Abstract
Exocytosis of neurotransmitter from a synaptic vesicle is followed by efficient retrieval of its constituent membrane and proteins. Real-time measurements indicate that fast and slow modes of retrieval operate in parallel at a number of presynaptic terminals. Two mechanisms can be distinguished by electron microscopy: clathrin-mediated retrieval of small vesicles and bulk retrieval of large cisternae. Methods that investigate the behaviour of individual vesicles have recently demonstrated a third route of retrieval: the rapid reversal of a pore-like connection between the vesicle and surface (‘kiss-and-run’). Key aims for the future are to identify the molecules underlying different mechanisms of endocytosis at the synapse and the signals that select between them.
Neurons transmit information at chemical synapses by exocytosis of small vesicles laden with neurotransmitter. This event is triggered by calcium influx and can occur at rates of hundreds of Hertz (Katz, 1969). The presynaptic terminal therefore faces a problem: in order to maintain the supply of secretion-competent vesicles and a normal morphology, the excess membrane and vesicle proteins must be recovered. The importance of recycling synaptic vesicles is illustrated by the fruit fly mutant shibire that cannot retrieve synaptic vesicles at high temperatures: on warming, the flies become paralysed and fall out of the air.
Exactly how the presynaptic terminal retrieves excess membrane is still a matter of debate. Three basic mechanisms of endocytosis are thought to operate: retrieval of small vesicles coated by clathrin, bulk retrieval of large portions of membrane and fast recapture of vesicles that do not fully collapse into the surface. In this review we examine the evidence for these three routes of endocytosis and ask how far they might account for the physiological properties of endocytosis measured at different synapses. Real-time measurements demonstrate that many synapses contain both fast and slow mechanisms of endocytosis and the relative importance of these different routes depends on how strongly the synapse is stimulated. The molecular mechanisms of fast and slow retrieval are less clear.
Mechanisms of endocytosis at the synaptic terminal
In the early 1970s, two groups used electron microscopy to examine how stimulation altered the ultrastructure of the frog neuromuscular junction (NMJ). Stimulating at 10 Hz for 1 min, Heuser & Reese (1973) observed decreased numbers of synaptic vesicles and the appearance of membranous cisternae emanating from the plasma membrane (Fig. 1B). If a rest period was allowed after stimulation, vesicles reappeared, apparently at the expense of the cisternae. Clathrin-coated pits and vesicles were frequently observed, particularly at sites removed from the active zone. In contrast, Ceccarelli et al. (1973) observed little change in the ultrastructure of the terminal following stimulation at lower frequency (2 Hz for up to 4 h; Fig. 1C). After release, vesicles were recycled fast enough to prevent depletion. These two sets of observations have been widely interpreted as indicating that two mechanisms of vesicle release and retrieval exist at the NMJ: some vesicles collapse fully into the plasma membrane and are then recycled by clathrin-mediated endocytosis (CME) while other vesicles release neurotransmitter without full collapse and are then retrieved by a direct and rapid reversal of this process. The latter mechanism has been poetically termed kiss-and-run (Fesce et al. 1994).
Figure 1. Models of synaptic vesicle retrieval at the frog NMJ.
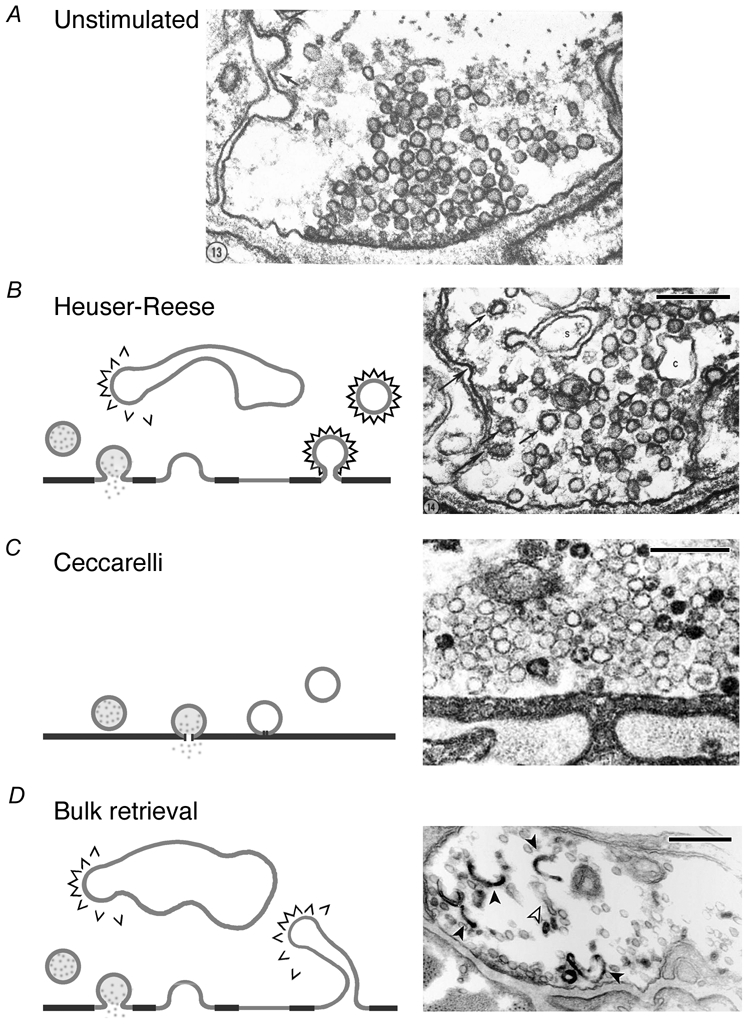
A, electron micrograph of the frog NMJ at rest. B, Heuser-Reese model for retrieval. Diagram (left) shows that vesicles fully collapse into the plasma membrane and are retrieved by CME. Coated vesicles may also form from cisternae. Electron micrograph (right) of frog NMJ following stimulation at 10 Hz for 1 min. Coated pits and vesicles (arrowed) and cisternae (c) are seen in the terminal. C, Ceccarelli model for retrieval. Diagram (left) shows a vesicle releasing neurotransmitter through a transient fusion pore by ‘kiss-and-run’; the vesicle is retrieved at the same site. Micrograph (right) of a terminal stimulated at 2 Hz for 2 h showing an absence of clathrin-coated vesicles and cisternae at low stimulation frequency. D, bulk membrane retrieval. In this model (left) large areas of membrane are internalised following complete vesicle collapse. Coated vesicles may bud from membrane invaginations or from large, internalised cisternae. Micrograph (right) shows internalised cisternae that are empty (open arrows) or filled with photoconverted FM1–43 (filled arrows). Scale bars, 250 nm in all cases. Figures reproduced from Heuser & Reese (1973). Ceccarelli et al. (1973) and Richards et al. (2000) with permission from the Rockefeller University Press and Elsevier Science/Cell Press.
