Abstract
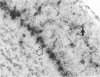
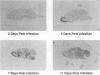
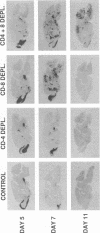
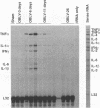
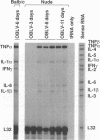
To investigate the mechanism by which viruses are cleared from neurons in the central nervous system, we have utilized a mouse model involving infection with a neurotropic variant of mouse hepatitis virus (OBLV60). After intranasal inoculation, OBLV60 grew preferentially in the olfactory bulbs of BALB/c mice. Using in situ hybridization, we found that viral RNA localized primarily in the outer layers of the olfactory bulb, including neurons of the mitral cell layer. Virus was cleared rapidly from the olfactory bulb between 5 and 11 days. Athymic nude mice failed to eliminate the virus, demonstrating a requirement for T lymphocytes. Immunosuppression of normal mice with cyclophosphamide also prevented clearance. Both CD4+ and CD8+ T-cell subsets were important, as depletion of either of these subsets delayed viral clearance. Gliosis and infiltrates of CD4+ and CD8+ cells were detected by immunohistochemical analysis at 6 days. The role of cytokines in clearance was investigated by using an RNase protection assay for interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, tumor necrosis factor alpha (TNF-alpha), TNF-beta, and gamma interferon (IFN-gamma). In immunocompetent mice there was upregulation of RNA for IL-1 alpha, IL-1 beta, IL-6, TNF-alpha, and IFN-gamma at the time of clearance. Nude mice had comparable increases in these cytokine messages, with the exception of IFN-gamma. Induction of major histocompatibility complex class I (MHC-I) molecules on cells in infected brains was demonstrated by immunohistochemical analyses in normal and nude mice, suggesting that IFN-gamma may not be necessary for induction of MHC-I on neural cells in vivo.
Full text
PDF

