Abstract
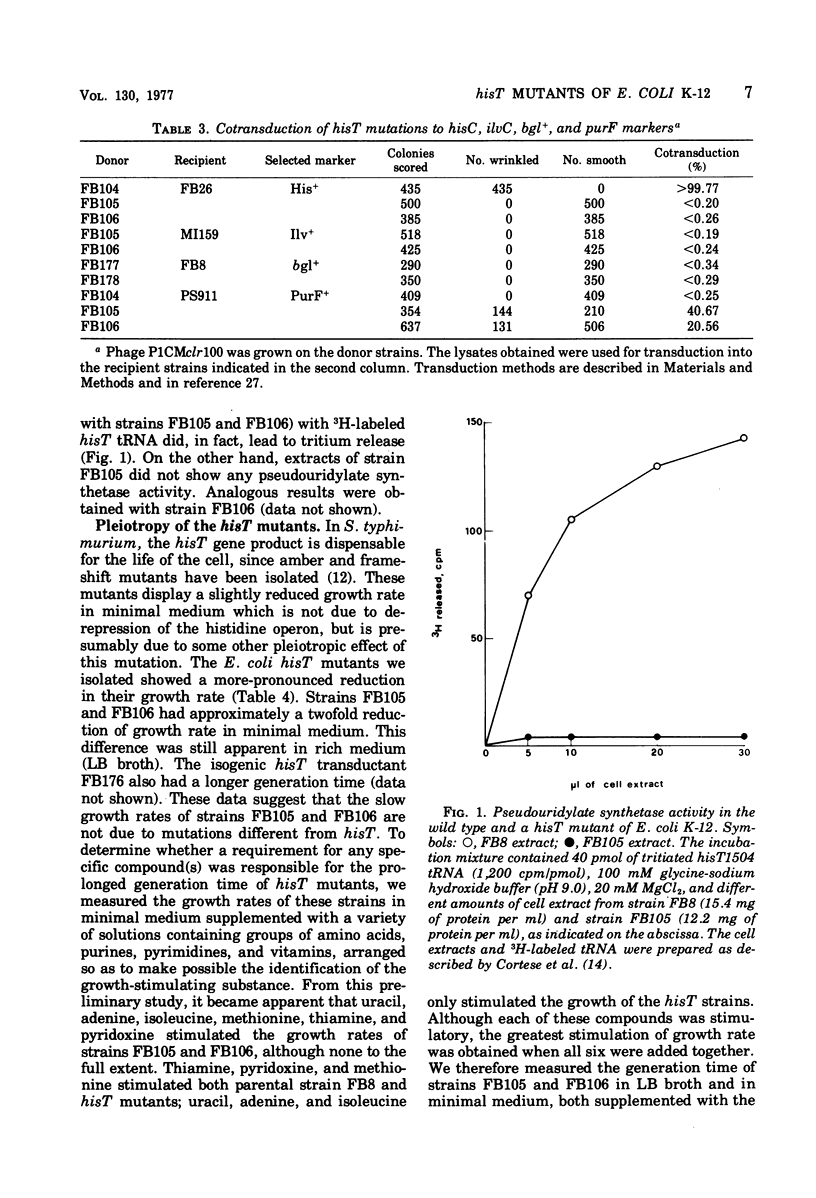
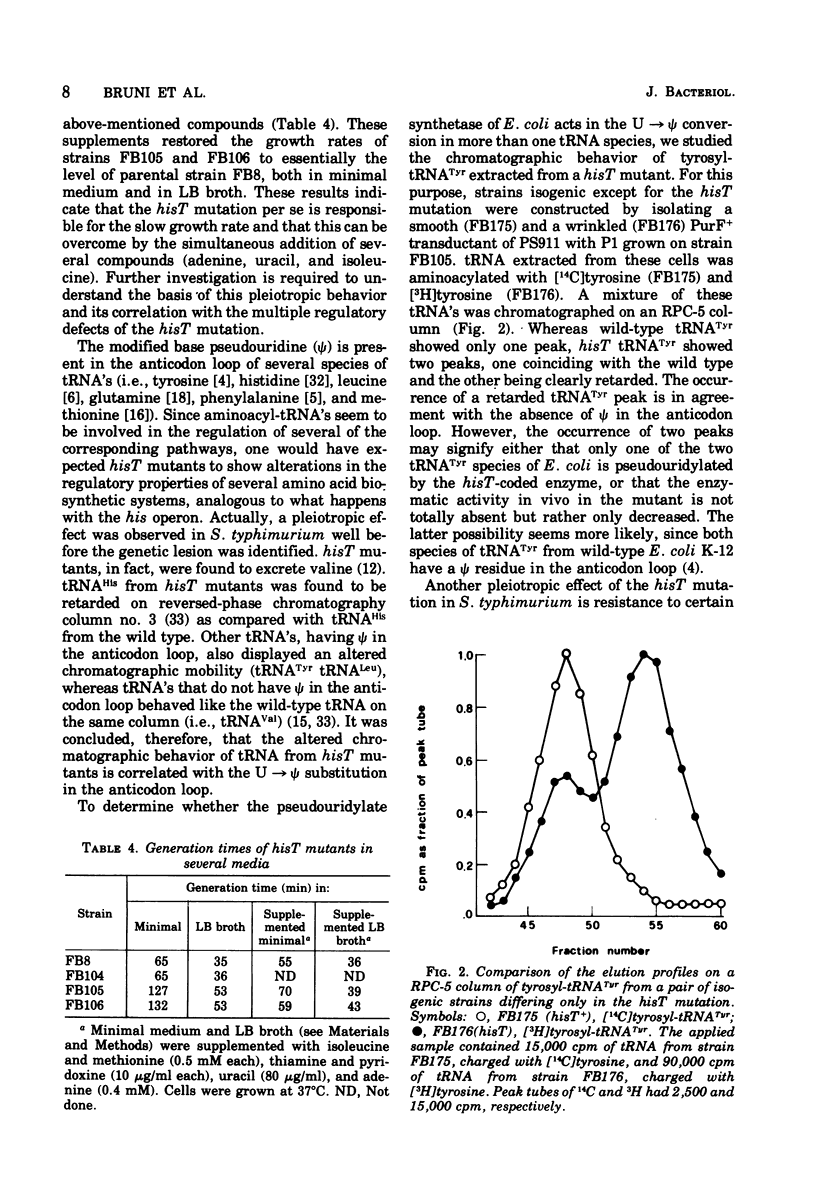
Escherichia coli K-12 hisT mutants were isolated, and their properties were studied. These mutants are derepressed for the histidine operon, map close to the purF locus at about 49.5 min on the E. coli linkage map, and lack pseudouridylate synthetase activity. The defect in this enzyme leads to the absence of pseudouridines in the anticodon loop of several transfer ribonucleic acid species, as evidenced by the altered elution profile on reversed-phase chromatography and resistance to amino acid analogues. Finally, the hisT mutants studied have a reduced growth rate that appears to be linked to hisT, although it is not known whether it is due to the same mutation. The normal generation time can be restored by supplementing the medium with adenine, uracil, and isoleucine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avitabile A., Bruni C. B., Covelli A., Di Nocera P. P., Sbordone L., Blasi F. In vitro transcription of the Escherichia coli histidine operon. Mol Gen Genet. 1974;132(1):1–12. doi: 10.1007/BF00268225. [DOI] [PubMed] [Google Scholar]
- Avitabile A., Carlomagno-Cerillo S., Favvre R., Blasi F. Isolation of transducing bacteriophages for the histidine and isoleucine-valine operons in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):40–47. doi: 10.1128/jb.112.1.40-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Sanger F. The sequence of phenylalanine tRNA from E. coli. FEBS Lett. 1969 Jun;3(4):275–278. doi: 10.1016/0014-5793(69)80157-2. [DOI] [PubMed] [Google Scholar]
- Blank H. U., Söll D. The nucleotide sequence of two leucine tRNA species from Escherichia coli K12. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1192–1197. doi: 10.1016/0006-291x(71)90589-4. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Brenner M., Lewis J. A., Straus D. S., De Lorenzo F., Ames B. N. Histidine regulation in salmonella typhimurium. XIV. Interaction of the histidyl transfer ribonucleic acid synthetase with histidine transfer ribonucleic acid. J Biol Chem. 1972 Jul 10;247(13):4333–4339. [PubMed] [Google Scholar]