The idea that endocytosis at the synapse might occur by a simple reversal of the fusion step is attractive because it would provide a rapid and economical way of recycling vesicles. The importance of kiss-and-run has been difficult to assess (Fesce et al. 1994), but a leap forward has recently been made by the use of methods that resolve the fusion and retrieval of individual vesicles. Capacitance measurements in pituitary nerve terminals show that microvesicles form a transient connection with the surface membrane in about 5 % of fusion events (Klyachko & Jackson, 2002). Imaging fusion of individual vesicles at the ribbon synapse of retinal bipolar cells also indicates that kiss-and-run is negligible under conditions where large numbers of vesicles are released (Zenisek et al. 2002). In hippocampal synapses, however, kiss-and-run accounts for a majority of release events when the release probability is low (Gandhi & Stevens, 2003).
A third mechanism of endocytosis, involving the formation of deep membrane infoldings, has also been consistently observed in the frog NMJ (Richards et al. 2000), as well as lamprey reticulospinal synapses (Gad et al. 1998), snake motor terminals (Teng et al. 1999; Teng & Wilkinson, 2000), rat hippocampal neurons (Takei et al. 1996) and goldfish bipolar cells (Paillart et al. 2003). These large invaginations occur away from the site of fusion and their formation is not thought to directly involve clathrin (Fig. 1D). The infoldings may be either pinched off from the surface, or may remain connected to the plasma membrane (Takei et al. 1996; Teng & Wilkinson, 2000). The current view is that the cisternae are not formed by the coalescence of internalised clathrin-coated vesicles as originally proposed (Heuser & Reese, 1973). Instead, synaptic vesicles are thought to be formed by CME from the infolded membrane, because clathrin-coated buds have been demonstrated on the cisternae in lamprey synapses and snake motor terminals (Shupliakov et al. 1997; Teng & Wilkinson, 2000).
Of these three basic mechanisms of retrieval, by far the best understood is CME. The molecules involved in CME are enriched at the synapse (Maycox et al. 1992; Ball et al. 1995; Ringstad et al. 1999; Roos & Kelly, 1999; Teng et al. 1999; Teng & Wilkinson, 2000) and clathrin-coated vesicles are observed at a number of synaptic terminals following stimulation (Heuser, 1989; Takei et al. 1996; Shupliakov et al. 1997). Rapid progress has been made in understanding how CME operates at the molecular level, although most of this information is derived from studies of endocytosis of transmembrane proteins in non-neuronal cells (Marsh & McMahon, 1999; Brodsky et al. 2001). The molecular mechanisms of CME are summarised in Fig. 2. The heterotetrameric adaptor complex AP-2 binds to transmembrane proteins at the surface and recruits clathrin triskelions that assemble in a lattice over the membrane surface. Formation of the lattice deforms the membrane to form a coated pit. Subsequent scission of the vesicle from the surface requires the large GTPase, dynamin. Within the cytoplasm, the clathrin coat is removed and the naked vesicle can then fuse with other endosomal structures. CME at the synapse is generally assumed to be similar to that in non-neuronal cells, although neuron-specific proteins may substitute for some of the key players shown in Fig. 2. An example is AP180, which can bind to clathrin, phosphoinositides in membranes (Ford et al. 2001) and possibly synaptobrevin, the v-SNARE in the vesicle membrane (Nonet et al. 1999). These properties suggest that AP180 may act together with AP-2 in the early stages of clathrin-coated vesicle formation.
Figure 2. Molecular mechanisms of clathrin-mediated endocytosis.
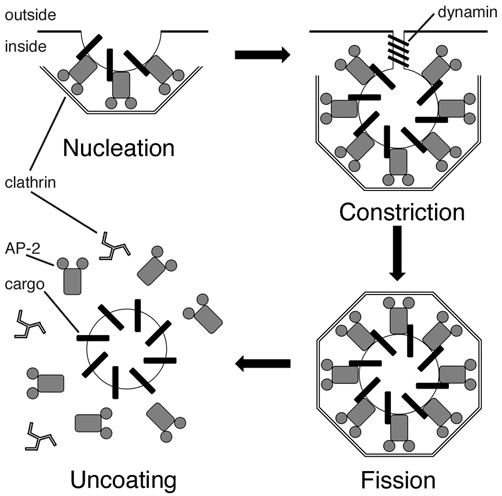
Schematic diagram to show the major steps of CME in non-neuronal cells. CME comprises four major steps: nucleation, constriction, fission and uncoating. Numerous other proteins such as amphiphysin, epsin and endophilin (Brodsky et al. 2001) have been omitted for clarity, and only the core molecular players are shown here. The transmembrane cargo protein has been proposed to be synaptotagmin in the case of synaptic vesicle retrieval.
