Abstract
In a previous study, we showed that the DNA molecule within a spherical virus (the minute virus of mice) plays an architectural role by anisotropically increasing the mechanical stiffness of the virus. A finite element model predicted that this mechanical reinforcement is a consequence of the interaction between crystallographically visible, short DNA patches and the inner capsid wall. We have now tested this model by using protein engineering. Selected amino acid side chains have been truncated to specifically remove major interactions between the capsid and the visible DNA patches, and the effect of the mutations on the stiffness of virus particles has been measured using atomic force microscopy. The mutations do not affect the stiffness of the empty capsid; however, they significantly reduce the difference in stiffness between the DNA-filled virion and the empty capsid. The results (i) reveal that intermolecular interactions between individual chemical groups contribute to the mechanical properties of a supramolecular assembly and (ii) identify specific protein–DNA interactions as the origin of the anisotropic increase in the rigidity of a virus. This study also demonstrates that it is possible to control the mechanical properties of a protein nanoparticle by the rational application of protein engineering based on a mechanical model.
Keywords: atomic force microscopy, nanomechanics, protein–DNA interactions
Viruses are extremely successful biological entities with an ubiquitous presence in the biosphere (1). The extracellular form of any virus (the virion) is a self-assembled nucleoprotein complex, with or without a lipid envelope. These supramolecular assemblies have evolved the ability to withstand the physicochemical aggressions they may encounter during organism-to-organism propagation (2). Some viruses can resist extremes of temperature, pH, radiation, or dehydration (3, 4). In addition, recent results indicate that virions may be also subjected to substantial mechanical stress (5–13). Investigation of the structural determinants that allow virus particles to deal with physicochemical extremes may be important for a better understanding of viruses as evolving biological machines and may benefit nanobiotechnological applications, such as the development of robust nanocontainers (14).
Very recently, the mechanical properties of virus particles have begun to be experimentally investigated. The protein shells, or capsids, of Φ29 and λ bacteriophages have been shown to withstand a very high internal pressure exerted by the encapsidated DNA (5, 9, 13). The stiffness/elasticity of a few viruses have been analyzed by atomic force microscopy (AFM), in experiments that involve the application of indentation forces. Nonenveloped virus particles, including those of phages Φ29 (15) and λ (13), cowpea chlorotic mottle virus (CCMV) (16), and the minute virus of mice [MVM, a single-stranded DNA (ssDNA) virus] (17), are mechanically robust, while possessing remarkable elastic properties. Interestingly, these properties can be biologically modulated. In two enveloped retroviruses (Moloney murine leukemia virus and HIV), the immature virion is relatively stiff, whereas the mature, infectious virion is considerably softer (18, 19).
Some molecular determinants of those mechanical properties have already been identified. The different stiffness of the immature and mature HIV virions is mediated by the cytoplasmic domain of the gp120 envelope protein (19). A variant, salt-stable CCMV virion whose capsid differed in a single amino acid residue per subunit was also stiffer (16). Comparison of the mechanical properties of the nucleic acid-filled virion of MVM with those of the empty capsid (devoid of DNA) showed that the enclosed nucleic acid molecule can play a structural role by increasing the stiffness of the particle (17). This mechanical reinforcement was anisotropic [i.e., higher when the force was applied along a capsid 2-fold (S2) symmetry axis, lower along a 3-fold (S3) axis, and insignificant along a 5-fold (S5) axis]. A nucleic acid-mediated overall increase in stiffness has also been also detected for phage λ (13) and CCMV (16).
