Abstract
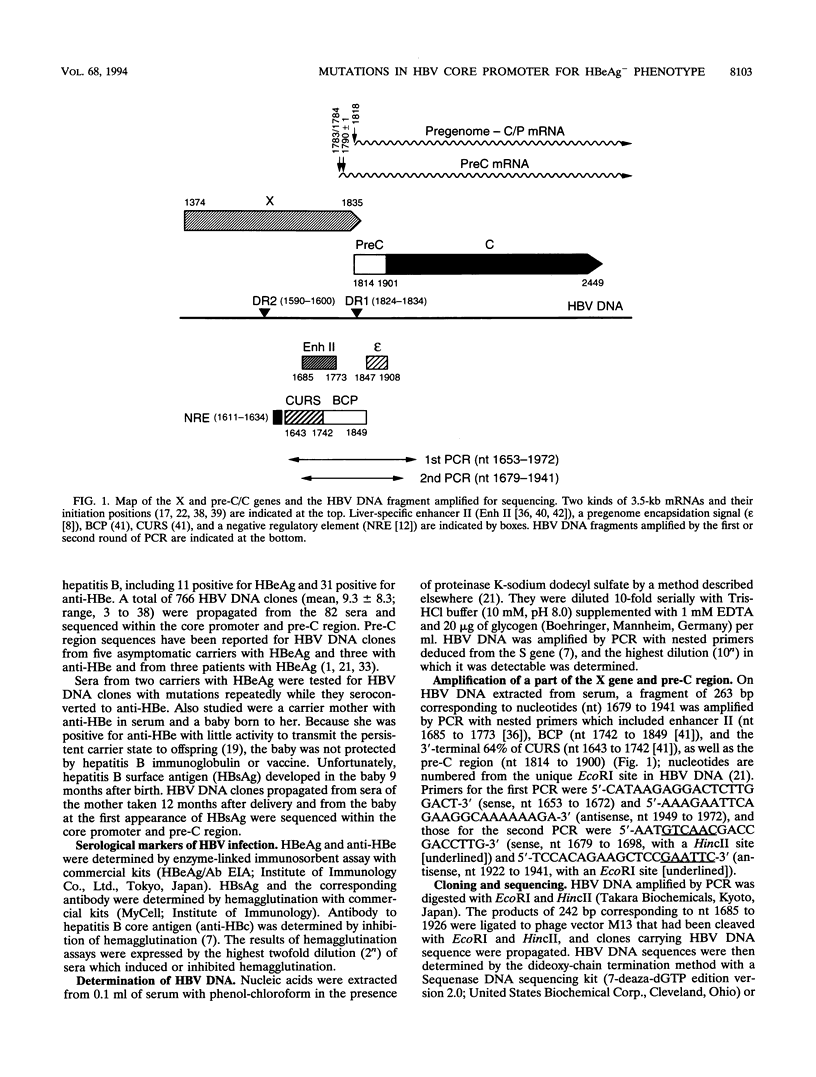
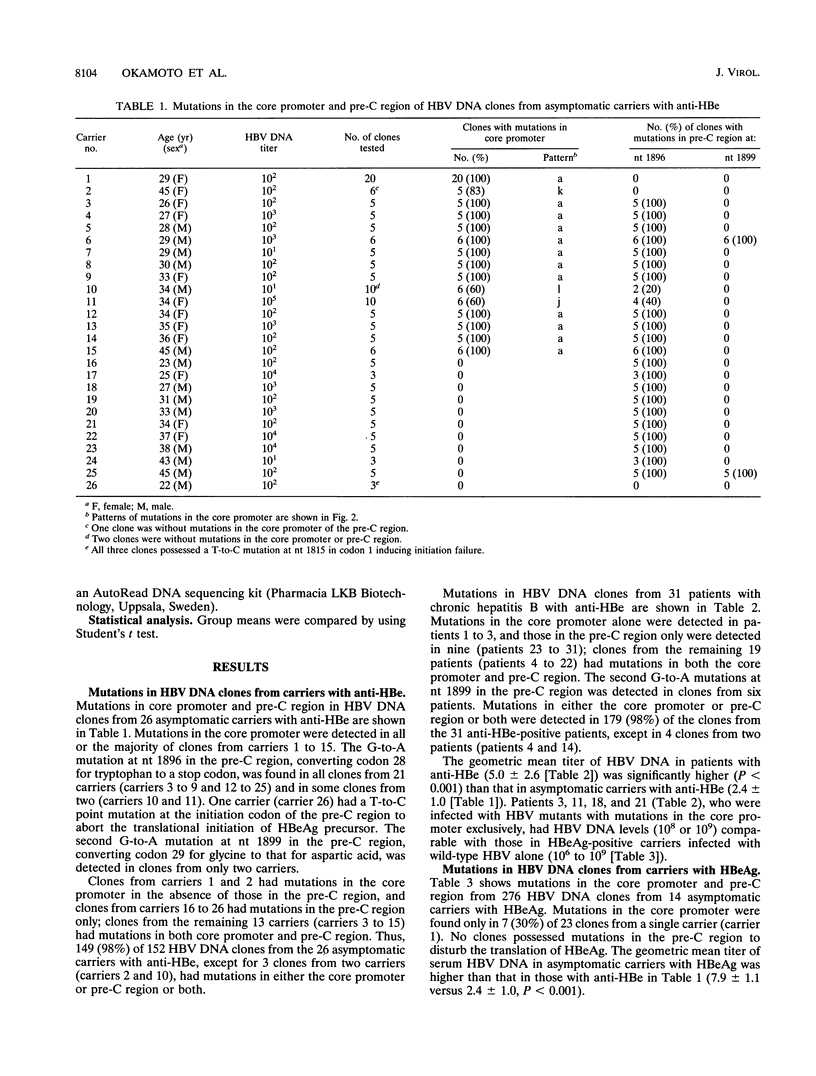
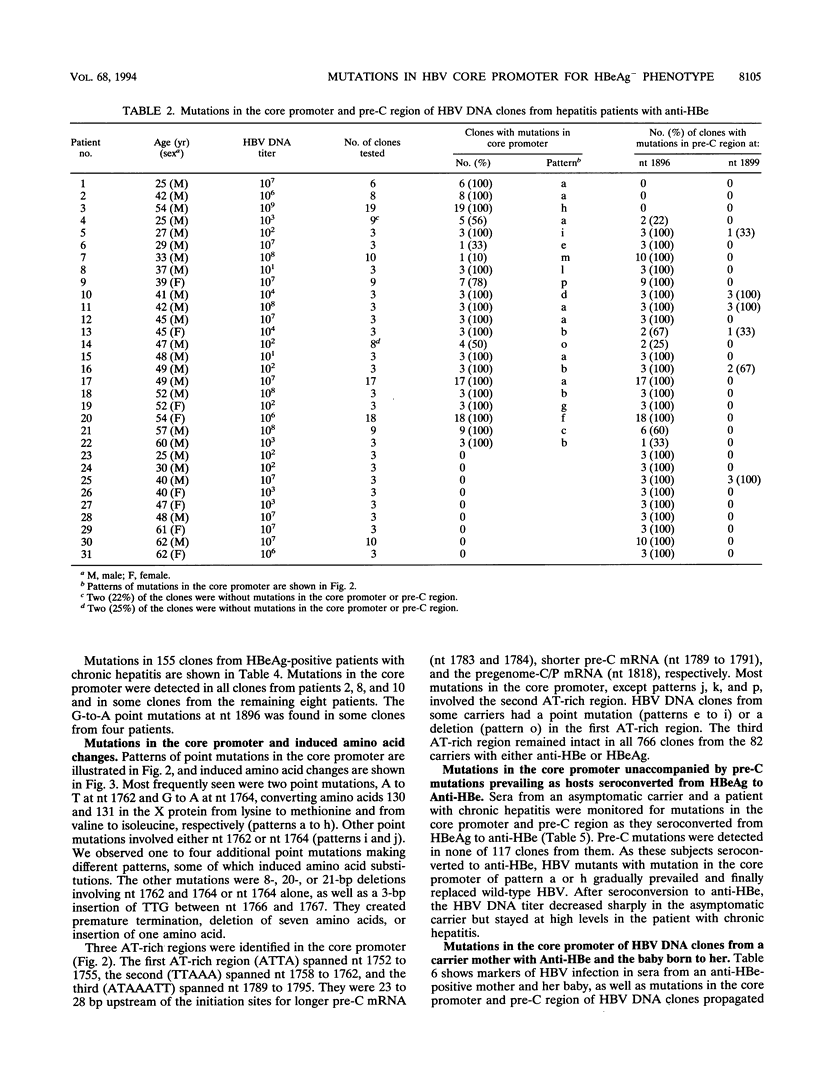
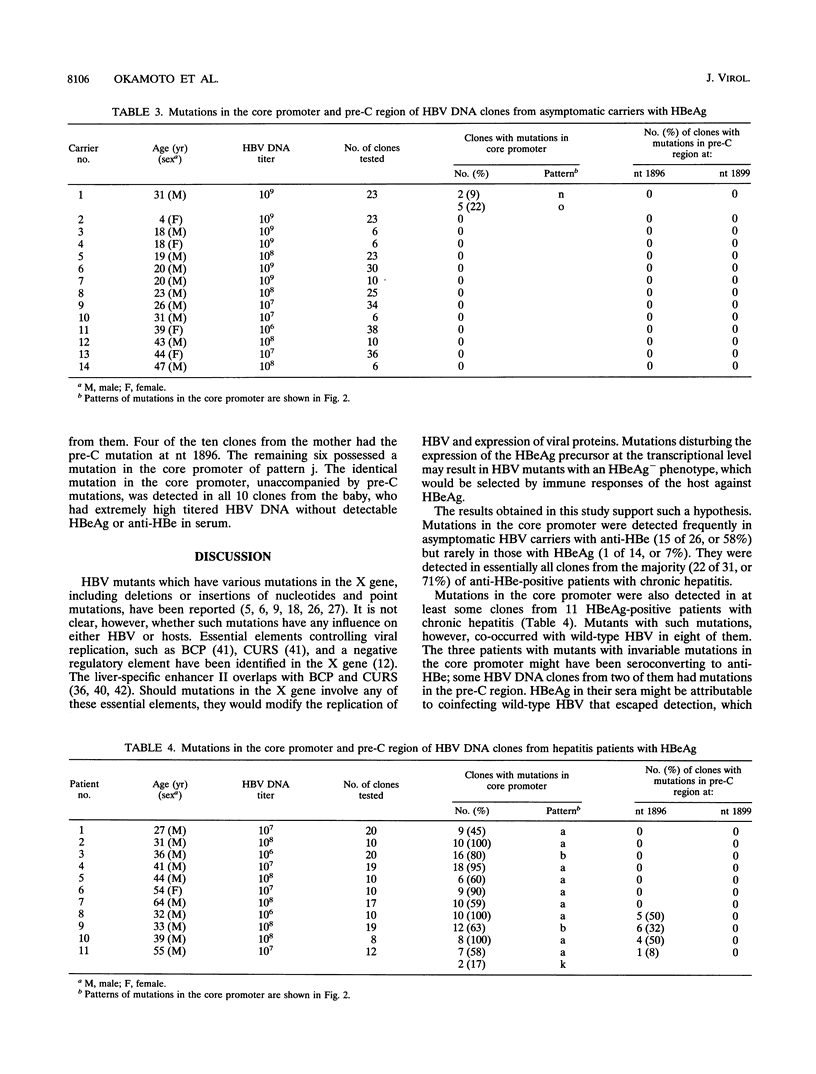
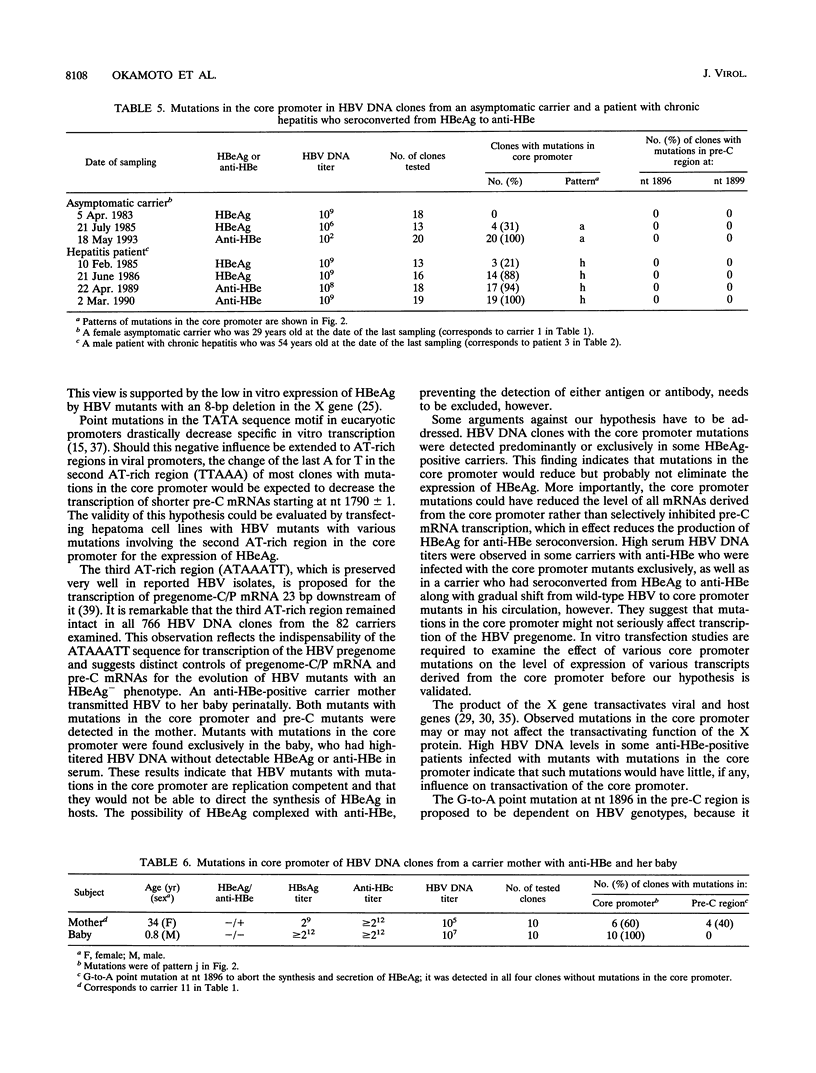
Hepatitis B virus (HBV) DNA clones were propagated from 57 carriers with antibody to hepatitis B e antigen (HBeAg) and sequenced within nucleotides (nt) 1685 to 1926 including the core promoter (nt 1742 to 1849) and the pre-C region (nt 1814 to 1900). Mutations in the core promoter or those in the pre-C region, or both, were detected in 328 (97.9%) of 335 clones from them. Five carriers were infected with HBV mutants with mutations in the core promoter alone, while 20 carriers were infected only with those in the pre-C region to abort the translation of HBeAg precursor; the remaining 32 carriers were infected with HBV mutants with mutations in both the core promoter and pre-C region. Some carriers infected with HBV with mutations in the core promoter exclusively had high HBV DNA titers, comparable with those in carriers infected with wild-type HBV, thereby indicating that such mutations would not affect the transcription of the HBV pregenome extensively. Two point mutations in the core promoter, from A to T at nt 1762 and from G to A at nt 1764, were most prevalent. The other mutations included a point mutation at either of the two nucleotides and their deletion. All of these mutations involved the TTAAA sequence (nt 1758 to 1762) at 28 bp upstream of the initiation site for shorter pre-C mRNAs (nt 1790 +/- 1). The ATAAATT sequence (nt 1789 to 1795) at 23 bp upstream