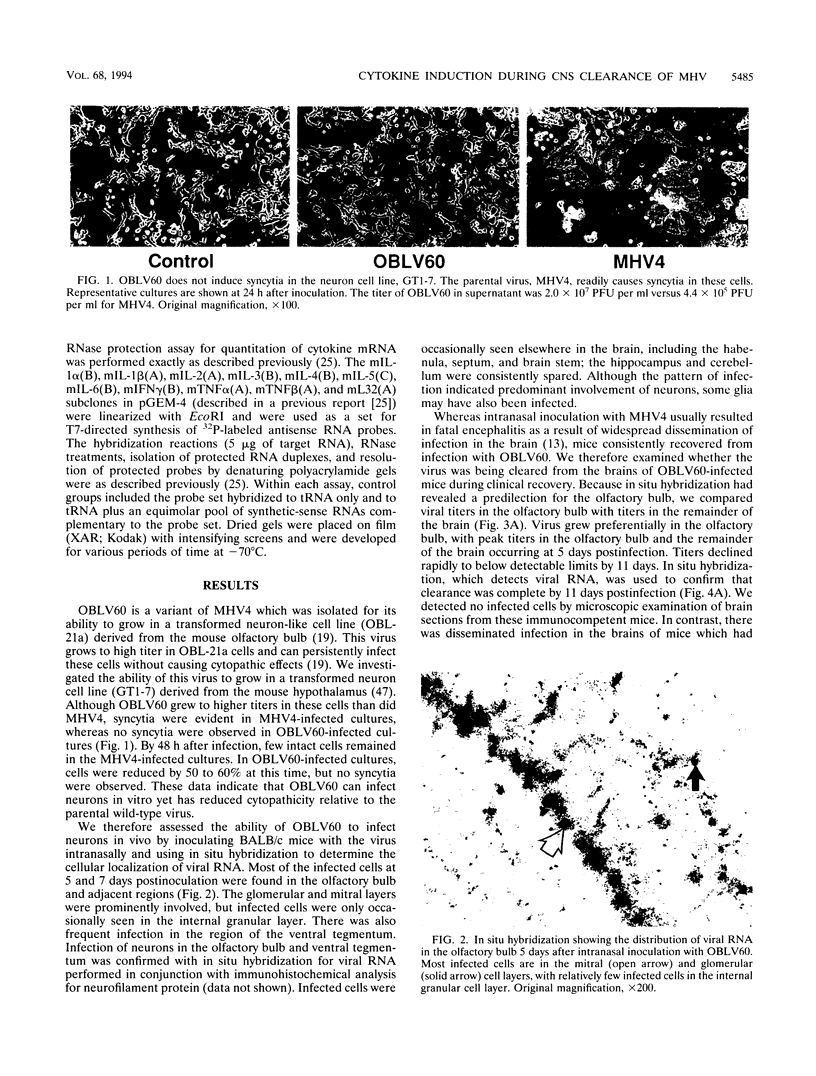
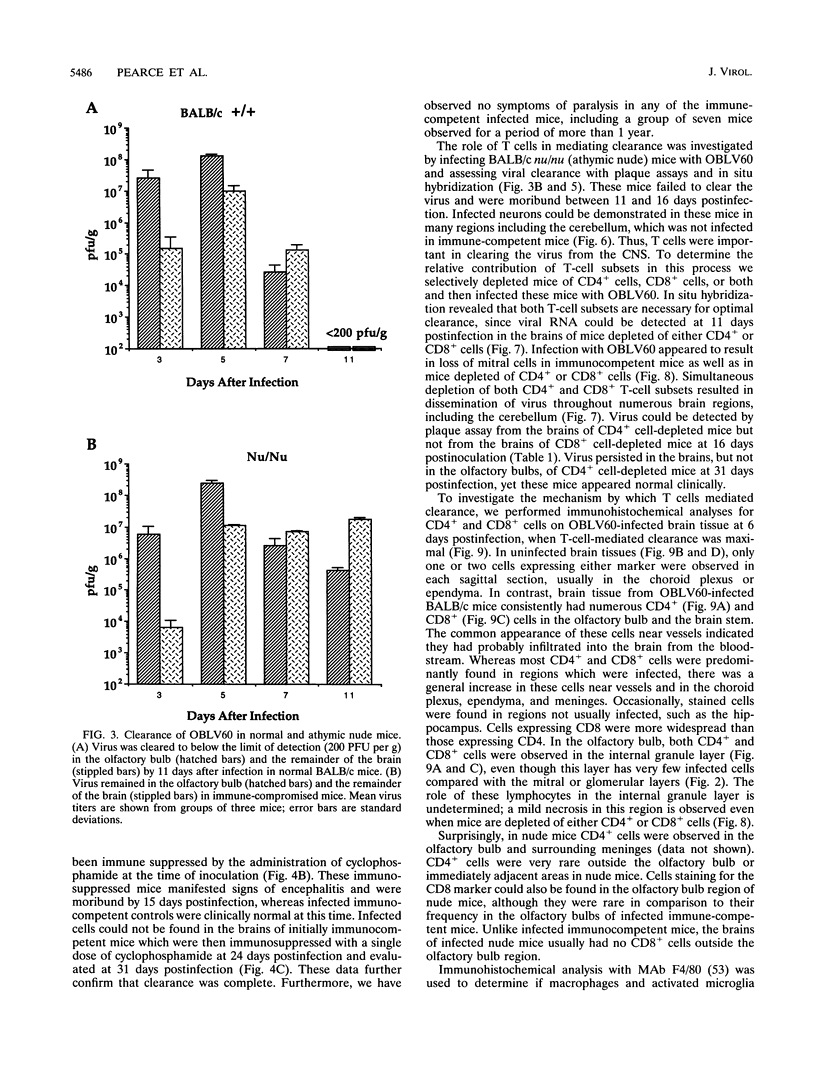

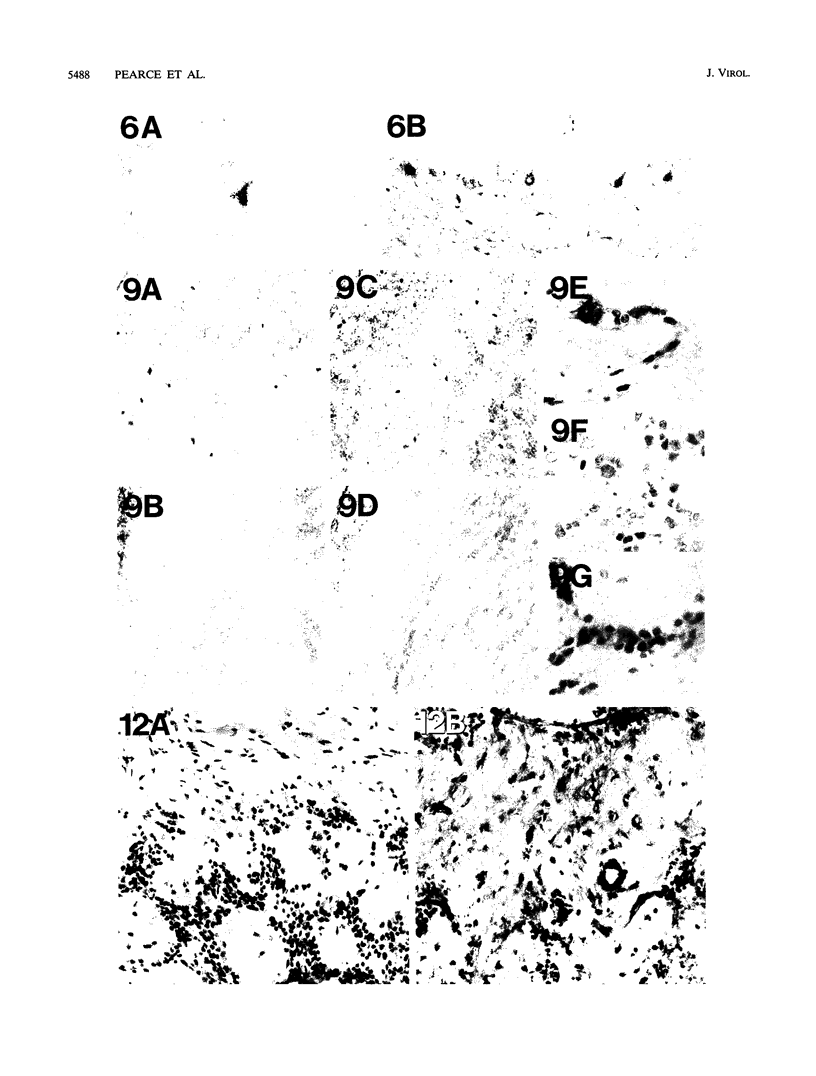

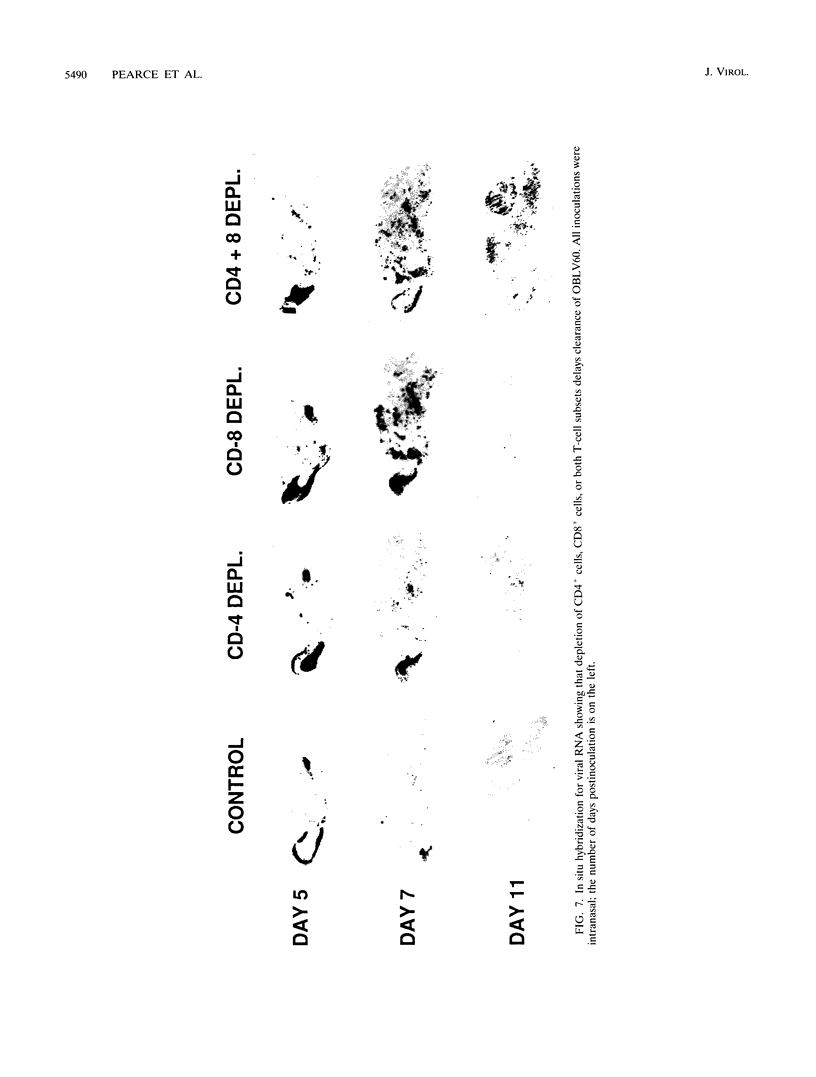
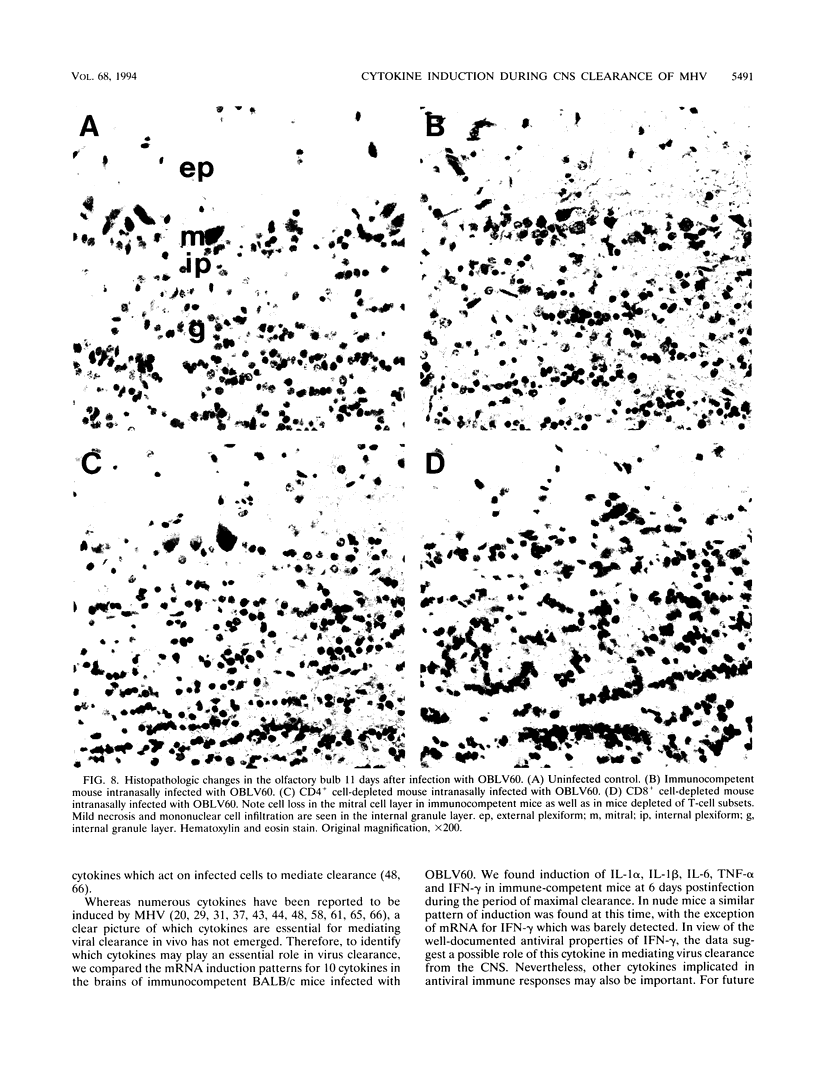
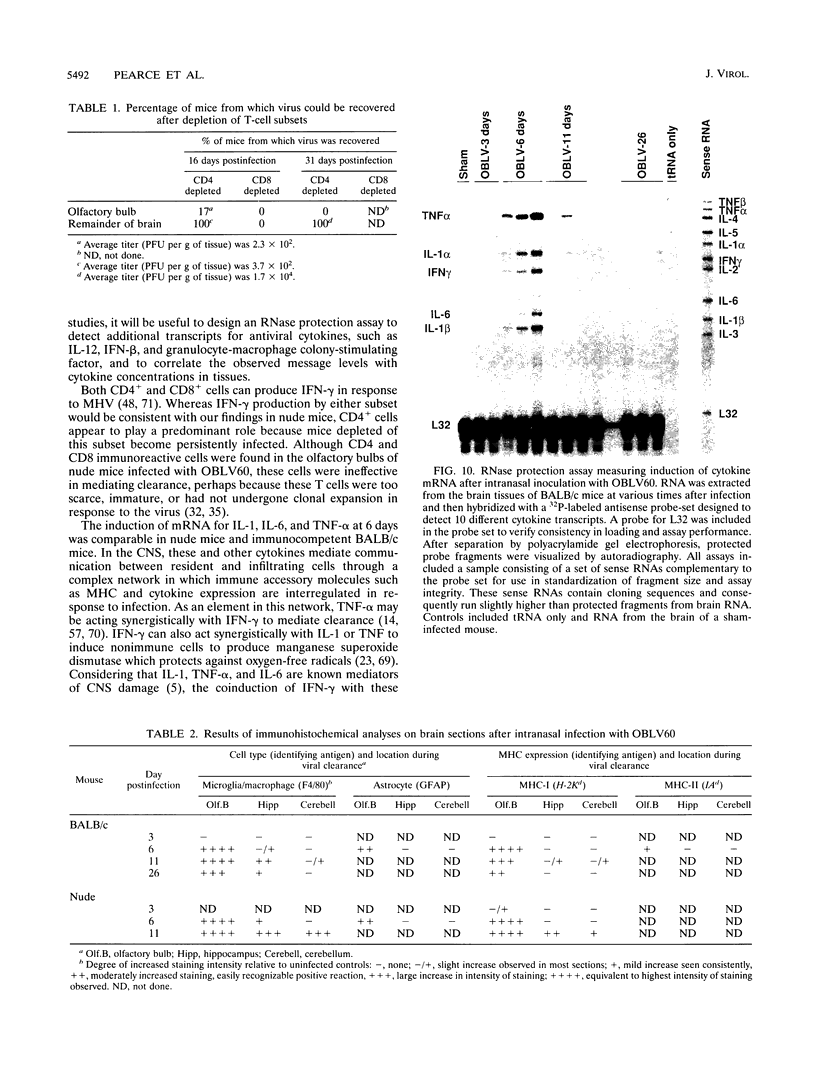



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett E. M., Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 1993 May;194(1):185–191. doi: 10.1006/viro.1993.1248. [DOI] [PubMed] [Google Scholar]
- Barthold S. W. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol. 1988;76(5):502–506. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Duration of mouse hepatitis virus infection: studies in immunocompetent and chemically immunosuppressed mice. Lab Anim Sci. 1990 Mar;40(2):133–137. [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch Virol. 1992;122(1-2):35–44. doi: 10.1007/BF01321116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste E. N. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992 Jul;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N., Sparacio S. M., Norris J. G., Grenett H. E., Fuller G. M. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol. 1990 Dec;30(2-3):201–212. doi: 10.1016/0165-5728(90)90104-u. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Dalziel R. G., Koolen M. J. Coronavirus-induced CNS disease: a model for virus-induced demyelination. J Neuroimmunol. 1988 Dec;20(2-3):111–116. doi: 10.1016/0165-5728(88)90141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I. Y., Benveniste E. N. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990 Apr 15;144(8):2999–3007. [PubMed] [Google Scholar]
- Compton S. R., Barthold S. W., Smith A. L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993 Feb;43(1):15–28. [PubMed] [Google Scholar]