The MVM capsid is formed by 60 structurally equivalent protein subunits arranged in a simple (T = 1) icosahedral symmetry (20–22). In the crystal structures of many icosahedral viruses, all, or a large part, of the nucleic acid is invisible because it is oriented randomly within the particles in the crystal. However, in MVM (as in other viruses; see refs. 23–28), some segments of the viral nucleic acid molecule adopt very similar conformations at symmetrically equivalent positions inside the capsid and have been crystallographically visualized. Each of these DNA patches is noncovalently bound to several amino acid residues located at one of 60 equivalent sites on the inner capsid wall (refs. 21 and 22, and see Fig. 1). Use of a finite element modeling approach allowed us to predict that the observed DNA-mediated mechanical reinforcement of MVM may be specifically attributed to those capsid-bound DNA patches (17). However, this approach was necessarily based on highly simplifying assumptions. The adequateness of any theoretical approach to predict whether, and how, the mechanical properties of a virus or supramolecular assembly are modified when intermolecular interactions are introduced or removed remained to be experimentally validated.
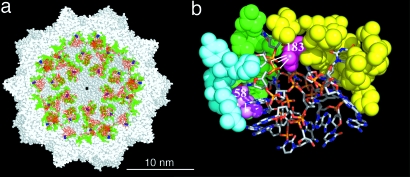
Fig. 1.
DNA–capsid interfaces in the MVM virion. (a) Cutaway section a crystallographic model of the MVM virion (22). The visible DNA stretches (red) are bound to amino acid residues (green, except Asn-183, blue) at equivalent sites on the inner capsid wall. The rest of the DNA molecule and the internal N-terminal segment of each capsid protein subunit are crystallographically invisible. For orientation, one of the capsid S5 axes is located at the center (black spot) of the model. (b) Close-up view of one of the equivalent DNA–capsid interfaces. The two ssDNA stretches that form each capsid-bound patch are shown as sticks models. The capsid residues that establish interactions with the DNA (except Asp-58 and Asn-183) are shown as cyan, green, or yellow spacefilling models, according to the subunit involved. The two amino acid residues we mutated (Asp-58 and Asn-183) are shown in violet and magenta, respectively, with the hydrogen bonds they establish with the DNA represented as white lines.
The present study provides experimental proof that, in the MVM virion, the DNA exerts its mechanical reinforcement effect mainly through interactions between the capsid-bound DNA patches and specific amino acid residues. It also provides proof of principle that the mechanical properties of a supramolecular biological assembly can be rationally modified by using protein engineering.
Results
In the refined crystallographic model of MVM (22), the visible ssDNA at each equivalent site includes two short stretches (of 11 and 8 nucleotides). This DNA patch adopts a wedge-like shape that penetrates a concavity of the inner capsid wall and establishes noncovalent interactions with several amino acid residues (refs. 21, 22, 29; and see Fig. 1). To determine whether these specific interactions are responsible for the DNA-mediated mechanical reinforcement of the virion, we have analyzed by AFM the effect of mutation of capsid residues on the mechanical stiffness of MVM particles. Two single mutations—Asn183Ala and Asp58Ala—were aimed at removing some of the major interactions between the capsid and the bound DNA patches, without compromising intracapsid interactions. Mutation to Ala involved simply the removal of either the amide group of Asn-183 or the carboxylate group of Asp-58 and was chosen to disrupt native interactions without introducing new interactions and because it had a very low probability of altering the conformation of the polypeptide backbone (30, 31). In the refined model of the MVM virion (22), the amide of Asn-183 and the carboxylate of Asp-58, respectively, are involved in hydrogen bonds with the sugar–phosphate backbone and with a purine base of the longer capsid-bound DNA stretch (Fig. 1b); they also establish several van der Waals contacts with the DNA. Similar interactions were observed between the DNA and the structurally equivalent capsid residues Asn-180 and Asn-56 in the refined structure of the homologous canine parvovirus (29, 32, 33). This finding provided further support for the presence and functional relevance of these interactions. None of the atoms that were removed by either mutation were found to be involved in intracapsid interactions.
An infectious DNA clone of MVM carrying either mutation, and the nonmutated clone as a control, were used to transfect susceptible cells. The virus particles produced consisted of both empty capsids and DNA-filled, infectious virions, which were purified and separated from each other based on their different buoyant densities. Detectable contamination of empty capsids by infectious virions was excluded by titration; significant contamination of infectious virions by empty capsids was equally excluded by recentrifuging the virion preparation and verifying the absence of MVM particles with a density corresponding to that of empty capsids (data not shown). The virion-free empty-capsid preparations, and the empty-capsid-free virion preparations obtained after the second density gradient, were used in the experiments described below.
