Abstract
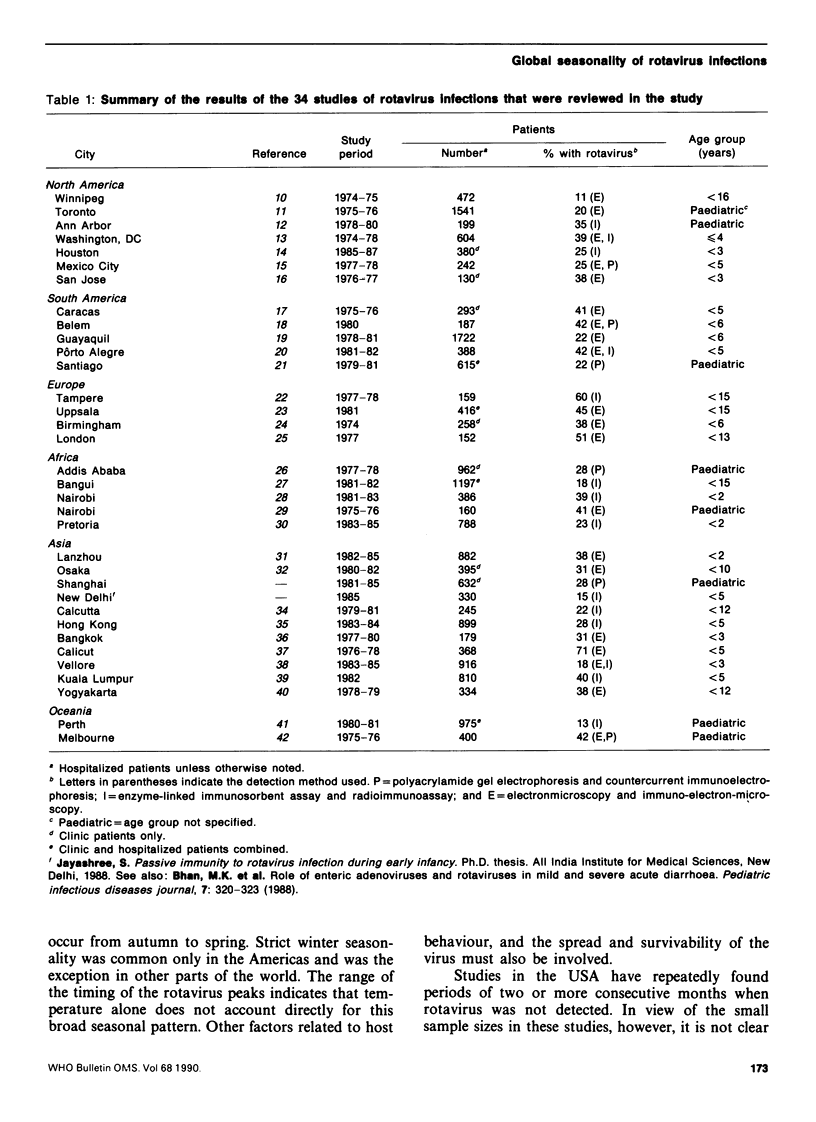
Data from 34 studies of the etiology of childhood diarrhoea were compiled in order to investigate the seasonal patterns of rotavirus gastroenteritis and consider their implications for transmission of the virus. Rotavirus was detected in 11-71% of children with diarrhoea, and the median rate of detection (33%) was independent of the level of economic development or geographical region of the study area, as well as of the method of detection used. While rotavirus infections have been called a winter disease in the temperate zones, we found that their incidence peaked in winter primarily in the Americas and that peaks in the autumn or spring are common in other parts of the world. In the tropics, the seasonality of such infections is less distinct and within 10 degrees latitude (north or south) of the equator, eight of the ten locations exhibited no seasonal trend. Throughout most of the world, rotavirus is present all the year round, which suggests that low-level transmission could maintain the chain of infection. The virus is spread by the faecal-oral route but airborne or droplet transmission has also been postulated. The epidemiology of rotavirus--its seasonality in the cooler months, its universal spread in temperate and tropical zones in developed and less developed settings--more closely resembles that of childhood viruses that are spread by the respiratory route (such as measles) than that of common enteric pathogens that are spread predominantly by the faecal-oral route.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan M. K., Raj P., Bhandari N., Svensson L., Stintzing G., Prasad A. K., Jayashree S., Srivastava R. Role of enteric adenoviruses and rotaviruses in mild and severe acute enteritis. Pediatr Infect Dis J. 1988 May;7(5):320–323. doi: 10.1097/00006454-198805000-00005. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974 Feb 2;1(7849):149–151. doi: 10.1016/s0140-6736(74)92440-4. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Parrott R. H. Rotavirus gastroenteritis and weather. J Clin Microbiol. 1982 Sep;16(3):478–482. doi: 10.1128/jcm.16.3.478-482.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Bryden A. S., Davies H. A., Hadley R. E., Flewett T. H. Rotavirus enteritis in the West Midlands during 1974. Lancet. 1975 Aug 9;2(7928):241–243. doi: 10.1016/S0140-6736(75)90959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke V., Gracey M., Robinson J., Peck D., Beaman J., Bundell C. The microbiology of childhood gastroenteritis: Aeromonas species and other infective agents. J Infect Dis. 1983 Jul;148(1):68–74. doi: 10.1093/infdis/148.1.68. [DOI] [PubMed] [Google Scholar]