Important questions regarding the role of CME remain. Clathrin-coated vesicles are not a consistent feature of stimulated synapses (Ceccarelli et al. 1973; Paillart et al. 2003) and where they do occur, it is often unclear whether they have budded directly from the surface membrane (Heuser & Reese, 1973), or from large invaginations still connected to the surface, or from endosomes (Takei et al. 1996). Another important issue is speed. The rate of receptor internalization by CME in non-neuronal cells is of the order of minutes, while many synapses possess a fast mode of endocytosis occurring on a time scale of 1 s or less.
The physiology of endocytosis at the synapse
For many years, our understanding of endocytosis at the synapse was limited by a lack of direct information about this process in living neurons. More recently, real-time measurements of endocytosis have been made at a number of synapses using electrophysiology and imaging methods. Fast and slow modes of endocytosis operate in parallel at a number of synapses, as summarized in Table 1 and described in more detail below.
Table 1.
Comparison of the kinetics of endocytosis at different presynaptic terminals
| Preparation | Endo τfast (S) | Endo τslow (S) | Effect of increased stimulation | Effect of increased [Ca2+]i |
|---|---|---|---|---|
| Goldfish bipolar cells | ∼1a,b | 10b,c | More endocytosis by slow mode. | [Ca2+]i stimulates fast mode c |
| No effect on rates c | ||||
| Mouse inner hair cells | 0.3e | 7.5d | — | Fast mode seen in more cells. |
| 15e | No effect on rates d | |||
| Frog NMJ | 20f | ∼5 minf | Slows rate | No effect of [Ca2+]ig |
| [Ca2+]i may inhibit fast endocytosis f | ||||
| Rat hippocampal neurons | 6h | 20–60j | Complex (see text) | Complex (see text) |
| 1i | 4–90k | |||
| 0.4–0.9l | 8→21l | |||
| Mouse calyx of Held | 0.1m | ∼14m | Slows rate | No effect |
| Rat posterior pituitary terminals | 0.3n | >2n | — | — |
References:
Wu & Betz, 1996
Giant synapses
The capacitance technique is a particularly direct method of monitoring membrane retrieval because the electrical capacitance of the surface membrane is directly proportional to its area, and changes can be measured on a time scale of tens of milliseconds (Neher & Marty, 1982). This method can be applied to large synaptic terminals, such as the ribbon synapse of bipolar cells from the goldfish retina. Using this preparation, von Gersdorff & Matthews (1994) were the first to directly demonstrate the speed of vesicle retrieval. Following a brief stimulus, all the excess membrane was retrieved with a time constant of ≈1 s. Neves & Lagnado (1999) then found that longer stimulation of this terminal (>100 ms) was followed by membrane retrieval occurring in two phases: fast endocytosis was followed by a slow mode, with a time constant of 10 s or more. An increase in stimulus duration increased the proportion of membrane retrieved by slow endocytosis and decreased the proportion retrieved by fast endocytosis, without altering the rate constant of either process. Fast and slow modes of endocytosis could also be distinguished by their dependence on Ca2+. Limiting the spread of Ca2+ by introducing calcium chelators caused a proportion of the excess membrane to be retrieved by slow endocytosis, even after a very brief stimulus (Neves et al. 2001; Fig. 3A). It therefore seems that the Ca2+ microdomain triggering fast exocytosis also selected vesicles for fast endocytosis. The idea that fast and slow endocytosis in bipolar cell terminals are mechanistically distinct is supported by the observation that the slow mode of retrieval is selectively inhibited by raising the hydrostatic pressure inside the terminal (Heidelberger et al. 2002). Fast and slow modes of endocytosis have also been observed at the ribbon synapse of inner hair cells from the cochlea of the mouse, where fast endocytosis is also triggered by Ca2+ (Moser & Beutner, 2000; Beutner et al. 2001).
Figure 3. Physiological measurements of endocytosis.
