Abstract
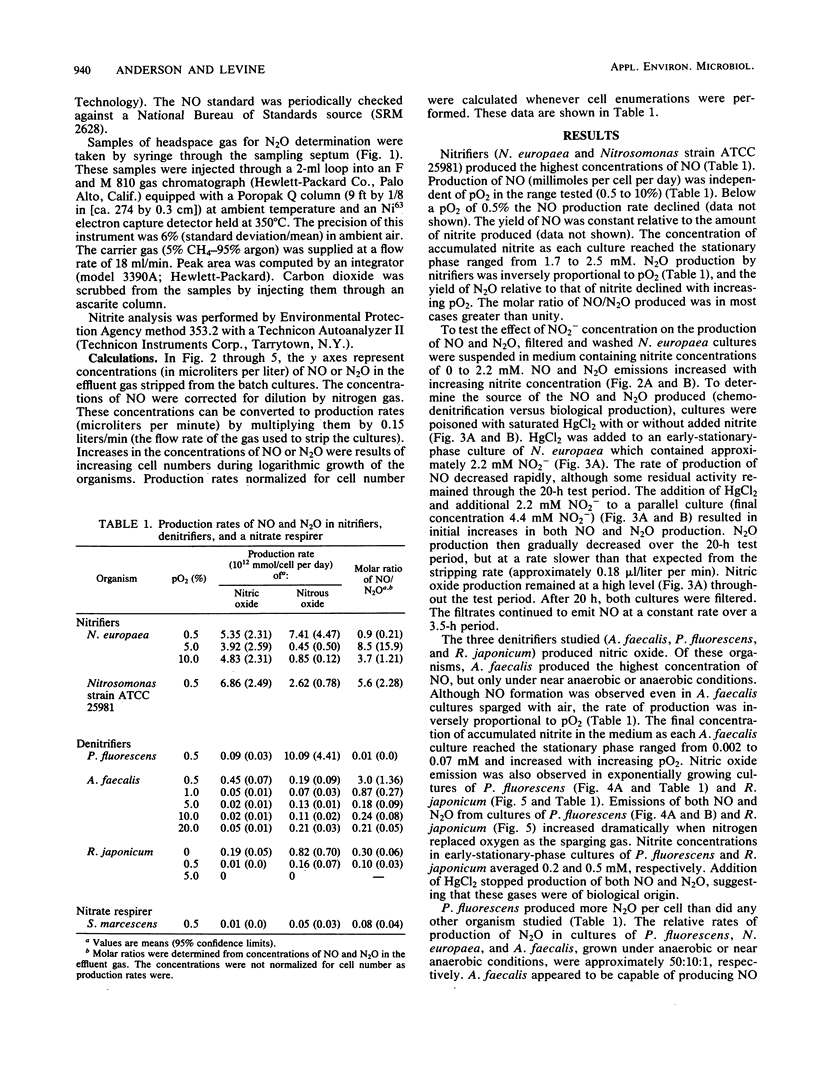
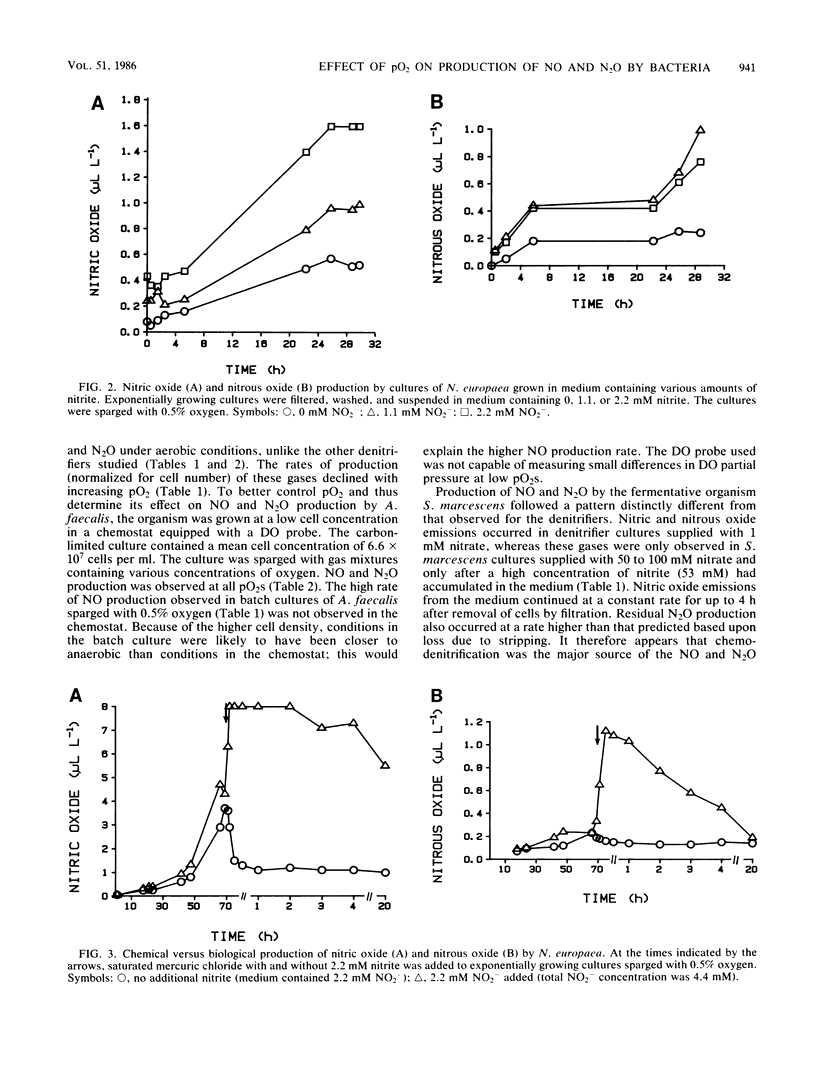
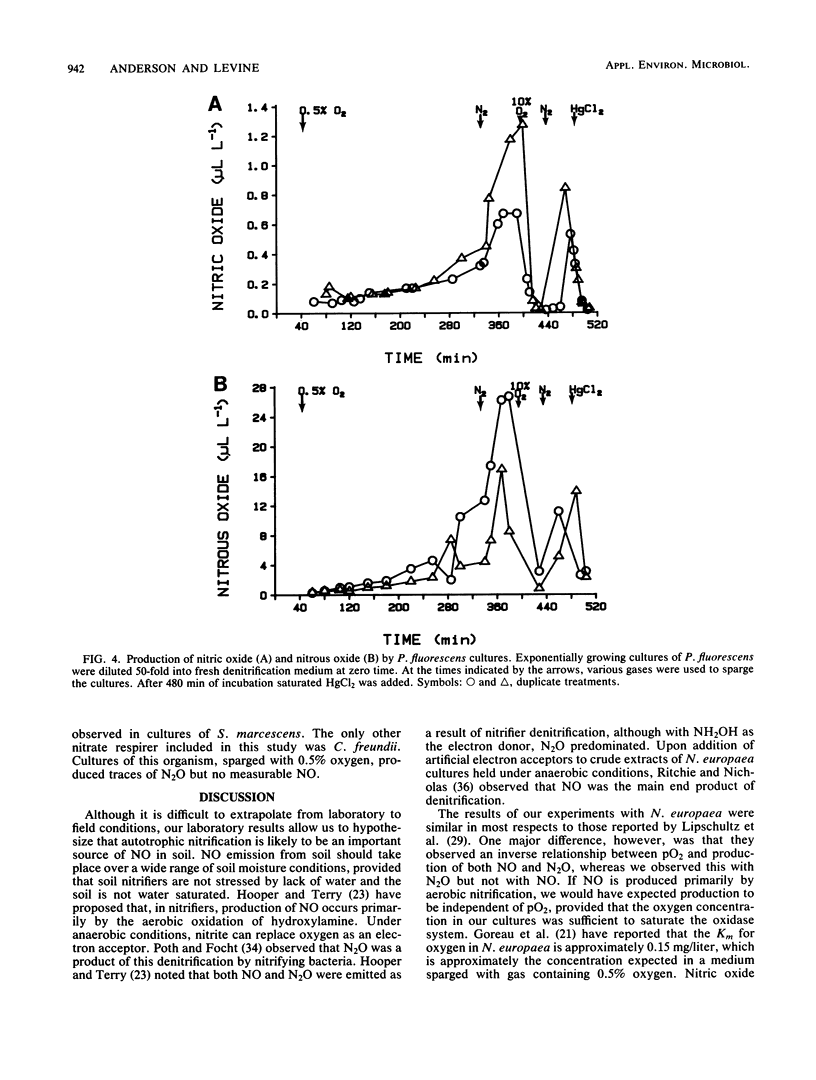
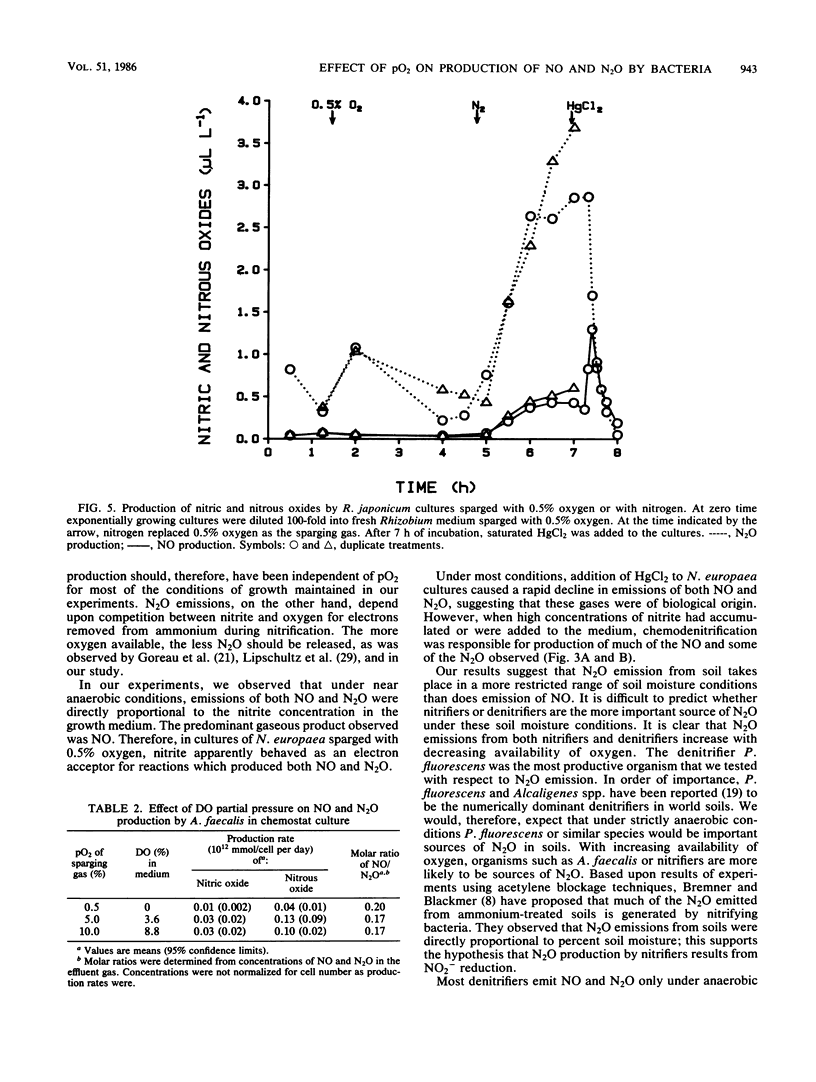
Biogenic emissions of nitric and nitrous oxides have important impacts on the photochemistry and chemistry of the atmosphere. Although biogenic production appears to be the overwhelming source of N2O, the magnitude of the biogenic emission of NO is very uncertain. In soils, possible sources of NO and N2O include nitrification by autotrophic and heterotrophic nitrifiers, denitrification by nitrifiers and denitrifiers, nitrate respiration by fermenters, and chemodenitrification. The availability of oxygen determines to a large extent the relative activities of these various groups of organisms. To better understand this influence, we investigated the effect of the partial pressure of oxygen (pO2) on the production of NO and N2O by a wide variety of common soil nitrifying, denitrifying, and nitrate-respiring bacteria under laboratory conditions. The production of NO per cell was highest by autotrophic nitrifiers and was independent of pO2 in the range tested (0.5 to 10%), whereas N2O production was inversely proportional to pO2. Nitrous oxide production was highest in the denitrifier Pseudomonas fluorescens, but only under anaerobic conditions. The molar ratio of NO/N2O produced was usually greater than unity for nitrifiers and