Abstract
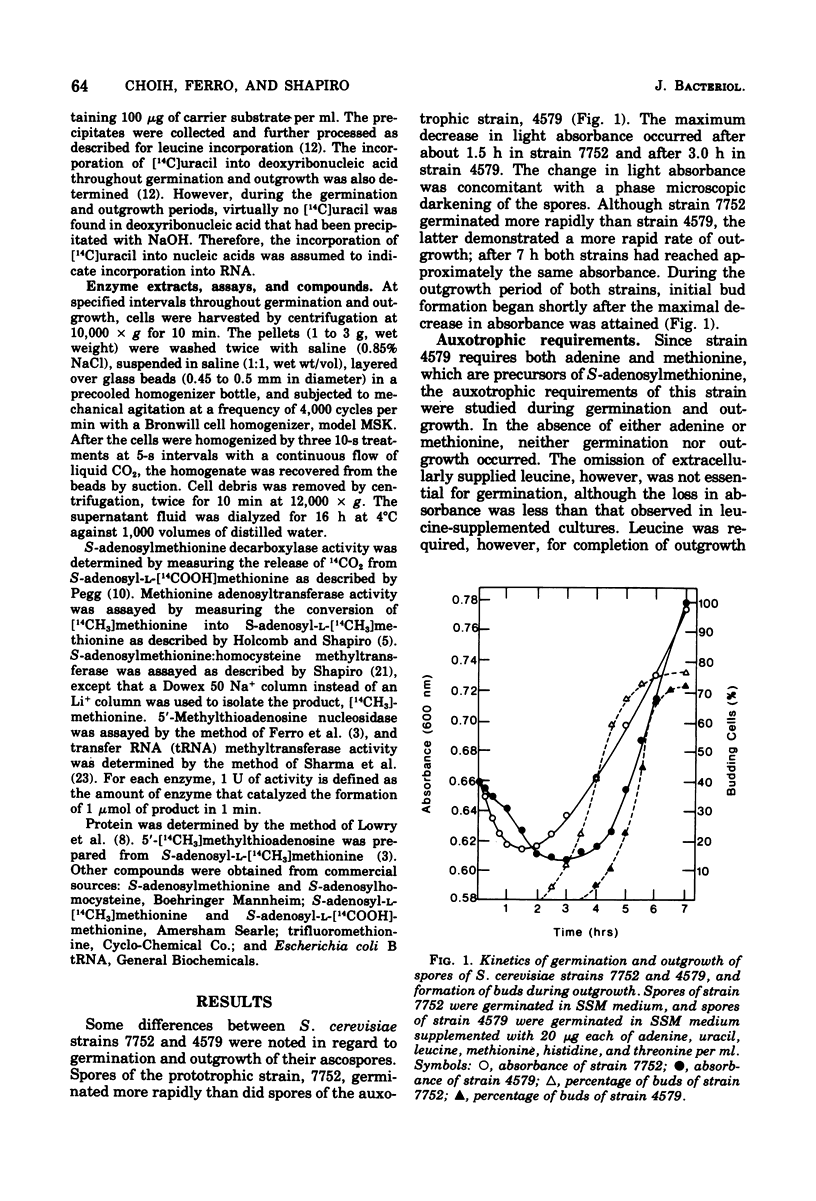
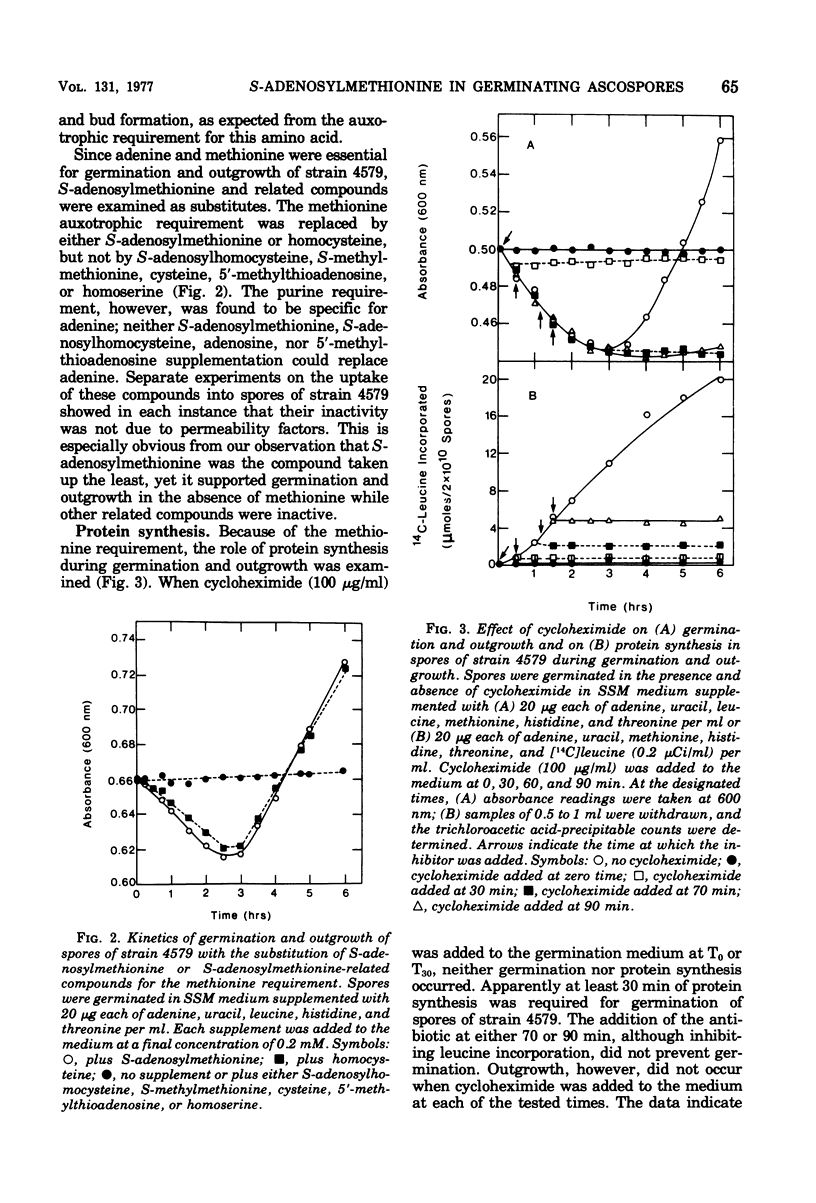
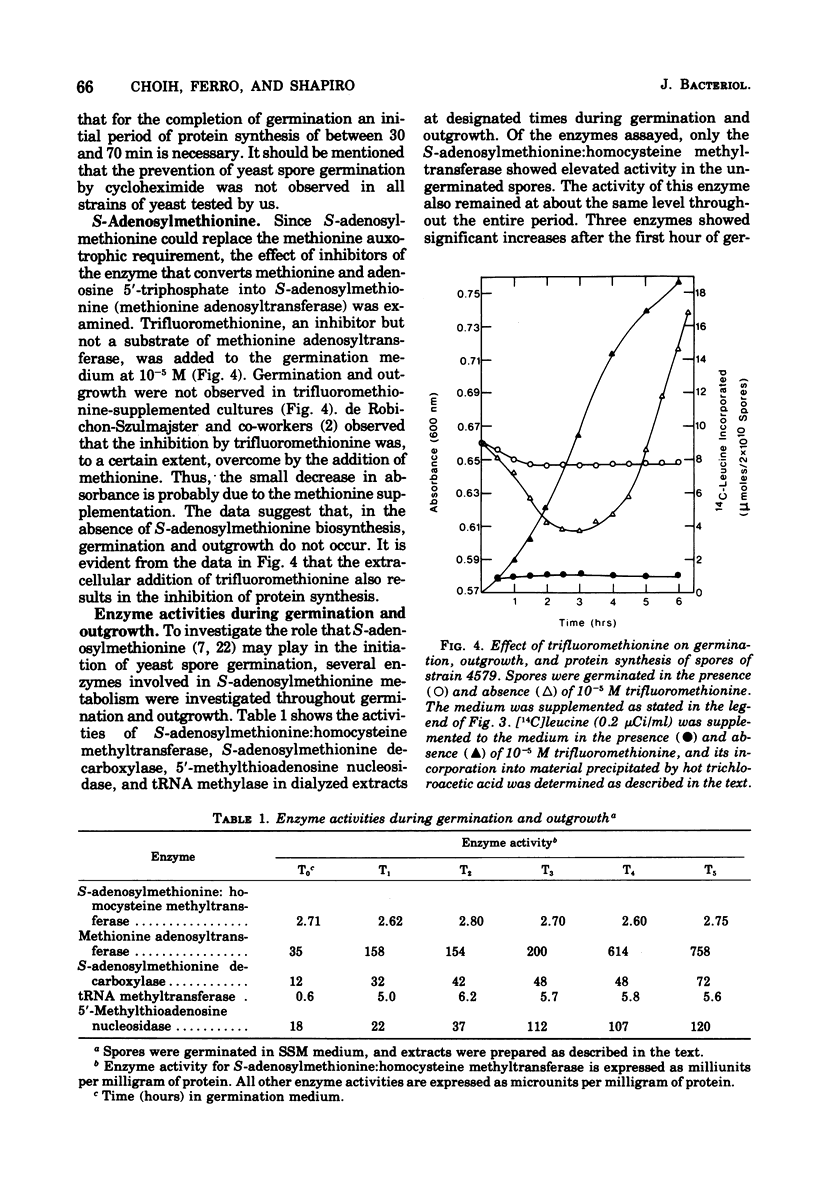
Germination and outgrowth of ascospores of Saccharomyces cerevisiae 4579 require both methionine and adenine, whereas leucine is only required for outgrowth. The methionine requirement may be satisfied by S-adenosylmethionine, but this sulfonium compound will not substitute for adenine. Between 30 and 70 min of protein synthesis is initially required for the completion of germination in strain 4579. The inhibition of S-adenosylmethionine synthetase by trifluoromethionine prevents both germination and protein synthesis. During the initial stages of germination, the S-adenosylmethionine synthetase, S-adenosylmethionine decarboxylase, and transfer ribonucleic acid methyltransferases increased significantly, indicating that polyamines and/or the methylation of transfer ribonucleic acid are required for the initiation of germination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek E. Transfer RNA and transfer RNA modification in differentiation and neoplasia. Introduction. Cancer Res. 1971 May;31(5):596–597. [PubMed] [Google Scholar]