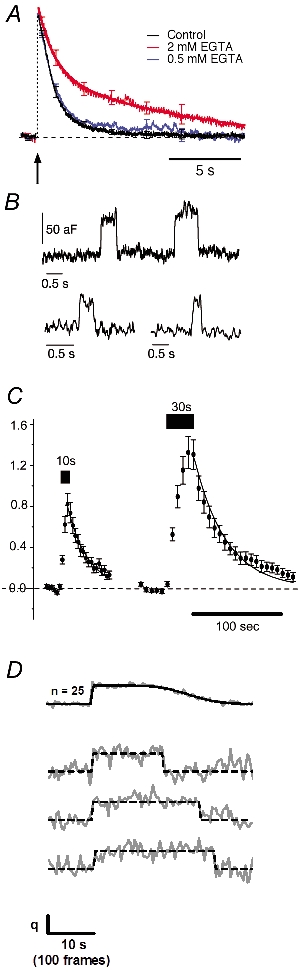
A, whole-cell capacitance recordings in goldfish retinal bipolar cells. A brief, 20 ms depolarisation (arrow) causes exocytosis and subsequent membrane retrieval by fast endocytosis. Introduction of EGTA to chelate intracellular calcium causes a proportion of membrane to be retrieved by slow endocytosis. B, low noise cell-attached capacitance recording in a pituitary nerve terminal reveals capacitance flickers that correspond to kiss-and-run of microvesicles. Note the similar size of the down-step following an up-step in the trace. C, average intensity of synapto-pHluorin fluorescence in hippocampal neurons. Transfected cells were imaged by confocal microscopy and stimulated at 10 Hz for 10 or 30 s. The decay of fluorescence at the end of the stimulus corresponds to reacidification of synaptic vesicles following endocytosis. D, exocytosis and endocytosis of single vesicles in hippocampal boutons. The top trace is an average of 25 events. The three individual traces below show stepwise changes in synapto-pHluorin fluorescence corresponding to exocytosis and endocytosis of individual vesicles. Figures reproduced from Neves et al. (2001). Klyachko & Jackson (2002). Sankaranarayanan & Ryan (2000) and Gandhi & Stevens (2003) with permission from the National Academy of Sciences and Nature Publishing Group.
Might the fast mode of endocytosis in bipolar cells occur by kiss-and-run? The possible existence of kiss-and-run has been tested using total internal reflection fluorescence microscopy (TIRFM), which allows fluorescent probes to be imaged within ≈100 nm of the plasma membrane. The resulting improvement in signal-to-noise allows the visualization of individual vesicles labelled with the membrane dye FM1–43 and the diffusion of FM1–43 into the surface membrane following exocytosis. Zenisek et al. (2000, 2002) found that vesicle fusion in response to a stimulus lasting 500 ms was followed by complete loss of FM1–43, indicating free exchange of lipid. It therefore seems unlikely that kiss-and-run is an important mechanism of vesicle retrieval in bipolar cells, at least in response to this relatively strong stimulus. The proviso may be important, because capacitance measurements show that a 500 ms stimulus is followed by both fast and slow modes of endocytosis (Neves & Lagnado, 1999).
The capacitance technique has also been applied successfully to the Calyx of Held, a large synaptic terminal in the auditory brainstem. Sun et al. (2002) measured capacitance in the presynaptic terminal while recording postsynaptic currents. By averaging the capacitance signal following hundreds of thousands of spontaneous miniature events, they measured the rate at which a single vesicle was retrieved. The time constant was surprisingly fast – just 56 ms at room temperature. The time constant of membrane retrieval increased to 115 ms at stimulation rates of 2 Hz, up to tens of seconds at higher frequencies. These findings were interpreted as indicating that a fast mechanism of endocytosis predominates during low frequency stimulation, but quickly saturates at higher frequencies, leaving vesicles to be retrieved by a slower mechanism (Sun et al. 2002). Buffering internal Ca2+ to different levels did not alter the rate of retrieval.
Capacitance recordings made in the cell-attached configuration have lower noise, allowing the fusion of individual vesicles to be detected (Neher & Marty, 1982; Albillos et al. 1997; Klyachko & Jackson, 2002). In chromaffin cells, the upward capacitance step caused by the fusion of a single granule is sometimes followed by a rapid downward step of equal size, indicating that exocytosis of individual granules can be rapidly reversed. However, only about 5 % of granules run away after the kiss (Ales et al. 1999), indicating that the great majority of exocytotic events in chromaffin cells involve full fusion. Until recently, it was unclear how far the behaviour of granules 200–300 nm in diameter could be related to synaptic vesicles about 30–40 nm in diameter. The first study to resolve retrieval of individual small vesicles was carried out by Klyachko & Jackson (2002) by making capacitance recordings from cell-attached patches on large terminals of posterior pituitary neurons, which contain microvesicles 50 nm in diameter that are similar to small vesicles found at synapses. About 5 % of the step increases in capacitance generated by fusion of a microvesicle were paired with down-steps of equal size occurring within 2 s (Fig. 3B). The connection between the microvesicle and surface (the fusion pore) was open for an average of 0.31 s. Curiously, the pore had a conductance of only 20 pS (≈0.3 nm radius), which would only allow a small trickle of neurotransmitter. The other 95 % of fusion events involving microvesicles were not obviously paired with a downward capacitance step, indicating that kiss-and-run was not the usual mode of exocytosis (Klyachko & Jackson, 2002).
Hippocampal boutons
The small presynaptic boutons of cultured hippocampal neurons have been studied by fluorescence imaging using FM1–43 (Cochilla et al. 1999) or fluorescent proteins associated with vesicles (Ryan, 2001). In earlier studies, the approach was to stain membrane compartments formed by endocytosis by applying FM1–43 at various times following strong stimulation. These experiments indicated that retrieval of stained membrane was relatively slow, of the order of 30–60 s (Ryan et al. 1993; Ryan & Smith, 1995; Ryan et al. 1996), similar to estimates of the rate of CME at the NMJ (Miller & Heuser, 1984). Later studies used synapto-pHluorin, a pH-sensitive form of GFP fused to the intraluminal side of the vesicle protein synaptobrevin-2 (Miesenbock et al. 1998; Sankaranarayanan et al. 2000). This probe is quenched at the acidic pH inside a vesicle and fluoresces more brightly when a vesicle fuses. Because vesicles are thought to be re-acidified rapidly after retrieval, the rate of endocytosis can be estimated from the rate at which synapto-pHluorin is quenched again. Sankaranarayanan & Ryan (2000) found the average rate of endocytosis to be graded with the length of the stimulus period, with a time constant of 4 s after a brief stimulus and 90 s after a long one (Fig. 3C). Lowering the external [Ca2+] during stimulation slowed the rate of retrieval (Sankaranarayanan & Ryan, 2001).