The nonmutated and mutant empty capsids and DNA-filled virions were visualized by transmission electron microscopy [see supporting information (SI) Figs. 6 and 7] and by AFM (Fig. 2). All of the particles were indistinguishable in dimensions, shape, and topography. AFM images of many individual particles clearly showed the expected topographic features, including the spikes located at the particle S3 axes and the cylindrical protrusions at the S5 axes. These features served to identify the specific orientation of virus particles that were positioned with a capsid S5, S3, or S2 axis on top (Fig. 2).
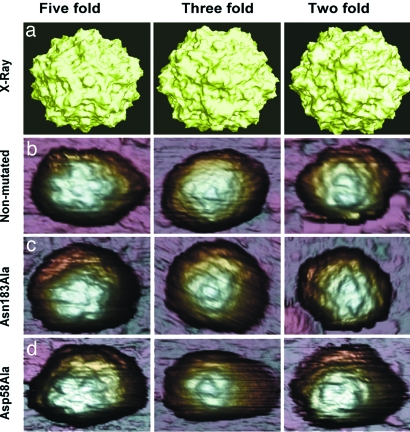
Fig. 2.
MVM virions imaged by AFM and viewed along an S5 (Left), S3 (Center), or S2 (Right) symmetry axis. (a) Molecular surface model of the nonmutated MVM virion derived from crystallographic data. (b–d) AFM images corresponding to nonmutated virion (b), Asn183Ala mutant (c), and Asp58Ala mutant (d). The MVM topography appears laterally expanded because of the usual tip-sample dilation effects. Only those particles with a symmetry axis at, or very close to, the top (center of the image) were selected for indentation. Most particles showed a symmetry axis clearly off-center, or no recognizable symmetry, and were discarded.
To quantitatively determine the mechanical stiffness of the modified MVM particles, we performed nanoindentations on individual intact particles in a physiological buffer, with the force applied along an S5, S3, or S2 symmetry axis (17). Fig. 3 shows examples of averaged indentation curves for MVM particles when probed along the three axis types, together with typical cantilever deflection curves. Examples for each virus particle type and symmetry axis are given as SI Figs. 8 and 9. The spring constants, k, obtained for each orientation were represented in a histogram, and a Gaussian fit of the histogram yielded the average spring constant of the particle when measured along that orientation (Fig. 4). Larger deviations of the k values were obtained for the virions relative to the empty capsids, especially when probed along an S2 axis (Fig. 4 and see SI Table 1). If the deviations were due solely to experimental error, those obtained by using empty capsids or virions should have been similar, at least when similar average k values were compared. Thus, the larger deviations probably reflect a broader Gaussian distribution of actual stiffness values. Likely causes are inaccuracies in the exact orientation of the viral particle being indented (the axis was not always exactly on top), as well as slight shifts in the orientations of both tip and particle after each indentation, which were clearly detected by imaging. Because the stiffness of the MVM virion (but not the empty capsid) is not isotropic (17), indentation at slightly different, off-axis positions could lead to small differences in the k value obtained in each indentation, even when the same individual particle was used. In addition, the intrinsic anisotropy of the single DNA molecule would make the 60 n-fold (n = 5, 3, or 2) axes not strictly equivalent in the virion (but not in the DNA-free capsid), potentially leading to slightly different k values, depending on the specific S2 (or S3, or S5) axis probed. Because the capsid-bound DNA segments are located closer to the S2 axes and farther from the S5 axes (Fig. 1a), this effect could be greater when the virions are probed along an S2 axis, as was actually observed.
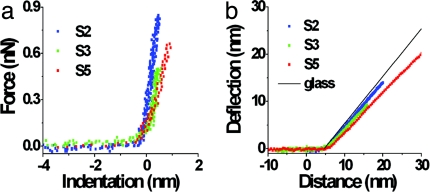
Fig. 3.