- Cserr H. F., Knopf P. M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992 Dec;13(12):507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Duguid J., Trzepacz C. Major histocompatibility complex genes have an increased brain expression after scrapie infection. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):114–117. doi: 10.1073/pnas.90.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Parker S. E., Bloom F., Buchmeier M. J. The V5A13.1 envelope glycoprotein deletion mutant of mouse hepatitis virus type-4 is neuroattenuated by its reduced rate of spread in the central nervous system. Virology. 1992 Mar;187(1):178–188. doi: 10.1016/0042-6822(92)90306-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduchi E., Alonso M. A., Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989 Mar;63(3):1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., Bradbury J., Stohlman S. A., Weiner L. P. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb Pathog. 1987 Jul;3(1):9–20. doi: 10.1016/0882-4010(87)90033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Wang F. I., Trousdale M. D., Hinton D. R., Stohlman S. A. Immunopathogenesis of demyelination induced by MHV-4. Adv Exp Med Biol. 1990;276:565–572. doi: 10.1007/978-1-4684-5823-7_78. [DOI] [PubMed] [Google Scholar]
- Frei K., Malipiero U. V., Leist T. P., Zinkernagel R. M., Schwab M. E., Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989 Apr;19(4):689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Gallagher T. M., Escarmis C., Buchmeier M. J. Alteration of the pH dependence of coronavirus-induced cell fusion: effect of mutations in the spike glycoprotein. J Virol. 1991 Apr;65(4):1916–1928. doi: 10.1128/jvi.65.4.1916-1928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlinghouse L. E., Jr, Smith A. L. Responses of mice susceptible or resistant to lethal infection with mouse hepatitis virus, strain JHM, after exposure by a natural route. Lab Anim Sci. 1985 Oct;35(5):469–472. [PubMed] [Google Scholar]