- Carr M. E., McKendrick G. D., Spyridakis T. The clinical features of infantile gastroenteritis due to rotavirus. Scand J Infect Dis. 1976;8(4):241–243. doi: 10.3109/inf.1976.8.issue-4.04. [DOI] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Measles in England and Wales--I: An analysis of factors underlying seasonal patterns. Int J Epidemiol. 1982 Mar;11(1):5–14. doi: 10.1093/ije/11.1.5. [DOI] [PubMed] [Google Scholar]
- Georges M. C., Wachsmuth I. K., Meunier D. M., Nebout N., Didier F., Siopathis M. R., Georges A. J. Parasitic, bacterial, and viral enteric pathogens associated with diarrhea in the Central African Republic. J Clin Microbiol. 1984 May;19(5):571–575. doi: 10.1128/jcm.19.5.571-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Ingraham H. S., Korns R. F. TRANSMISSION OF EPIDEMIC GASTROENTERITIS TO HUMAN VOLUNTEERS BY ORAL ADMINISTRATION OF FECAL FILTRATES. J Exp Med. 1947 Oct 31;86(5):409–422. doi: 10.1084/jem.86.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M. J., Williams T. W. Gastroenteritis in children: a two-year review in Manitoba. I. Etiology. J Infect Dis. 1977 Aug;136(2):239–247. doi: 10.1093/infdis/136.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. S., Glass R. I., Pinsky P. F., Anderson L. J. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis. 1988 Nov;158(5):1112–1116. doi: 10.1093/infdis/158.5.1112. [DOI] [PubMed] [Google Scholar]
- Jayavasu C., Hooniwat Y., Sagaunwong S., Jayavasu J., Chatiyanonda K. A long term study of rotavirus infection in Thai infants and children with diarrhoea. Southeast Asian J Trop Med Public Health. 1982 Sep;13(3):373–376. [PubMed] [Google Scholar]
- KRAFT L. M. Studies on the etiology and transmission of epidemic diarrhea of infant mice. J Exp Med. 1957 Nov 1;106(5):743–755. doi: 10.1084/jem.106.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno T., Suzuki H., Katsushima N., Imai A., Tazawa F., Kutsuzawa T., Kitaoka S., Sakamoto M., Yazaki N., Ishida N. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis. 1983 Jan;147(1):125–128. doi: 10.1093/infdis/147.1.125. [DOI] [PubMed] [Google Scholar]
- Koopman J. S., Turkish V. J., Monto A. S., Gouvea V., Srivastava S., Isaacson R. E. Patterns and etiology of diarrhea in three clinical settings. Am J Epidemiol. 1984 Jan;119(1):114–123. doi: 10.1093/oxfordjournals.aje.a113712. [DOI] [PubMed] [Google Scholar]
- Lewis H. M., Parry J. V., Davies H. A., Parry R. P., Mott A., Dourmashkin R. R., Sanderson P. J., Tyrrell D. A., Valman H. B. A year's experience of the rotavirus syndrome and its association with respiratory illness. Arch Dis Child. 1979 May;54(5):339–346. doi: 10.1136/adc.54.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke E., Blomberg J., Berg G., Eriksson A., Madsen L. Epidemic acute diarrhoea in adults associated with infantile gastroenteritis virus. Lancet. 1978 Nov 11;2(8098):1056–1057. doi: 10.1016/s0140-6736(78)92389-9. [DOI] [PubMed] [Google Scholar]
- Mitchell C. D., Balfour H. H., Jr Measles control: so near and yet so far. Prog Med Virol. 1985;31:1–42. [PubMed] [Google Scholar]