much less than unity for denitrifiers. Chemodenitrification was the major source of both the NO and N2O produced by the nitrate respirer Serratia marcescens. Chemodenitrification was also a possible source of NO and N2O in nitrifier cultures but only when high concentrations of nitrite had accumulated or were added to the medium. Although most of the denitrifiers produced NO and N2O only under anaerobic conditions, chemostat cultures of Alcaligenes faecalis continued to emit these gases even when the cultures were sparged with air. Based upon these results, we predict that aerobic soils are primary sources of NO and that N2O is produced only when there is sufficient soil moisture to provide the anaerobic microsites necessary for denitrification by either denitrifiers or nitrifiers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M. W., Kator H. I. Seasonal variation in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983 Jun;45(6):1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill B. A., Tiedje J. M. The chemical mechanism of microbial denitrification. FEBS Lett. 1982 Feb 8;138(1):8–12. doi: 10.1016/0014-5793(82)80383-9. [DOI] [PubMed] [Google Scholar]
- BAALSRUD K., BAALSRUD K. S. Studies on Thiobacillus denitrificans. Arch Mikrobiol. 1954;20(1):34–62. doi: 10.1007/BF00412265. [DOI] [PubMed] [Google Scholar]
- Betlach M. R., Tiedje J. M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981 Dec;42(6):1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakley B. H., Tiedje J. M. Nitrous oxide production by organisms other than nitrifiers or denitrifiers. Appl Environ Microbiol. 1982 Dec;44(6):1342–1348. doi: 10.1128/aem.44.6.1342-1348.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castignetti D., Hollocher T. C. Nitrogen Redox Metabolism of a Heterotrophic, Nitrifying-Denitrifying Alcaligenes sp. from Soil. Appl Environ Microbiol. 1982 Oct;44(4):923–928. doi: 10.1128/aem.44.4.923-928.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitric oxide as an intermediate in denitrification: evidence from nitrogen-13 isotope exchange. Biochem Biophys Res Commun. 1979 Nov 14;91(1):10–16. doi: 10.1016/0006-291x(79)90575-8. [DOI] [PubMed] [Google Scholar]
- Gamble T. N., Betlach M. R., Tiedje J. M. Numerically dominant denitrifying bacteria from world soils. Appl Environ Microbiol. 1977 Apr;33(4):926–939. doi: 10.1128/aem.33.4.926-939.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Hollocher T. C. 15N tracer studies on the role of NO in denitrification. J Biol Chem. 1981 Jun 10;256(11):5459–5465. [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Hydroxylamine oxidoreductase of Nitrosomonas. Production of nitric oxide from hydroxylamine. Biochim Biophys Acta. 1979 Nov 9;571(1):12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- Hutchinson G. L., Mosier A. R. Nitrous oxide emissions from an irrigated cornfield. Science. 1979 Sep 14;205(4411):1125–1127. doi: 10.1126/science.205.4411.1125. [DOI] [PubMed] [Google Scholar]
