Abstract
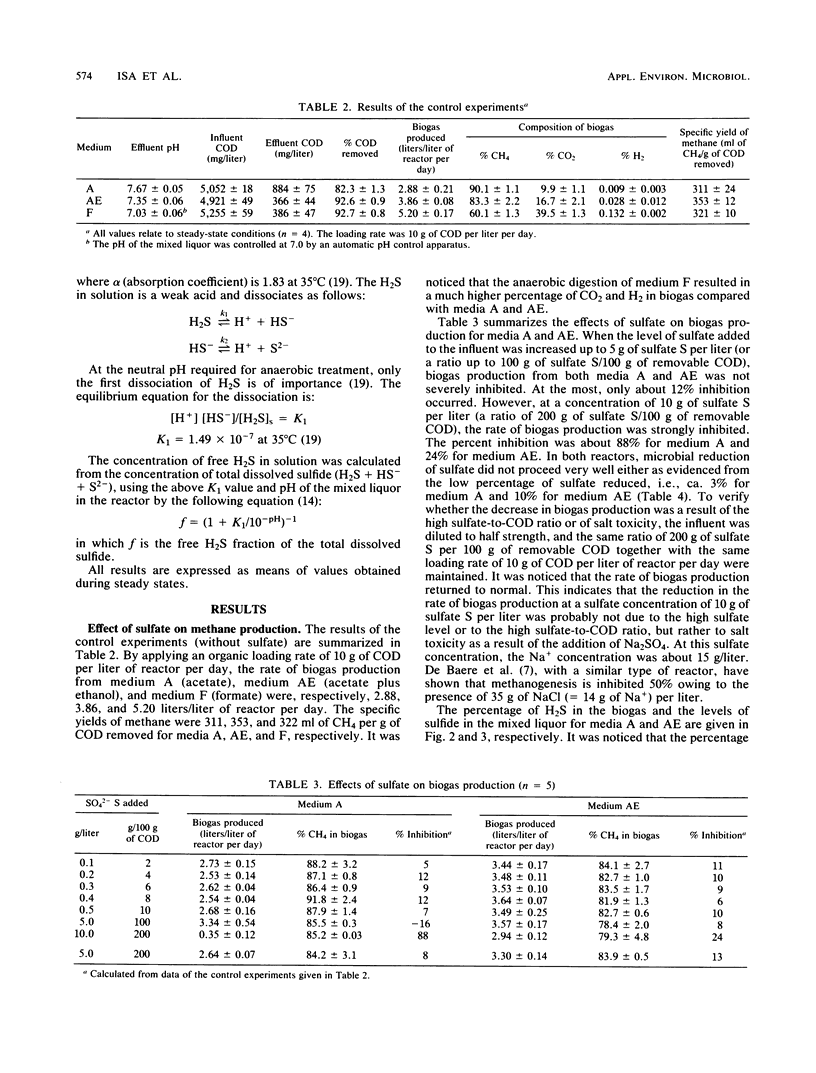
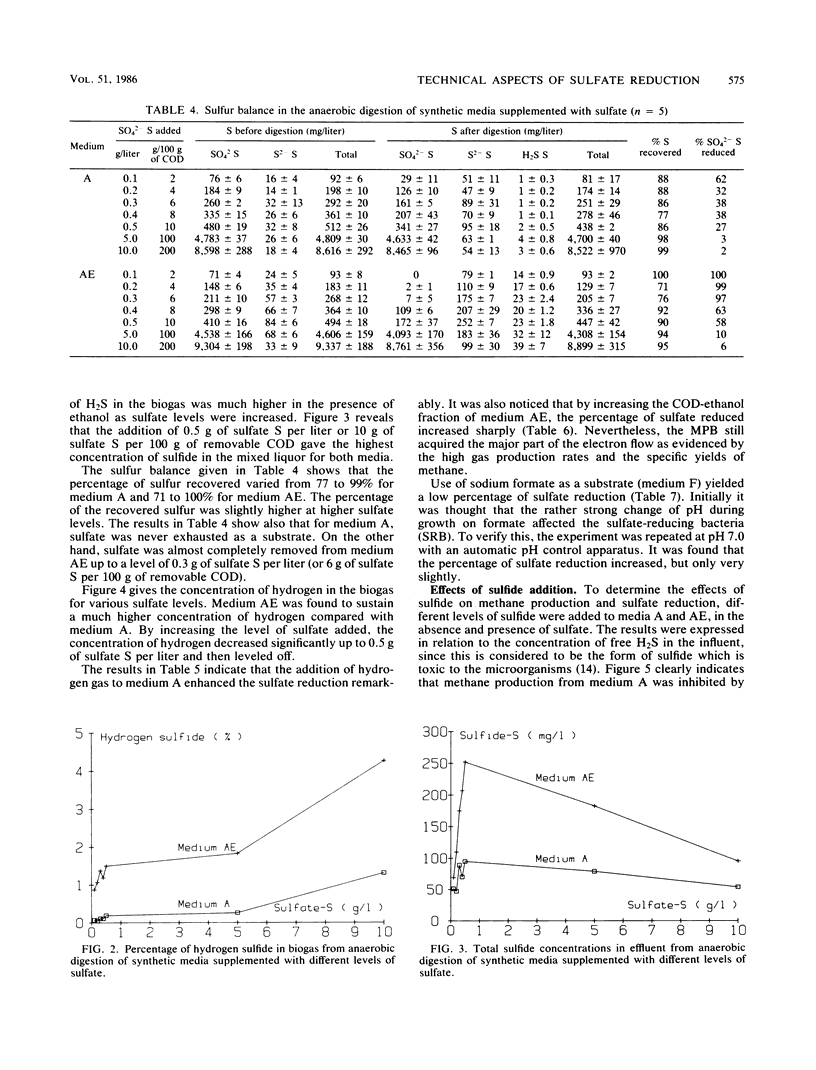
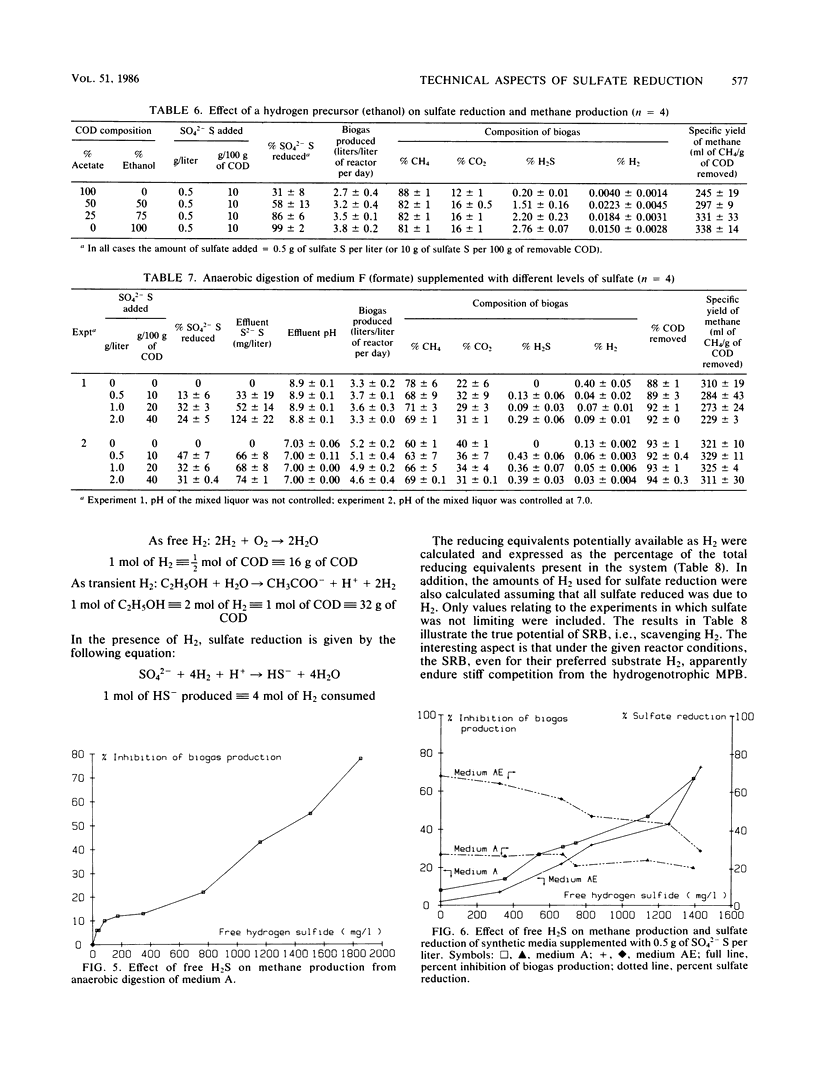
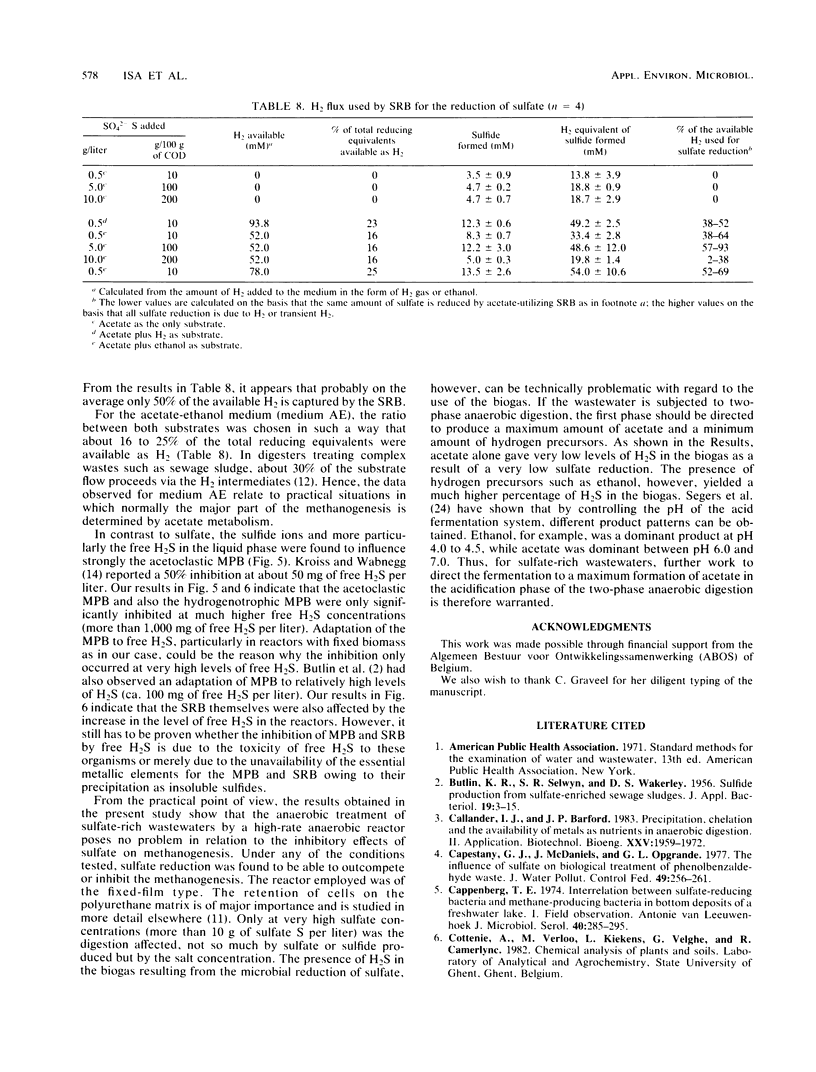
The effect of different substrates and different levels of sulfate and sulfide on methane production relative to sulfate reduction in high-rate anaerobic digestion was evaluated. Reactors could be acclimated so that sulfate up to a concentration of 5 g of sulfate S per liter did not significantly affect methanogenesis. Higher levels gave inhibition because of salt toxicity. Sulfate reduction was optimal at a relatively low level of sulfate, i.e., 0.5 g of sulfate S per liter, but was also not significantly affected by higher levels. Both acetoclastic and hydrogenotrophic methane-producing bacteria adapted to much higher levels of free H2S than the values reported in the literature (50% inhibition occurred only at free H2S levels of more than 1,000 mg/liter). High levels of free H2S affected the sulfate-reducing bacteria only slightly. Formate and acetate supported the sulfate-reducing bacteria very poorly. In the high-rate reactors studied, intensive H2S formation occurred only when H2 gas or an H2 precursor such as ethanol was supplied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Isa Z., Grusenmeyer S., Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: microbiological aspects. Appl Environ Microbiol. 1986 Mar;51(3):580–587. doi: 10.1128/aem.51.3.580-587.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. W., Trottier T. M. Effect of sulfur-containing compounds on anaerobic degradation of cellulose to methane by mixed cultures obtained from sewage sludge. Appl Environ Microbiol. 1978 Jun;35(6):1027–1034. doi: 10.1128/aem.35.6.1027-1034.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnow P. H., Gunnarsson L. A. Sulfide-dependent methane production and growth of a thermophilic methanogenic bacterium. Appl Environ Microbiol. 1981 Oct;42(4):580–584. doi: 10.1128/aem.42.4.580-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K. Dissimilatory sulphate reduction with acetate as electron donor. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):467–471. doi: 10.1098/rstb.1982.0092. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


