Abstract
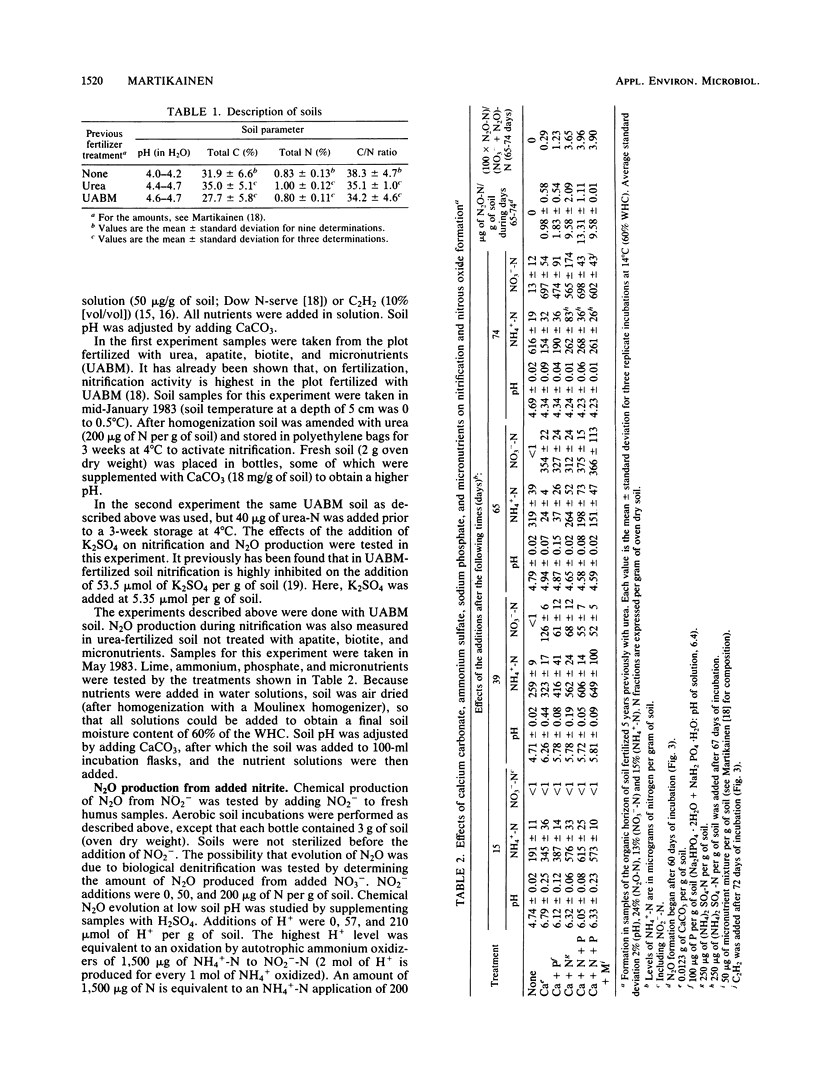
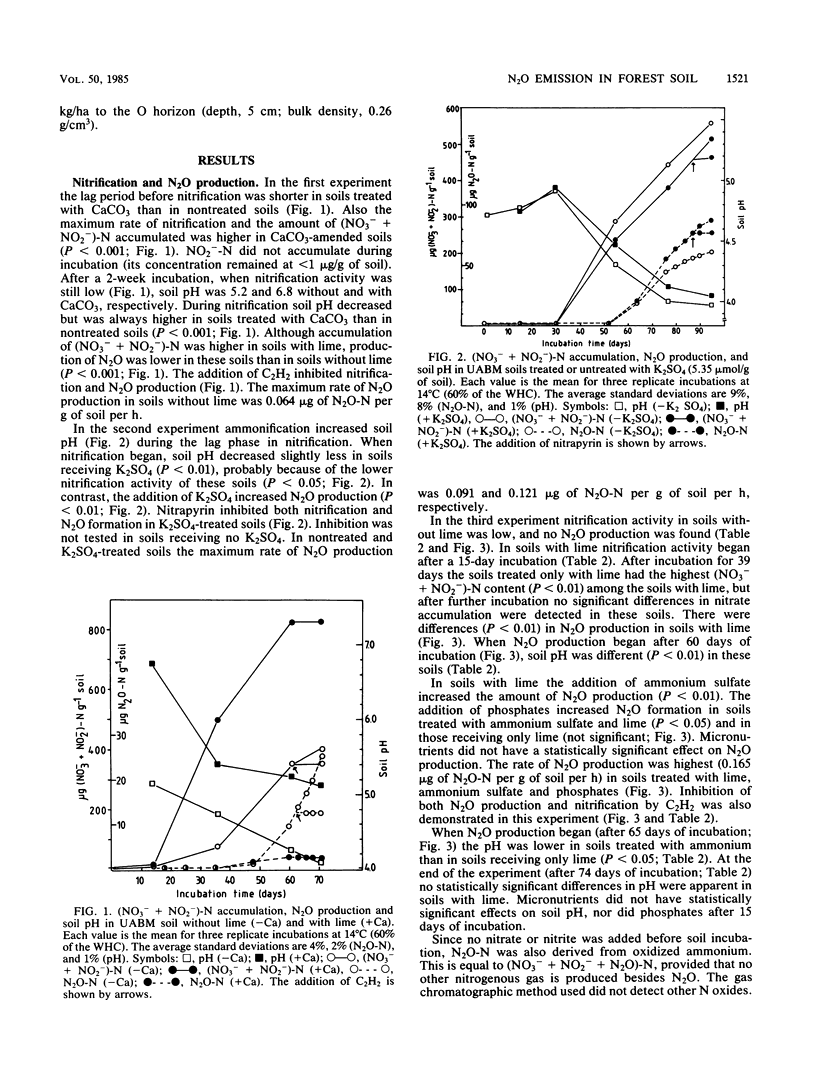
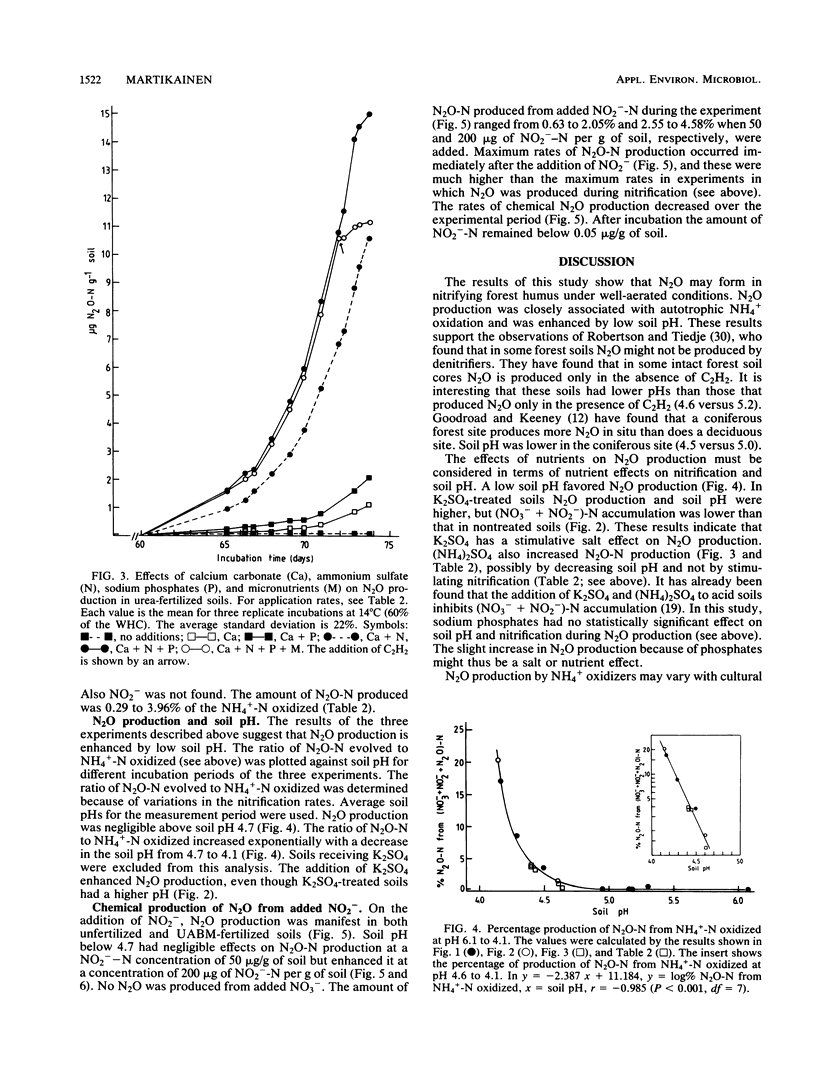
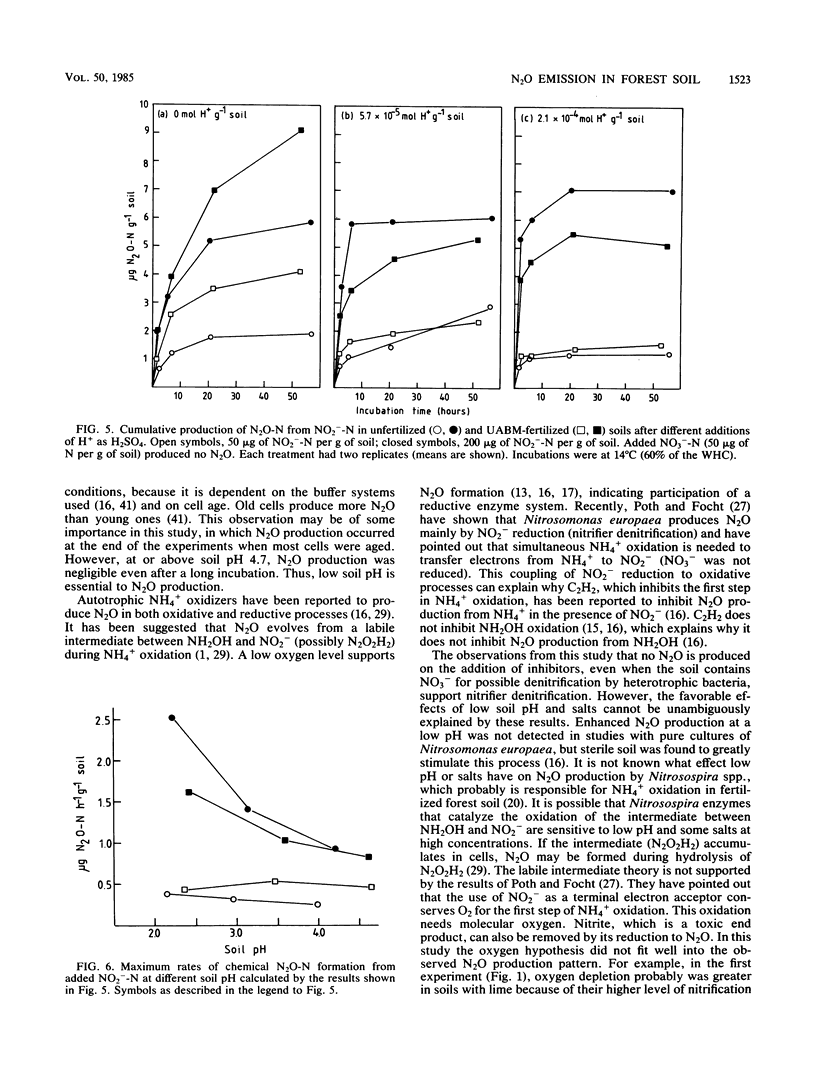
Aerobic N2O production was studied in nitrifying humus from urea-fertilized pine forest soil. Acetylene and nitrapyrin inhibited both NH4+ oxidation and N2O production, indicating that N2O production was closely associated with autotrophic NH4+ oxidation. N2O production was enhanced by low soil pH; it was negligible above pH 4.7. When soil pH decreased from 4.7 to 4.1, the relative amount of N2O-N produced from NH4+-N oxidized increased exponentially to 20%. There was also some evidence that N2O formation was stimulated by salts (potassium sulfate and sodium phosphates). The maximum rate of N2O-N production was 0.17 μg of N2O-N per g of soil per h. When humus was treated with NO2−, N2O evolved immediately, indicating