Indentation curves (a) and deflection curves (b) for the nonmutated virion probed along an S2, S3, or S5 axis. Each indentation graph is the average of five curves carried out with the same cantilever to probe particles along each type of symmetry axis in a same experimental session. Each deflection curve shown is a typical curve obtained in one of these five measurements. The thin line in b corresponds to the deflection curve when the force was applied on the glass surface.
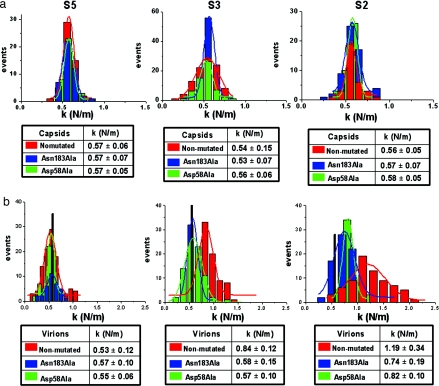
Fig. 4.
Comparison of the mechanical properties of nonmutated and mutant MVM particles. (a) Comparison of the mechanical properties of the nonmutated and mutant empty capsids of MVM. Histograms depict the stiffness (k value) obtained for individual empty capsids along different symmetry axes. Red, nonmutated; blue, Asn183Ala mutant; green, Asp58Ala mutant. The associated tables indicate the average k values and standard deviations obtained from Gaussian fits for indentations along an S5 (Left), S3 (Center), or S2 (Right) axis. (b) Comparison of the mechanical properties of nonmutated and mutant DNA-filled virions of MVM. The corresponding histograms and tables for the virions are depicted as in a. The vertical black bar in each histogram indicates the k value for the nonmutated empty capsid (to facilitate comparison between virions and empty capsids); the mutant empty capsids yielded k values that were indistinguishable from this value (compare a). A Student t test for samples having an unequal variance reveals, with >99% confidence, that the mean k values obtained for the nonmutated virions and for either mutant along the 2-fold or 3-fold axes do not overlap.
To validate a comparative analysis, it was important to ascertain to what approximation the quantitative value of k could be reproduced. The results from using nonmutated capsids and virions in independent experiments showed an excellent agreement in the average k values (see SI Table 1). This reproducibility, observed even in those cases where a broad Gaussian distribution occurred, experimentally validated any statistically significant difference that could be obtained when comparing the stiffness of different particles under the same experimental conditions.
We then compared the stiffness of the mutants vs. nonmutated virus particles. Any difference found in the stiffness of the mutant virions relative to the nonmutated virion could be due, in principle, to the removal of interactions between the truncated groups and other capsid residues and/or the bound DNA segments. The MVM empty capsid and virion are structurally almost indistinguishable, even at atomic resolution (22). Thus, if a mutation affects the stiffness of the virion because of the disruption of intracapsid interactions, it would equally affect the stiffness of the empty capsid. We carried out indentations on several individual intact empty capsids carrying no mutations, or including either the Asn183Ala or Asp58Ala mutations (Fig. 4a and see SI Table 2). Gaussian fits of the histograms yielded k values along the S5, S3, and S2 axes that were indistinguishable from each other. The k values were also indistinguishable when the two mutant empty capsids and the nonmutated empty capsid were compared (Fig. 4a). To confirm that the number of measurements carried out was sufficient to obtain good fitting values, in one arbitrary case (mutant Asp58Ala oriented along the 3-fold axis) much larger numbers of force-vs.-distance curves and individual particles were subsequently used in the calculation; no significant differences in the fitting k value, or in the distribution of individual measurements, were obtained. The same was observed for other particle or symmetry axis types when the number of particles indented and the total number of indentations was increased (data not shown). The above results indicate that any effect of these mutations on the stiffness of the MVM virion would be due to the removal of DNA–capsid interactions and not to removal of intracapsid interactions.