The resolution of measurements using synapto-pHluorin has been dramatically improved by Gandhi & Stevens (2003). who measured the retrieval and reacidification of individual vesicles in response to single action potentials (Fig. 3D). Exocytosis of a single vesicle leads to a jump in fluorescence of fixed size (q) caused by unquenching of one vesicle's worth of synapto-pHluorin. The signal then abruptly steps down by an amount -q on one of two time scales: fast endocytosis occurred within 400–860 ms and slow (‘compensatory’) endocytosis occurred within 8–21 s. The rapid events were identified as kiss-and-run involving a selective fusion pore because the retrieved vesicle could be loaded with the pH buffer Tris but not Hepes. Two other events triggered by an action potential were also identified; a step of q that did not decline, representing a ‘stranded’ vesicle, and a spontaneous step of -q, representing triggered retrieval and reacidification of a vesicle uncoupled from an exocytotic event. The frequency of ‘stranded’ vesicles and -q events were similar, suggesting that ‘stranded’ vesicles were left on the surface until an action potential triggered their retrieval.
A notable feature of the results of Gandhi & Stevens (2003) is that stimulation with just one action potential could trigger full fusion of a vesicle followed by slow retrieval, which argues against the idea that full collapse of vesicles only occurs during strong stimulation. Intriguingly, the proportion of vesicles retrieved by each of the three modes of endocytosis differed according to the release probability of the bouton under study (Gandhi & Stevens, 2003). Synapses with a release probability of 0.2 used kiss-and-run in about 70 % of events, with the remainder of vesicles being ‘stranded’. In contrast, synapses with a release probability of 0.42 only used kiss-and-run in about 25 % of events, with the remainder being ‘compensatory’.
Another approach to investigating endocytosis in hippocampal boutons has been to analyse the kinetics with which FM1–43 and its analogues are released from vesicles during stimulation of exocytosis (Klingauf et al. 1998; Pyle et al. 2000; Stevens & Williams, 2000). FM2–10 is more hydrophilic than FM1–43 and therefore partitions out of membrane more rapidly. Klingauf et al. (1998) found that destaining of boutons loaded with FM1–43 tended to occur more slowly than destaining of FM2–10, and suggested that FM1–43 was not always completely discharged from a vesicle during the time it was in contact with the external medium. By comparison with measurements of the rate at which FM1–43 partitions out of membrane, they estimated that a proportion of vesicles were retrieved by a mechanism with a time constant of ≈1 s, and the remainder with a time constant of ≈30 s. Aravanis et al. (2003) improved the resolution of this approach to the level of single vesicles, and found incomplete discharge of FM1–43 to be a common event in response to single action potentials. Some synaptic vesicles could fuse repeatedly with the plasma membrane, only losing a small proportion of FM1–43 each time.
The neuromuscular junction
A number of studies indicate that there are two distinct vesicle populations at the NMJ of a number of species. Richards et al. (2000) used FM1–43 and FM2–10 to distinguish these populations at the frog NMJ. They found that FM1–43 applied for 10 min after a 1 min tetanus and then washed away became trapped in large cisternae within the terminal, while FM2–10 applied in this way did not (Fig. 1D). These cisternae originated from deep invaginations of the surface membrane, from which FM1–43 was washed out more slowly than its more hydrophilic analogue FM2–10. This slow route of endocytosis operated long after the stimulus and regenerated vesicles slowly. However, during stimulation, a rapid endocytotic mechanism retrieved FM1–43 and FM2–10 with similar efficiency. This fast route of endocytosis selectively refilled the 20 % of vesicles in the terminal comprising the readily releasable pool, while the slow route refills the reserve pool. Rapid and selective recycling of vesicles into the readily releasable pool is qualitatively similar to the role of fast endocytosis in hippocampal boutons (Pyle et al. 2000). Recent data indicate that fast recycling of the readily releasable pool is sufficient to maintain transmitter output during relatively weak (2–5 Hz) stimulation, suggesting that the reserve pool need not be mobilised under these conditions (Richards et al. 2003). It remains to be seen whether the fast mode of retrieval at the frog NMJ is also kiss-and-run. Teng & Wilkinson (2003) have suggested that at the snake NMJ, clathrin-mediated endocytosis accounts for the ‘delayed’ route of endocytosis, which is sensitive to cooling to below 7 °C; while bulk retrieval or macropinocytosis may account for the pathway that is insensitive to this manipulation.
Electron microscopy and FM1–43 imaging experiments indicate that the Drosophila NMJ also contains two pools of vesicles, as do photoreceptor terminals in this species. Vesicles available for immediate release are thought to be replenished by fast endocytosis close to the active zone (the cycling pool), while the reserve pool is refilled by a slower pathway away from the active zone (Koenig & Ikeda, 1999; Kuromi & Kidokoro, 1999, 2002). There is good evidence that the replenishment of the reserve pool is by CME (Koenig & Ikeda, 1996, 1999). The advantages of Drosophila for studying synaptic function rest with the genetic manipulations that this organism allows (Richmond & Broadie, 2002) and such experiments are discussed below.