chemical formation, but no N2O was formed on the addition of NO3−. The amount of N2O-N evolved was 0.6 to 4.6% of NO2−-N added. A high concentration of NO2− and low soil pH enhanced chemical production of N2O. There was no accumulation of NO2− during nitrification. The calculations indicated that chemical formation of N2O was not the main source of N2O during NH4+ oxidation. After the addition of inhibitors of NH4+ oxidation the soils contained NO3−, but no N2O was produced. The results suggest that enhanced autotrophic NH4+ oxidation is a potential source of N2O in fertilized acid forest soil.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H. The metabolism of hydroxylamine to nitrite by Nitrosomonas. Biochem J. 1964 Apr;91(1):8–17. doi: 10.1042/bj0910008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer A. M., Bremner J. M., Schmidt E. L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980 Dec;40(6):1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward E., Whitwell H., Paul K. S., Barnes D. M. Steroid receptors in human meningioma. Clin Neuropharmacol. 1984;7(4):351–356. doi: 10.1097/00002826-198412000-00014. [DOI] [PubMed] [Google Scholar]
- Henniger N. M., Bollag J. M. Effect of chemicals used as nitrification inhibitors on the denitrification process. Can J Microbiol. 1976 May;22(5):668–672. doi: 10.1139/m76-098. [DOI] [PubMed] [Google Scholar]
- Poth M., Focht D. D. N Kinetic Analysis of N(2)O Production by Nitrosomonas europaea: an Examination of Nitrifier Denitrification. Appl Environ Microbiol. 1985 May;49(5):1134–1141. doi: 10.1128/aem.49.5.1134-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Nicholas D. J. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972 Mar;126(5):1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]