of the initiation site for the pregenome RNA (nt 1818), however, remained intact in all 335 HBV DNA clones. HBV mutants with mutations in the core promoter, unaccompanied by pre-C mutations, prevailed and replaced wild-type HBV in two carriers as they seroconverted from HBeAg to the corresponding antibody. These results indicate that HBV mutants with an HBeAg- phenotype would be generated by mutations in the core promoter which might abort the transcription of pre-C mRNA but do not seriously affect that of pregenome RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane Y., Yamanaka T., Suzuki H., Sugai Y., Tsuda F., Yotsumoto S., Omi S., Okamoto H., Miyakawa Y., Mayumi M. Chronic active hepatitis with hepatitis B virus DNA and antibody against e antigen in the serum. Disturbed synthesis and secretion of e antigen from hepatocytes due to a point mutation in the precore region. Gastroenterology. 1990 Oct;99(4):1113–1119. doi: 10.1016/0016-5085(90)90632-b. [DOI] [PubMed] [Google Scholar]
- Bruss V., Gerlich W. H. Formation of transmembraneous hepatitis B e-antigen by cotranslational in vitro processing of the viral precore protein. Virology. 1988 Apr;163(2):268–275. doi: 10.1016/0042-6822(88)90266-8. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Jacyna M. R., Hadziyannis S., Karayiannis P., McGarvey M. J., Makris A., Thomas H. C. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989 Sep 9;2(8663):588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Feitelson M., Duan L. X., Horiike N., Clayton M. Hepatitis B X open reading frame deletion mutants isolated from atypical hepatitis B virus infections. J Hepatol. 1991;13 (Suppl 4):S58–S60. doi: 10.1016/0168-8278(91)90025-7. [DOI] [PubMed] [Google Scholar]