- Giri J. G., Lomedico P. T., Mizel S. B. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985 Jan;134(1):343–349. [PubMed] [Google Scholar]
- Gombold J. L., Weiss S. R. Mouse hepatitis virus A59 increases steady-state levels of MHC mRNAs in primary glial cell cultures and in the murine central nervous system. Microb Pathog. 1992 Dec;13(6):493–505. doi: 10.1016/0882-4010(92)90015-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. A., Derbin K. S., Hunte-McDonough B., Krauss M. R., Chen K. T., Smith D. M., Epstein L. B. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol. 1991 Jul 1;147(1):149–154. [PubMed] [Google Scholar]
- Hazuda D. J., Strickler J., Kueppers F., Simon P. L., Young P. R. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990 Apr 15;265(11):6318–6322. [PubMed] [Google Scholar]
- Hobbs M. V., Weigle W. O., Noonan D. J., Torbett B. E., McEvilly R. J., Koch R. J., Cardenas G. J., Ernst D. N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993 Apr 15;150(8 Pt 1):3602–3614. [PubMed] [Google Scholar]
- Höfler H., Pütz B., Ruhri C., Wirnsberger G., Klimpfinger M., Smolle J. Simultaneous localization of calcitonin mRNA and peptide in a medullary thyroid carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(3):144–151. doi: 10.1007/BF02899206. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Hirano N., Tsukamoto T., Haga S. Vacuolar degeneration induced by JHM-CC virus in the CNS of cyclophosphamide-treated mice. Adv Exp Med Biol. 1990;276:601–607. doi: 10.1007/978-1-4684-5823-7_82. [DOI] [PubMed] [Google Scholar]
- Joly E., Mucke L., Oldstone M. B. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991 Sep 13;253(5025):1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Joseph J., Grun J. L., Lublin F. D., Knobler R. L. Interleukin-6 induction in vitro in mouse brain endothelial cells and astrocytes by exposure to mouse hepatitis virus (MHV-4, JHM). J Neuroimmunol. 