- Colombani F., Cherest H., de Robichon-Szulmajster H. Biochemical and regulatory effects of methionine analogues in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):375–384. doi: 10.1128/jb.122.2.375-384.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A. J., Barrett A., Shapiro S. K. Kinetic properties and the effect of substrate analogues on 5'-methylthioadenosine nucleosidase from Escherichia coli. Biochim Biophys Acta. 1976 Jul 8;438(2):487–494. doi: 10.1016/0005-2744(76)90264-3. [DOI] [PubMed] [Google Scholar]
- Holcomb E. R., Shapiro S. K. Assay and regulation of S-adenosylmethionine synthetase in Saccharomyces cerevisiae and Candida utilis. J Bacteriol. 1975 Jan;121(1):267–271. doi: 10.1128/jb.121.1.267-271.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen R. C., Moore K., Yall I. Uptake and utilization of S-adenosyl-L-methionine and S-adenosyl-L-homocysteine in an adenine mutant of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):629–636. doi: 10.1128/jb.98.2.629-636.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell J. L., Rusch H. P. Regulation of polyamine synthesis in Physarum polyciphalum during growth and differentiation. Biochim Biophys Acta. 1973 Feb 28;297(2):503–516. doi: 10.1016/0304-4165(73)90098-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O. Macromolecular synthesis during the germanation of Saccharomyces cerevisiae spores. J Bacteriol. 