- Horikita M., Itoh S., Yamamoto K., Shibayama T., Tsuda F., Okamoto H. Differences in the entire nucleotide sequence between hepatitis B virus genomes from carriers positive for antibody to hepatitis B e antigen with and without active disease. J Med Virol. 1994 Sep;44(1):96–103. doi: 10.1002/jmv.1890440118. [DOI] [PubMed] [Google Scholar]
- Iizuka H., Ohmura K., Ishijima A., Satoh K., Tanaka T., Tsuda F., Okamoto H., Miyakawa Y., Mayumi M. Correlation between anti-HBc titers and HBV DNA in blood units without detectable HBsAg. Vox Sang. 1992;63(2):107–111. doi: 10.1111/j.1423-0410.1992.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990 Oct;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Hong S. P., Kim S. K., Lee W. S., Rho H. M. Replication of a mutant hepatitis B virus with a fused X-C reading frame in hepatoma cells. J Gen Virol. 1992 Sep;73(Pt 9):2421–2424. doi: 10.1099/0022-1317-73-9-2421. [DOI] [PubMed] [Google Scholar]
- Laskus T., Rakela J., Tong M. J., Nowicki M. J., Mosley J. W., Persing D. H. Naturally occurring hepatitis B virus mutants with deletions in the core promoter region. J Hepatol. 1994 Jun;20(6):837–841. doi: 10.1016/s0168-8278(05)80158-8. [DOI] [PubMed] [Google Scholar]