1993 Jan;42(1):47–52. doi: 10.1016/0165-5728(93)90211-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Knobler R. L., Lublin F. D., Hart M. N. Mouse hepatitis virus (MHV-4, JHM) blocks gamma-interferon-induced major histocompatibility complex class II antigen expression on murine cerebral endothelial cells. J Neuroimmunol. 1991 Sep;33(3):181–190. doi: 10.1016/0165-5728(91)90105-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Knobler R. L., Lublin F. D., Hart M. N. Regulation of MHC class I and II antigens on cerebral endothelial cells and astrocytes following MHV-4 infection. Adv Exp Med Biol. 1990;276:579–591. doi: 10.1007/978-1-4684-5823-7_80. [DOI] [PubMed] [Google Scholar]
- Kennedy J. D., Pierce C. W., Lake J. P. Extrathymic T cell maturation. Phenotypic analysis of T cell subsets in nude mice as a function of age. J Immunol. 1992 Mar 15;148(6):1620–1629. [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kung J. T. Impaired clonal expansion in athymic nude CD8+CD4- T cells. J Immunol. 1988 Jun 1;140(11):3727–3735. [PubMed] [Google Scholar]
- Kuper C. F., Koornstra P. J., Hameleers D. M., Biewenga J., Spit B. J., Duijvestijn A. M., van Breda Vriesman P. J., Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992 Jun;13(6):219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- Kyuwa S., Yamaguchi K., Hayami M., Hilgers J., Fujiwara K. Spontaneous production of interleukin-2 and interleukin-3 by spleen cells from mice infected with mouse hepatitis virus type 4. J Virol. 1988 Sep;62(9):3506–3508. doi: 10.1128/jvi.62.9.3506-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Fishman P. S., Highkin M. K., Weiss S. R. Limbic encephalitis after inhalation of a murine coronavirus. Lab Invest. 