Similarities and differences in synaptic vesicle endocytosis
The pattern that emerges from real-time measurements is that many synapses possess both fast and slow modes of endocytosis, with the fast mode predominating after weak stimulation, and the slow mode becoming more important after stronger stimulation (Table 1). The yawning gap in our understanding comes in attempting to relate these kinetically distinct modes of retrieval with the three basic modes of endocytosis described above. For instance, the kinetics of endocytosis in hippocampal boutons and bipolar cells is strikingly similar. In bipolar cells, increased stimulation causes a shift from fast endocytosis (τ = 1 s) to slow endocytosis (τ > 10 s) without altering the rate of either process (Neves & Lagnado, 1999; Neves et al. 2001). Fast and slow modes of endocytosis are also observed when retrieval of individual vesicles is imaged in hippocampal boutons, and these also have time constants of 0.4–0.8 and 10 s (Gandhi & Stevens, 2003). These similarities beg an obvious question – do fast and slow endocytosis in hippocampal boutons and bipolar cell terminals occur by the same mechanisms? While experiments in hippocampal boutons provide strong evidence that fast retrieval after a single action potential is by kiss-and-run (Aravanis et al. 2003; Gandhi & Stevens, 2003), TIRFM experiments in bipolar cells have failed to detect such a mechanism after stimuli lasting 500 ms (Zenisek et al. 2002). It therefore seems that these two terminals may possess different mechanisms of fast endocytosis, although before completely ruling out the existence of kiss-and-run in bipolar cells it would be good to test the effects of briefer stimuli that trigger exocytosis followed exclusively by fast endocytosis (Neves & Lagnado, 1999). The mechanism of slow endocytosis is just as elusive. Does this occur by a clathrin-dependent mechanism? And how rapidly might mechanisms of bulk retrieval operate?
It may be that synapses from different parts of the nervous system employ different mechanisms of endocytosis, depending on how the synapse operates. Strong stimuli applied to hippocampal boutons only cause the cycling of a subpopulation of vesicles (Harata et al. 2001a, b), and long depolarisations in high potassium solution cause little change to the ultrastructure (Sara et al. 2002). In contrast, strong stimulation of the frog NMJ and of bipolar cells causes release of many more vesicles than are originally docked to the membrane, as well as build-up of internal cisternae (Heuser & Reese, 1973; Lagnado et al. 1996; Paillart et al. 2003; Holt et al. 2003). These differences may indicate that hippocampal boutons rely more on a local and fast form of recycling compared to other synapses (Pyle et al. 2000). Gandhi & Stevens (2003) even found important heterogeneities within one type of synapse: kiss-and-run exocytosis was less prevalent at hippocampal boutons with high release probability compared with boutons with low release probability. It will be interesting to test whether stronger stimulation of boutons with low release probability causes a switch to the slow mode of endocytosis.
The gap between physiology and molecules
Although dynamin and clathrin have established roles in endocytosis at the synapse, we are still a long way from understanding the physiology of vesicle recycling in molecular terms.
A universal role for dynamin?
Dynamin is a GTPase that is involved in membrane scission and is thought to play an essential role in a wide range of endocytotic events, both at the synapse and in non-neuronal cells (Urrutia et al. 1997; Brodsky et al. 2001). The essential role for dynamin in retrieval of synaptic vesicles has been studied extensively using the Drosophila orthologue, shibire. A temperature-sensitive mutant of shibire exhibits paralysis at temperatures > 29 °C. Exocytosis at the NMJ is intact, but endocytosis is inhibited at > 29 °C and a total depletion of vesicles is seen following stimulation at 33 °C (Poodry & Edgar, 1979; Koenig et al. 1983).
Evidence from Drosophila indicates that dynamin may be essential for both fast and slow modes of endocytosis at the synapse. CME away from the active zone is blocked in Drosophila with mutations in the gene for endophilin, a protein that binds dynamin, resulting in enlarged boutons and fewer synaptic vesicles per bouton (Guichet et al. 2002; Verstreken et al. 2002). In the face of this, evoked release is still maintained at 15–20 % of wild-type levels, although FM1–43 is no longer taken up (Verstreken et al. 2002). These results indicate that while the majority of retrieval requires CME, a fast kiss-and-run mechanism of endocytosis continues to operate after CME is blocked. Interestingly, all endocytosis was blocked in double shibire/endophilin mutants, indicating that all forms of endocytosis at the Drosophila NMJ involve the GTPase activity of dynamin (Verstreken et al. 2002).
A universal role for dynamin in endocytosis at the synapse has been called into question by Heidelberger (2001) who carried out capacitance experiments indicating that endocytosis at the ribbon synapse of bipolar cells was dependent on ATP but not GTP. The possibility that membrane scission at this ribbon synapse depends on an enzyme other than dynamin is intriguing.
Clathrin-mediated endocytosis
Evidence for a key role of CME at the synapse comes both from electron microscopy (Fig. 1B) and from experiments disrupting this mechanism in living neurons (Brodin et al. 2000; Richmond & Broadie, 2002). In the giant reticulospinal synapse of the lamprey, introduction of proteins and peptides that inhibit the interaction between amphiphysin and the proline-rich domain of dynamin cause an activity-dependent accumulation of clathrin-coated vesicles (Shupliakov et al. 1997). Similarly, peptides that block the interaction between clathrin heavy chain and AP180 or AP2 were found to inhibit clathrin-coated vesicle formation and reduce the number of synaptic vesicles at the squid giant synapse (Morgan et al. 1999, 2000). Peptides that inhibit the uncoating of clathrin-coated vesicles by auxilin also cause an increase in the number of clathrin-coated vesicles per active zone (Morgan et al. 2001). However, it seems likely that CME is not the only mechanism of endocytosis in the lamprey and squid giant synapse. Vesicles still remain after perturbation of CME at lamprey synapses (Shupliakov et al. 1997; Ringstad et al. 1999) and squid terminals have been reported to contain a clathrin-independent pathway involving the vesicle protein synaptophysin (Daly et al. 2000).