- Levine J. S., Augustsson T. R., Anderson I. C., Hoell J. M., Jr Tropospheric sources of NOx: lightning and biology. Atmos Environ. 1984;18(9):1797–1804. doi: 10.1016/0004-6981(84)90355-x. [DOI] [PubMed] [Google Scholar]
- NAJJAR V. A., ALLEN M. B. Formation of nitrogen, nitrous oxide, and nitric oxide by extracts of denitrifying bacteria. J Biol Chem. 1954 Jan;206(1):209–214. [PubMed] [Google Scholar]
- Poth M., Focht D. D. N Kinetic Analysis of N(2)O Production by Nitrosomonas europaea: an Examination of Nitrifier Denitrification. Appl Environ Microbiol. 1985 May;49(5):1134–1141. doi: 10.1128/aem.49.5.1134-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E. D., Becker G. E. Production of nitric oxide and nitrous oxide during denitrification by Corynebacterium nephridii. J Bacteriol. 1970 Mar;101(3):821–826. doi: 10.1128/jb.101.3.821-826.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Nicholas D. J. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972 Mar;126(5):1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S. Dissimilatory Reduction of NO(2) to NH(4) and N(2)O by a Soil Citrobacter sp. Appl Environ Microbiol. 1982 Apr;43(4):854–860. doi: 10.1128/aem.43.4.854-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