1988 Jan;58(1):31–36. [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Wroblewska Z., Rorke L. B., Weiss S. R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984 May;34(5):597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchiari M. A., Martin J. P., Modolell M., Pereira C. A. Acquired immunity of A/J mice to mouse hepatitis virus 3 infection: dependence on interferon-gamma synthesis and macrophage sensitivity to interferon-gamma. J Gen Virol. 1991 Jun;72(Pt 6):1317–1322. doi: 10.1099/0022-1317-72-6-1317. [DOI] [PubMed] [Google Scholar]
- Lucchiari M. A., Modolell M., Eichmann K., Pereira C. A. In vivo depletion of interferon-gamma leads to susceptibility of A/J mice to mouse hepatitis virus 3 infection. Immunobiology. 1992 Sep;185(5):475–482. doi: 10.1016/S0171-2985(11)80089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlen J., Nennesmo I., Olsson A. B., Olsson T., Schröder H. D., Kristensson K. Peripheral nerve injury causes transient expression of MHC class I antigens in rat motor neurons and skeletal muscles. Brain Res. 1989 Mar 6;481(2):368–372. doi: 10.1016/0006-8993(89)90816-0. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Brinkmann R., ter Meulen V. Inducibility of Ia antigen on astrocytes by murine coronavirus JHM is rat strain dependent. J Exp Med. 1987 Jul 1;166(1):259–264. doi: 10.1084/jem.166.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990 Jul;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Mobley J., Evans G., Dailey M. O., Perlman S. Immune response to a murine coronavirus: identification of a homing receptor-negative CD4+ T cell subset that responds to viral glycoproteins. Virology. 1992 Apr;187(2):443–452. doi: 10.1016/0042-6822(92)90446-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Rous-Whipple Award Lecture. Viruses and diseases of the twenty-first century. Am J Pathol. 1993 Nov;143(5):1241–1249. [PMC free article] [PubMed] [Google Scholar]