We then performed many indentations on several DNA-filled virions carrying no mutations or including either the Asn183Ala or Asp58Ala mutations (Fig. 4b and see SI Table 2). Remarkably, introduction of either mutation substantially reduced the increase in the k value that was observed for the nonmutated virion along the S3 axes and, to a greater extent, the S2 axes, relative to the empty capsid (Fig. 4b and Fig. 5). The presence of the DNA in the nonmutated particle led to increases of ≈60% and 110% when probed along S3 and S2 axes, respectively. Mutations Asn183Ala or Asp58Ala reduced those values to ≈30% and 40% or 30% and 30%, respectively. A Student t test revealed that the average k values obtained along the 2-fold axis for the mutant virions and the nonmutated virion are significantly different (with a 99% confidence). The reduced k values cannot be due to a change in the particle surface electrostatics because the mutations removed only neutral groups from internal residues of the capsid and did not affect any of the charged groups of the capsid or the DNA, which remained unmodified. We conclude that removal of major noncovalent interactions between specific amino acid residues located at the inner wall of the MVM capsid, and the capsid-bound DNA patches, led to a very substantial reduction in the DNA-mediated mechanical reinforcement of the virus particle.
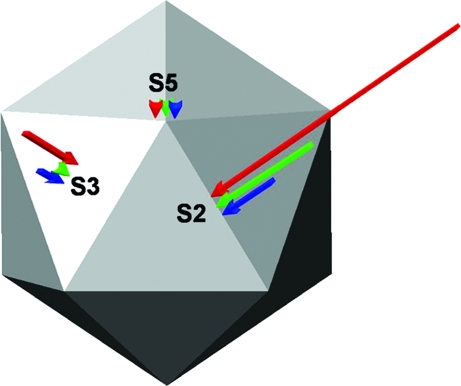
Fig. 5.
Schematic representation of the increase in stiffness of the DNA-filled virion, relative to the empty capsid, along the 5-fold (S5), 3-fold (S3), or 2-fold (S2) symmetry axes. Arrows refer to unmodified MVM (red), Asn183Ala mutant (blue), or Asp58Ala mutant (green). Arrow length is proportional to Δk, the difference in k value between the virion and the empty capsid. If Δk is not significantly different from zero according to a Student t test, an arrowhead is used.
Discussion
A finite element calculation allowed us to propose that the DNA-mediated mechanical reinforcement of the MVM virion could be specifically due to small DNA patches intimately bound to the inner capsid wall. These DNA patches would effectively increase the local thickness of the capsid wall, acting as molecular buttresses (17). The results of the present study experimentally confirm such prediction and show that specific noncovalent interactions holding the capsid and those DNA segments together are directly responsible for the observed reinforcement. In the Asn183Ala and Asp58Ala mutant virions, the absence of some of the major capsid–DNA interactions could cause those DNA segments to be more loosely bound to their recognition sites in the capsid wall, thus reducing their reinforcing (buttress) effect. In either mutant, only some of the major interactions between the capsid and each DNA patch were removed and, consequently, the reinforcing effect of the DNA was not expected to be completely prevented. However, the stiffness of the N183A virion was reduced to the point of making it statistically indistinguishable from that of the empty capsid; the stiffness of the D58A virion was also substantially reduced, although it was still significantly higher than that of the capsid, according to a Student t test (99% confidence). Loss of cooperative effects between different intermolecular interactions at each equivalent DNA–capsid interface may explain such a large mechanical action, in the same way that it explains equally drastic reductions in binding affinity caused by single-residue mutations in many protein–ligand interfaces. In addition, a single amino acid replacement will remove in the MVM virion equivalent sets of interactions between the only DNA molecule and up to 60 sites in the capsid wall. Cooperative effects due to the loss of interactions at so many sites may also amplify the effect of each mutation (34).