- Moe K., Shirley J. A. The effects of relative humidity and temperature on the survival of human rotavirus in faeces. Arch Virol. 1982;72(3):179–186. doi: 10.1007/BF01348963. [DOI] [PubMed] [Google Scholar]
- Mutanda L. N. Epidemiology of acute gastroenteritis in early childhood in Kenya. III. Distribution of the aetiological agents. East Afr Med J. 1980 May;57(5):317–326. [PubMed] [Google Scholar]
- Mutanda L. N., Kinoti S. N., Gemert W., Lichenga E. O. Age distribution and seasonal pattern of rotavirus infection in children in Kenya. J Diarrhoeal Dis Res. 1984 Sep;2(3):147–150. [PubMed] [Google Scholar]
- Paniker C. K., Mathew S., Mathan M. Rotavirus and acute diarrhoeal disease in children in a southern Indian coastal town. Bull World Health Organ. 1982;60(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Saha M. R., Sen D., Datta P., Datta D., Pal S. C. Role of rotavirus as the cause of acute paediatric diarrhoea in Calcutta. Trans R Soc Trop Med Hyg. 1984;78(6):818–820. doi: 10.1016/0035-9203(84)90031-2. [DOI] [PubMed] [Google Scholar]
- Spencer E., Avendaño F., Araya M. Characteristics and analysis of electropherotypes of human rotavirus isolated in Chile. J Infect Dis. 1983 Jul;148(1):41–48. doi: 10.1093/infdis/148.1.41. [DOI] [PubMed] [Google Scholar]
- Stintzing G., Bäck E., Tufvesson B., Johnsson T., Wadström T., Habte D. Seasonal fluctuations in the occurrence of enterotoxigenic bacteria and rotavirus in paediatric diarrhoea in Addis Ababa. Bull World Health Organ. 1981;59(1):67–73. [PMC free article] [PubMed] [Google Scholar]
- Sutmoller F., Azeredo R. S., Lacerda M. D., Barth O. M., Pereira H. G., Hoffer E., Schatzmayr H. G. An outbreak of gastroenteritis caused by both rotavirus and Shigella sonnei in a private school in Rio de Janeiro. J Hyg (Lond) 1982 Apr;88(2):285–293. doi: 10.1017/s0022172400070145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. S., Kum W. W., Lam B., Yeung C. Y., Ng M. H. Molecular epidemiology of human rotavirus infection in children in Hong Kong. J Clin Microbiol. 1986 Mar;23(3):660–664. doi: 10.1128/jcm.23.3.660-664.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres B. V., Ilja R. M., Esparaza J. Epidemiological aspects of rotavirus infection in hospitalized Venezuelan children with gastroenteritis. Am J Trop Med Hyg. 1978 May;27(3):567–572. doi: 10.4269/ajtmh.1978.27.567. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Olding-Stenkvist E., Ekwall E., Mölby R. Aetiology and epidemiology of acute gastro-enteritis in Swedish children. J Infect. 1986 Jul;13(1):73–89. doi: 10.1016/s0163-4453(86)92348-0. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Sabil D., Muthu P. A. Human rotavirus infection in Malaysia. III. A one year survey on the prevalence of rotavirus enteritis in children. Southeast Asian J Trop Med Public Health. 1983 Dec;14(4):467–469. [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]