- Li J. S., Tong S. P., Wen Y. M., Vitvitski L., Zhang Q., Trépo C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: possible contribution of a single nucleotide in the precore region. J Virol. 1993 Sep;67(9):5402–5410. doi: 10.1128/jvi.67.9.5402-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W. Y., Ting L. P. Repression of enhancer II activity by a negative regulatory element in the hepatitis B virus genome. J Virol. 1994 Mar;68(3):1758–1764. doi: 10.1128/jvi.68.3.1758-1764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Naoumov N. V., Schneider R., Grötzinger T., Jung M. C., Miska S., Pape G. R., Will H. Precore mutant hepatitis B virus infection and liver disease. Gastroenterology. 1992 Feb;102(2):538–543. doi: 10.1016/0016-5085(92)90101-4. [DOI] [PubMed] [Google Scholar]
- Nassal M., Junker-Niepmann M., Schaller H. Translational inactivation of RNA function: discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell. 1990 Dec 21;63(6):1357–1363. doi: 10.1016/0092-8674(90)90431-d. [DOI] [PubMed] [Google Scholar]
- Ogata N., Miller R. H., Ishak K. G., Purcell R. H. The complete nucleotide sequence of a pre-core mutant of hepatitis B virus implicated in fulminant hepatitis and its biological characterization in chimpanzees. Virology. 1993 May;194(1):263–276. doi: 10.1006/viro.1993.1257. [DOI] [PubMed] [Google Scholar]
- Okada K., Kamiyama I., Inomata M., Imai M., Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976 Apr 1;294(14):746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Tsuda F., Sakugawa H., Sastrosoewignjo R. I., Imai M., Miyakawa Y., Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988 Oct;69(Pt 10):2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Yotsumoto S., Akahane Y., Yamanaka T., Miyazaki Y., Sugai Y., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990 Mar;64(3):1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Bao H., Shih C., Tahara S. M. Preferred translation of human hepatitis B virus polymerase from core protein- but not from precore protein-specific transcript. J Virol. 