The physicochemical cause for a nucleic acid-mediated mechanical reinforcement may depend on the virus and nucleic acid involved. A recently developed analytical model indicated that the double-stranded DNA-mediated mechanical reinforcement of phage λ is due to the osmotic pressure generated by DNA-hydrating water molecules (13). The ssDNA inside MVM is also densely packed, and the presence of a substantial internal pressure in MVM is a definite, albeit unproven, possibility (17). However, when the interaction between the bound DNA segments and the MVM capsid was debilitated through mutation, the stiffness of the virion was reduced to a value similar to that of the empty capsid, even though the complete, unmodified DNA molecule was still inside the virus particle. Thus, the ssDNA inside MVM may not generate substantial internal pressure. Alternatively, if a DNA-mediated internal pressure exists in MVM, it may not significantly contribute to the mechanical stiffness of this virus.
It may be important to relate the observed mechanical effects to the biology of MVM. We had shown previously (29) that capsid residues involved in interactions with DNA patches, including N183 and D58 involved in the DNA-mediated stiffening effect, contribute to virion stability against thermal inactivation and to virus infectivity, which was reduced >10-fold by truncation of individual side chains interacting with the DNA. Also, each visible DNA patch binds the inner capsid wall by penetrating and filling a concavity and establishing multiple interactions with residues from three neighboring capsid subunits, contributing to cementing together of these subunits. Thus, one possibility is that the bound DNA serves to mechanically and thermically reinforce the capsid, allowing more extracellular virions to remain infectious until they reach their host cells. A nonexclusive possibility is that binding of the DNA patches could have a role in freezing the capsid in a stiffer, mechanically more stable conformation and prevent it from undergoing unproductive conformational changes during the infection cycle. A similar situation may occur in bean pod mottle virus (BPMV). Biophysical evidence has suggested that the BPMV empty capsid has a highly dynamic structure, as opposed to the ssRNA-filled virion, and the presence of nucleic acid in the BPMV virion was shown to stabilize the capsid against thermal denaturation (35, 36).
In the above scenario, the absence of a DNA-mediated increase in the stiffness of the MVM virion around the S5 axes could also be explained in biological terms. The pores located at the S5 axes are used for externalization of capsid polypeptide N-terminal segments and for entry and/or exit of the viral DNA. These events are critically necessary for infection (21, 22, 37–41) and depend on the occurrence of local conformational rearrangements modulated by some capsid residues lining the pores (39, 41–43), as well as by the size and shape of capsid cavities located nearby (44). Those conformational changes may require a certain mechanical flexibility in the regions around the pores, precisely the only large areas of the capsid where bound DNA is not observed (Fig. 1a). Thus, it is tempting to suggest the action on MVM of a selective pressure to keep the capsid regions that are closer to the 5-fold axis pores free from binding, stiffening DNA segments and thus flexible enough to allow those biologically critical translocation events. In short, MVM and other parvoviruses may have evolved a functionally compatible, multiple-site interaction between their DNA genomes and the inner capsid wall, leading to improved mechanical and thermal stability and contributing to the biological success of these viruses.
From a nanobiotechnological perspective, the present study demonstrates that the mechanical properties of a biomolecular complex can be rationally manipulated using a protein engineering approach. The predictions of a simple mechanical model led us to obtain, by site-directed mutagenesis, a modified virus particle that is mechanically softer than the natural virus. We propose that protein engineering may be a suitable approach to tailoring the mechanical properties of protein nanoparticles.
Materials and Methods
Recombinant Plasmids and Mutagenesis.
Site-directed mutagenesis of the VP1/VP2 gene of MVM (strain p) was carried out using the QuikChange system (Stratagene) on recombinant plasmid pSVtk-VP1/2 originally provided by J. M. Almendral (Centro de Biología Molecular, Madrid, Spain) (45). The mutations were introduced by subcloning in an MVMp infectious clone originally provided by P. Tattersall (Yale University Medical School, New Haven, CT) (46) and then modified (45). The presence of the mutations was confirmed by sequencing.
Electroporation of Mammalian Cells and Infectivity Assays.
NB324K cells were electroporated with the MVM infectious plasmid carrying the appropriate mutations (47). Virions were recovered from transfected monolayers and titrated in plaque assays.
Production and Purification of MVM Empty Capsids and Virions.