- Pasick J. M., Wilson G. A., Morris V. L., Dales S. SJL/J resistance to mouse hepatitis virus-JHM-induced neurologic disease can be partially overcome by viral variants of S and host immunosuppression. Microb Pathog. 1992 Jul;13(1):1–15. doi: 10.1016/0882-4010(92)90027-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Ramsay A. J., Ruby J., Ramshaw I. A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993 Apr;14(4):155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- Ryder E. F., Snyder E. Y., Cepko C. L. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol. 1990 Mar;21(2):356–375. doi: 10.1002/neu.480210209. [DOI] [PubMed] [Google Scholar]
- Schijns V. E., Van der Neut R., Haagmans B. L., Bar D. R., Schellekens H., Horzinek M. C. Tumour necrosis factor-alpha, interferon-gamma and interferon-beta exert antiviral activity in nervous tissue cells. J Gen Virol. 1991 Apr;72(Pt 4):809–815. doi: 10.1099/0022-1317-72-4-809. [DOI] [PubMed] [Google Scholar]
- Schindler L., Engler H., Kirchner H. Activation of natural killer cells and induction of interferon after injection of mouse hepatitis virus type 3 in mice. Infect Immun. 1982 Mar;35(3):869–873. doi: 10.1128/iai.35.3.869-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan D. J., Wood M. J., Charlton H. M. Leucocyte recruitment and inflammation in the CNS. Trends Neurosci. 1992 Aug;15(8):276–278. doi: 10.1016/0166-2236(92)90075-j. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Barthold S. W., de Souza M. S., Bottomly K. The role of gamma interferon in infection of susceptible mice with murine coronavirus, MHV-JHM. Arch Virol. 1991;121(1-4):89–100. doi: 10.1007/BF01316746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Matsushima G. K., Casteel N., Weiner L. P. In vivo effects of coronavirus-specific T cell clones: DTH inducer cells prevent a lethal infection but do not inhibit virus replication. J Immunol. 1986 Apr 15;136(8):3052–3056. [PubMed] [Google Scholar]