1990 Sep;64(9):4578–4581. doi: 10.1128/jvi.64.9.4578-4581.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993 Jun;67(6):3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler-Adams S., Schlayer H. J., Peters T., Hettler F., Gerok W., Rasenack J. Sequence analysis of hepatitis B virus DNA in immunologically negative infection. Arch Virol. 1993;133(3-4):385–396. doi: 10.1007/BF01313777. [DOI] [PubMed] [Google Scholar]
- Repp R., Keller C., Borkhardt A., Csecke A., Schaefer S., Gerlich W. H., Lampert F. Detection of a hepatitis B virus variant with a truncated X gene and enhancer II. Arch Virol. 1992;125(1-4):299–304. doi: 10.1007/BF01309646. [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Imai M., Miyakawa Y., Iwakiri S., Mayumi M. Duality of hepatitis B e antigen in serum of persons infected with hepatitis B virus: evidence for the nonidentity of e antigen with immunoglobulins. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1952–1956. doi: 10.1073/pnas.75.4.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Takeda K., Akahane Y., Suzuki H., Okamoto H., Tsuda F., Miyakawa Y., Mayumi M. Defects in the precore region of the HBV genome in patients with chronic hepatitis B after sustained seroconversion from HBeAg to anti-HBe induced spontaneously or with interferon therapy. Hepatology. 1990 Dec;12(6):1284–1289. doi: 10.1002/hep.1840120606. [DOI] [PubMed] [Google Scholar]