It is also unclear whether disruption of CME affects fast or slow modes of retrieval. An acute disruption of synaptic transmission would be expected if CME played an immediate role in vesicle retrieval, but it is also possible that fast endocytosis occurs by a mechanism that is independent of clathrin, with CME having a longer-term role, such as retrieval of vesicle proteins that leak into the surface membrane (Li & Murthy, 2001). Studies at squid and lamprey synapses have not assayed endocytosis directly; gross changes in the rate of retrieval were inferred from changes in postsynaptic responses measured electrophysiologically and changes in the ultrastructure revealed by electron microscopy. To determine the role of clathrin it will be necessary to disrupt CME at synapses in which endocytosis can be assayed in real-time using capacitance or fluorescence imaging techniques.
A key step in CME is the recognition by the AP-2 adaptor complex of a molecule in the surface membrane, usually a receptor (Marsh & McMahon, 1999; Brodsky et al. 2001). What molecule(s) could fulfil this role when vesicles are retrieved at the synapse? Interest has centred on synaptotagmin as a membrane receptor for AP-2 (Zhang et al. 1994), although it is not clear whether the high affinity of this interaction in vitro accurately reflects the situation in vivo. While synaptotagmin remains an attractive candidate to trigger CME (Jorgensen et al. 1995), other molecules resident on the synaptic vesicle membrane, such as neurotransmitter transporters or proton pumps, might also play this role.
Several observations suggest that actin may play a role in CME of synaptic vesicles, the strongest evidence coming from electron microscopy at the lamprey reticulospinal synapse (Slepnev & De Camilli, 2000; Shupliakov et al. 2002). Disruption of F-actin inhibits vesicle cycling at the Drosophila and snake NMJ (Kuromi & Kidokoro, 1998; Cole et al. 2000), although these results might be explained by inhibition of exocytosis rather than endocytosis. In contrast, capacitance measurements in bipolar cells and FM1–43 measurements in hippocampal neurons demonstrate that exocytosis, endocytosis and refilling of release sites at the active zone all continue at normal rates after the actin cytoskeleton has been disrupted (Job & Lagnado, 1998; Holt et al. 2003; Sankaranarayanan et al. 2003). A lack of actin-dependence does not necessarily rule out CME at these synapses, because clathrin-dependent internalization of membrane receptors is not always sensitive to disruption of the actin cytoskeleton (Fujimoto et al. 2000).
Unidentified molecules
We have very little understanding of the mechanisms of bulk retrieval at the synapse. The formation of large invaginations of the surface appears to be independent of clathrin because the infolded membranes are not coated (Fig. 1D). Although the molecules tubulating membrane at the synapse have not been identified, liposomes containing phosphoinositides can be tubulated in vitro by epsin and amphiphysin, two proteins that are enriched at the synapse (Takei et al. 1999; Ford et al. 2002). This tubulating activity occurs in the absence of clathrin or other factors, so one might speculate that retrieval of large sections of uncoated membrane might represent a ‘stripped-down’ mechanism that occurs when other elements of the clathrin-dependent pathway are depleted. It seems likely that dynamin is required for scission of these large compartments, because tubular invaginations remain connected to the plasma membrane in shibire mutants at the restrictive temperature (Koenig & Ikeda, 1996).
Bulk retrieval at the synapse may be related to macropinocytosis in non-neuronal cells, which is dependent on the actin cytoskeleton and the activity of phosphatidylinositol 3-kinase (Nichols & Lippincott-Schwartz, 2001). In terminals of goldfish bipolar cells, inhibitors of actin remodelling and phosphatidylinositol 3-kinase activity block bulk retrieval measured by uptake of large fluorescent dextrans (Holt et al. 2003). In contrast, inhibition of phosphatidylinositol 3-kinase at the frog NMJ blocks a clathrin-dependent step that occurs after bulk internalisation (Rizzoli & Betz, 2002). The role of phosphoinositides in regulating endocytosis at the synapse will certainly be an important area of future research.
An important factor regulating exocytosis at many synapses is the Ca2+ signal triggering exocytosis (Table 1). The evidence for a stimulatory effect of Ca2+ at some synapses is strong enough to ask what is the calcium-sensitive molecule linked to fast endocytosis? Synapto-tagmins are attractive candidates, given their role in exocytosis (Chapman, 2002), but there are several other calcium-binding proteins associated with vesicles and the presynaptic membrane (Marks & McMahon, 1998). The identification of the Ca2+-binding molecule(s) involved in regulating endocytosis may become clearer when the fast and slow modes of endocytosis are defined more clearly at the molecular level.
Conclusions
Our understanding of endocytosis at the synapse is still at a relatively rudimentary stage. The evidence for neurotransmitter release by kiss-and-run has become much firmer in the last year (Klyachko & Jackson, 2002; Aravanis et al. 2003; Gandhi & Stevens, 2003), but we do not know what molecules are involved. What is the fusion pore made of? The role of bulk retrieval in recycling vesicles to a reserve pool has been characterized (Richards et al. 2000; Paillart et al. 2003), but we do not understand the mechanisms by which large membrane compartments are formed. The mechanism of clathrin-mediated endocytosis is understood in much greater detail through work on non-neuronal cells, but we do not have a clear understanding of the importance of this process in retrieval of synaptic vesicles. How rapidly does CME recover vesicles following fast exocytosis triggered by calcium?