- Chang G. W., Roth J. R., Ames B. N. Histidine regulation in Salmonella typhimurium. 8. Mutations of the hisT gene. J Bacteriol. 1971 Oct;108(1):410–414. doi: 10.1128/jb.108.1.410-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Straus D., Ames B. N. Enriched selection of dominant mutations: histidine operator mutations. J Bacteriol. 1971 Aug;107(2):578–579. doi: 10.1128/jb.107.2.578-579.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Kammen H. O., Spengler S. J., Ames B. N. Biosynthesis of pseudouridine in transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1103–1108. [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Marcker K. A. The nucleotide sequence of methionine transfer RNA-M. Eur J Biochem. 1970 Jan;12(1):177–194. doi: 10.1111/j.1432-1033.1970.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Ely B., Fankhauser D. B., Hartman P. E. A fine structure map of the salmonella histidine operator-promoter. Genetics. 1974 Oct;78(2):607–631. doi: 10.1093/genetics/78.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Yaniv M. Coding properties and nucleotide sequences of E. coli glutamine tRNAs. Nat New Biol. 1972 Jun 7;237(75):165–166. doi: 10.1038/newbio237165a0. [DOI] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger R. F., Kovach J. S. Regulation of histidine biosynthesis in Salmonella typhimurium. Curr Top Cell Regul. 1972;5:285–308. doi: 10.1016/b978-0-12-152805-8.50014-9. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E. P., Cater M. S., Matney T. S., Butler M. A., Greene A. Genetic analysis of the histidine operon in Escherichia coli K12. Genetics. 1970 Oct;66(2):219–229. doi: 10.1093/genetics/66.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R. Histidine regulation in Salmonella typhimurium. 13. Nucleotide sequence of histidine transfer ribonucleic acid. J Biol Chem. 1972 May 25;247(10):2989–3000. [PubMed] [Google Scholar]
- Umbarger H. E. Metabolite analogs as genetic and biochemical probes. Adv Genet. 1971;16:119–140. doi: 10.1016/s0065-2660(08)60356-9. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


