fuel gauge of the mammalian cell. Eur J Biochem 246 259–273 [DOI] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego [DOI] [PubMed]
- Irihimovitch V, Stern DB (2006) The sulfur acclimation SAC3 kinase is required for chloroplast transcriptional repression under sulfur limitation in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103 7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Noble ME, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85 149–158 [DOI] [PubMed] [Google Scholar]
- Kimura T, Shibagaki N, Ohkama-Ohtsu N, Hayashi H, Yoneyama T, Davies JP, Fujiwara T (2006) Arabidopsis SNRK2.3 protein kinase is involved in the regulation of sulfur-responsive gene expression and O-acetyl-L-serine accumulation under limited sulfur supply. Soil Sci Plant Nutr 52 211–220 [Google Scholar]
- Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 87 1228–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Assmann SM (1996) An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kinoshita T, Pandey S, Ng CK, Gygi SP, Shimazaki K, Assmann SM (2002) Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418 793–797 [DOI] [PubMed] [Google Scholar]
- Lumbreras V, Stevens DR, Purton S (1998) Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J 14 441–447 [Google Scholar]
- Mahler RJ, Maples RL (1986) Responses of wheat to sulfur fertilization. Commun Soil Sci Plant Anal 17 975–988 [Google Scholar]
- Mahler RJ, Maples RL (1987) Effect of sulfur additions on soil and the nutrition of wheat. Commun Soil Sci Plant Anal 18 653–673 [Google Scholar]
- Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol 47 127–158 [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52 711–760 [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G (2000) Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12 165–178 [PMC free article] [PubMed] [Google Scholar]
- Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE (2001) Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell 13 1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WJ, Noggle SA, Maddox DM, Condie BG (2005) The mouse vesicular inhibitory amino acid transporter gene: expression during embryogenesis, analysis of its core promoter in neural stem cells and a reconsideration of its alternate splicing. Gene 351 39–49 [DOI] [PubMed] [Google Scholar]
- Padegirnas LS, Reichert NA (1998) Adaptor ligation-based polymerase chain reaction-mediated walking. Anal Biochem 260 149–153 [DOI] [PubMed] [Google Scholar]
- Peltier G, Schmidt GW (1991) Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 88 4791–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock SV, Colombo SL, Prout DL Jr, Godfrey AC, Moroney JV (2003) Rubisco activase is required for optimal photosynthesis in the green alga Chlamydomonas reinhardtii in a low-CO2 atmosphere. Plant Physiol 133 1854–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock SV, Pootakham W, Shibagaki N, Moseley JL, Grossman AR (2005) Insights into the acclimation of Chlamydomonas reinhardtii to sulfur deprivation. Photosynth Res 86 475–489 [DOI] [PubMed] [Google Scholar]
- Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73 149–156 [DOI] [PubMed] [Google Scholar]
- Ravina CG, Chang CI, Tsakraklides GP, McDermott JP, Vega JM, Leustek T, Gotor C, Davies JP (2002) The sac mutants of Chlamydomonas reinhardtii reveal transcriptional and posttranscriptional control of cysteine biosynthesis. Plant Physiol 130 2076–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schloss JA, Silflow CD, Rosenbaum JL (1984) mRNA abundance changes during flagellar regeneration in Chlamydomonas reinhardtii. Mol Cell Biol 4 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Quiclet-Sire B, Kondorosi E, Virelizer H, Glushka JN, Endre G, Gero SD, Kondorosi A (1992) Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci USA 89 192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawara K, Fujiwara S, Grossman AR, Usuda H (1998) High efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148 1821–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow CD (1998) Organization of the Nuclear Genome. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Sizova I, Fuhrmann M, Hegemann P (2001) A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene 277 221–229 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Braby CE, Grossman AR (2001) Sulfur economy and cell wall biosynthesis during sulfur limitation of Chlamydomonas reinhardtii. Plant Physiol 127 665–673 [PMC free article] [PubMed] [Google Scholar]
- Warman PR, Sampson HG (1994) Effect of sulfur additions on the yield and elemental composition of canola and spring wheat. J Plant Nutr 17 1817–1825 [Google Scholar]
- Werner R, Mergenhagen D (1998) Mating type determination of Chlamydomonas reinhardtii by PCR. Plant Mol Biol Rep 16 295–299 [Google Scholar]
- Wykoff D, Davies J, Grossman A (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F, Davies JP, Grossman AR (1994) Characterization of sulfate transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol 104 981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR (1996) Sulfur availability and the SAC1 gene control adenosine triphosphate sulfurylase gene expression in Chlamydomonas reinhardtii. Plant Physiol 112 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shrager J, Jain M, Chang CW, Vallon O, Grossman AR (2004) Insights into the survival of Chlamydomonas reinhardtii during sulfur starvation based on microarray analysis of gene expression. Eukaryot Cell 3 1331–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.