We also have little understanding of the ways in which these various routes of retrieval interact. It is clear that the relative importance of fast and slow endocytosis depends on the strength of stimulation (Table 1), and it seems that after fusion, a vesicle can ‘choose’ between different routes of retrieval (Neves et al. 2001; Gandhi & Stevens, 2003). How is this choice made? Might different synapses employ different mechanisms of endocytosis according to their release properties, as suggested by Gandhi & Stevens (2003)?
How will we obtain answers to these basic questions? In our opinion, a major bottleneck in our understanding of endocytosis at the synapse is the gap between physiology and molecules. Bridging this gap will require the coupling of real-time methods for directly assaying endocytosis to preparations that allow manipulation of specific proteins. The search for answers to these questions will surely provide exciting times for the scientists that pursue them.
Acknowledgments
We would like to thank Dr Anne Cooke for her help in preparing Fig. 1 and Vahri Beaumont, Wolf Jockusch, Artur Llobet and Minnie Wu for helpful discussions. S.J.R. is supported by the Human Frontiers Science Program (HFSP: RGP0045/2002-C). We also thank the two anonymous reviewers for their helpful suggestions.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez De Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez De Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Ball CL, Hunt SP, Robinson MS. Expression and localization of α-adaptin isoforms. J Cell Sci. 1995;108:2865–2875. doi: 10.1242/jcs.108.8.2865. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Brodin L, Low P, Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol. 2000;10:312–320. doi: 10.1016/s0959-4388(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Cole JC, Villa BR, Wilkinson RS. Disruption of actin impedes transmitter release in snake motor terminals. J Physiol. 2000;525:579–586. doi: 10.1111/j.1469-7793.2000.t01-2-00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Sugimori M, Moreira JE, Ziff EB, Llinas R. Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles. Proc Natl Acad Sci USA. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4, 5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Guichet A, Wucherpfennig T, Dudu V, Etter S, Wilsch-Brauniger M, Hellwig A, Gonzalez-Gaitan M, Huttner WB, Schmidt AA. Essential role of endophilin A in synaptic vesicle budding at the Drosophila neuromuscular junction. EMBO J. 2002;21:1661–1672. doi: 10.1093/emboj/21.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001a;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. Visualizing recycling synaptic vesicles in hippocampal neurons by FM1–43 photoconversion. Proc Natl Acad Sci USA. 2001b;98:12748–12753. doi: 10.1073/pnas.171442798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Zhou ZY, Matthews G. Multiple components of membrane retrieval in synaptic terminals revealed by changes in hydrostatic pressure. J Neurophysiol. 2002;88:2509–2517. doi: 10.1152/jn.00267.2002. [DOI] [PubMed] [Google Scholar]
- Heuser J. The role of coated vesicles in recycling of synaptic vesicle membrane. Cell Biol Int Rep. 1989;13:1063–1076. doi: 10.1016/0309-1651(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool: Liverpool University Press; 1969. [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81:1495–1505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Saito K, Ikeda K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J Cell Biol. 1983;96:1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. The optically determined size of exo/endo cycling vesicle pool correlates with the quantal content at the neuromuscular junction of Drosophila larvae. J Neurosci. 1999;19:1557–1565. doi: 10.1523/JNEUROSCI.19-05-01557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Selective replenishment of two vesicle pools depends on the source of Ca2+ at the Drosophila synapse. Neuron. 2002;35:333–343. doi: 10.1016/s0896-6273(02)00777-8. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Gomis A, Job C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron. 1996;17:957–967. doi: 10.1016/s0896-6273(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Li Z, Murthy VN. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Link E, Reetz A, Morris SA, Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Miller TM, Heuser JE. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984;98:685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Hao W, Augustine GJ, Lafer EM. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J Neurosci. 2000;20:8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM. Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron. 2001;32:289–300. doi: 10.1016/s0896-6273(01)00467-6. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci. 1999;19:10201–10212. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci USA. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Lagnado L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. J Physiol. 1999;515:181–202. doi: 10.1111/j.1469-7793.1999.181ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poodry CA, Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ. Synaptic vesicle pools at the frog neuromuscular junction. Neuron. 2003;39:529–541. doi: 10.1016/s0896-6273(03)00405-7. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Broadie KS. The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans. Curr Opin Neurobiol. 2002;12:499–507. doi: 10.1016/s0959-4388(02)00360-4. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction. J Neurosci. 2002;22:10680–10689. doi: 10.1523/JNEUROSCI.22-24-10680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, Kelly RB. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr Biol. 1999;9:1411–1414. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Ryan TA. Presynaptic imaging techniques. Curr Opin Neurobiol. 2001;11:544–549. doi: 10.1016/s0959-4388(00)00247-6. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ, Reuter H. The timing of synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sara Y, Mozhayeva MG, Liu X, Kavalali ET. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci USA. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, DeCamilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Williams JH. ‘Kiss and run’ exocytosis at hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- Teng H, Cole JC, Roberts RL, Wilkinson RS. Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals. J Neurosci. 1999;19:4855–4866. doi: 10.1523/JNEUROSCI.19-12-04855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J Neurosci. 2000;20:7986–7993. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. ‘Delayed’ endocytosis is regulated by extracellular Ca2+ in snake motor boutons. J Physiol. 2003;551:103–114. doi: 10.1113/jphysiol.2003.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994;367:735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Feldman ME, Almers W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron. 2002;35:1085–1097. doi: 10.1016/s0896-6273(02)00896-6. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Sudhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]