1973 Mar;113(3):1289–1295. doi: 10.1128/jb.113.3.1289-1295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O. Physiological changes following the breaking of dormancy of Saccharomyces cerevisiae ascospores. Can J Microbiol. 1973 May;19(5):547–555. doi: 10.1139/m73-091. [DOI] [PubMed] [Google Scholar]
- Savarese J. J. Germination studies on pure yeast ascospores. Can J Microbiol. 1974 Nov;20(11):1517–1522. doi: 10.1139/m74-237. [DOI] [PubMed] [Google Scholar]
- Sebastian J., Carter B. L., Halvorson H. O. Use of yeast populations fractionated by zonal centrifugation to study the cell cycle. J Bacteriol. 1971 Dec;108(3):1045–1050. doi: 10.1128/jb.108.3.1045-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel J. L., Miller J. J. Observations on acid-fastness and respiration of germinating yeast ascospores. Can J Microbiol. 1971 Jul;17(7):837–845. doi: 10.1139/m71-135. [DOI] [PubMed] [Google Scholar]
- Sharma O. K., Loeb L. A., Borek E. Transfer RNA methylases during sea urchin embryogenesis. Biochim Biophys Acta. 1971 Jul 29;240(4):558–563. doi: 10.1016/0005-2787(71)90713-1. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Tingle M. A., Küenzi M. T., Halvorson H. O. Germination of yeast spores lacking mitochondrial deoxyribonucleic acid. J Bacteriol. 1974 Jan;117(1):89–93. doi: 10.1128/jb.117.1.89-93.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