- Sussman M. A., Shubin R. A., Kyuwa S., Stohlman S. A. T-cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J Virol. 1989 Jul;63(7):3051–3056. doi: 10.1128/jvi.63.7.3051-3056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Bhat S., Murasko D., Weiss S. R., Silberberg D. H. Induction of glial cell MHC antigen expression in neurotropic coronavirus infections. Characterization of the H-2-inducing soluble factor elaborated by infected brain cells. J Immunol. 1988 Mar 15;140(6):2068–2072. [PubMed] [Google Scholar]
- Virelizier J. L., Virelizier A. M., Allison A. C. The role of circulating interferon in the modifications of immune responsiveness by mouse hepatitis virus (MHV-3). J Immunol. 1976 Sep;117(3):748–753. [PubMed] [Google Scholar]
- Williamson J. S., Stohlman S. A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990 Sep;64(9):4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. S., Sykes K. C., Stohlman S. A. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J Neuroimmunol. 1991 Jun;32(3):199–207. doi: 10.1016/0165-5728(91)90189-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. S. Virus-specific T cells in the central nervous system following infection with an avirulent neurotropic mouse hepatitis virus. Reg Immunol. 1992 May-Jun;4(3):145–152. [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Krowka J. F., Stites D. P., Goeddel D. V. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-alpha and interferon-gamma. J Immunol. 1988 Jan 1;140(1):120–124. [PubMed] [Google Scholar]
- Yamaguchi K., Goto N., Kyuwa S., Hayami M., Toyoda Y. Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J Neuroimmunol. 1991 Apr;32(1):1–9. doi: 10.1016/0165-5728(91)90065-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza M. S., Smith A. L., Bottomly K. Infection of BALB/cByJ mice with the JHM strain of mouse hepatitis virus alters in vitro splenic T cell proliferation and cytokine production. Lab Anim Sci. 1991 Apr;41(2):99–105. [PubMed] [Google Scholar]