- Tong S. P., Li J. S., Vitvitski L., Kay A., Treépo C. Evidence for a base-paired region of hepatitis B virus pregenome encapsidation signal which influences the patterns of precore mutations abolishing HBe protein expression. J Virol. 1993 Sep;67(9):5651–5655. doi: 10.1128/jvi.67.9.5651-5655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen P., Wu X., Sun A. L., Wang H., Zhu Y. A., Li Z. P. A new enhancer element, ENII, identified in the X gene of hepatitis B virus. J Virol. 1990 Aug;64(8):3977–3981. doi: 10.1128/jvi.64.8.3977-3981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. A T to A base substitution and small deletions in the conalbumin TATA box drastically decrease specific in vitro transcription. Nucleic Acids Res. 1981 Apr 24;9(8):1813–1824. doi: 10.1093/nar/9.8.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Reiser W., Weimer T., Pfaff E., Büscher M., Sprengel R., Cattaneo R., Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987 Mar;61(3):904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- Yuh C. H., Chang Y. L., Ting L. P. Transcriptional regulation of precore and pregenomic RNAs of hepatitis B virus. J Virol. 1992 Jul;66(7):4073–4084. doi: 10.1128/jvi.66.7.4073-4084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh C. H., Ting L. P. The genome of hepatitis B virus contains a second enhancer: cooperation of two elements within this enhancer is required for its function. J Virol. 1990 Sep;64(9):4281–4287. doi: 10.1128/jvi.64.9.4281-4287.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