MVM empty capsids and virions were obtained as described in ref. 17, with some modifications. Briefly, MVM particles were produced by infection of NB324K cells at a low multiplicity of infection. After adsorption of the virus and incubation at 37°C, the cells were suspended in culture medium, plated at low density, and incubated at 37°C until complete cytopathic effect. The empty capsids and virions obtained from the remaining infected cells and those obtained from the supernatant of infection were mixed, supplemented with SDS to 0.5%, deposited on layers containing 20% sucrose in TE buffer (50 mM Tris·HCl pH 8.0, 0.5 mM EDTA) plus 0.1 M NaCl and centrifuged for 5.5 h at 35,000 rpm in an SW40 rotor (Beckman) at 10°C. The sediment was thoroughly resuspended in TE buffer containing 0.2% Sarkosyl, and the suspension was centrifuged in a cesium chloride gradient in the same buffer for 24 h at 50,000 rpm in a TFT 75.13 rotor (Kontron) at 10°C. The gradient was fractionated, and the aliquots were analyzed for the presence of empty capsids (buoyant density 1.363) or virions (density 1.373) by assaying their hemagglutination activity. The fractions of interest were extensively dialyzed against PBS (pH 7.2). To exclude any cross-contamination of virions and capsids, only the central fractions of well resolved peaks were used. Titration in plaque assays was used to ascertain the absence of virions in the empty-capsid preparation. To exclude empty capsids from the virion preparation, the latter was layered on a second cesium chloride gradient, recentrifuged as above, and extensively dialyzed again. The purity, integrity, and concentration of MVM particles were assessed by electron microscopy.
AFM of Viral Particles.
AFM experiments were carried out essentially as described in ref. 17. Briefly, one drop (20 μl) of diluted stock of purified empty capsids or virions in PBS was deposited on a sylanized glass surface. The drop was left on the surface for 30 min and then rinsed twice with 20 μl of PBS. The tip was also prewetted with 20 μl of PBS. The atomic force microscope (Nanotec Electrónica) was operated in jumping mode (48) in liquid. We used rectangular cantilevers (RC800PSA; Olympus) with spring constant of 0.05 ± 0.01 N/m. The maximum normal force during AFM imaging was always ≈100 pN. Dynamic mode could not be used because the softer cantilevers required for reproducible imaging using this mode would have led to increased errors in the k values. AFM images were processed by using WSxM software (49).
To determine the stiffness of empty capsids and virions once individual particles were located on the surface, the lateral piezo scan was stopped when the tip was on top of the equatorial area of the particle. Then, force-vs.-distance curves were obtained by elongating the z-piezo until the tip established mechanical contact with the virus particle, and a nanoindentation was performed. To avoid particle damage, the maximum applied force was limited to 0.9 nN, with typical indentations of ≈2 nm. We observed that, after a few contact events, the force-vs.-distance curve exhibited marked steps, which corresponded to an irreversible modification of the virus particle. In this case, we moved to another particle. The curves were processed assuming the cantilever and the virus to be two springs in series, to obtain the stiffness (spring constant, k) of the virus particle along the direction of the applied force (15). Each cantilever was calibrated as described in ref. 50 and as implemented online at www.ampc.ms.unimelb.edu.au/afm/theory.html#normal.
Molecular Graphics and Structural Analyses.
The refined PDB coordinates of the of MVMi virion (1Z1C) and of the MVMp empty capsid (1Z14) (22), as well as the software programs Insight II (Biosym Technologies), RasMol (51), WHAT IF (52), and PyMOL (DeLano Scientific), were used.
Supplementary Material
Acknowledgments.
We thank J. Gómez-Herrero, P. A.Serena, and I. A. T. Schaap for fruitful discussions and critical reading of the manuscript; P. Tattersall, J. M. Almendral, and J. Reguera (Centro Nacional de Biotecnología, Madrid, Spain) for providing MVM plasmids; and L. Riolobos and E. Grueso for technical advice. M.C. is a predoctoral fellow from Comunidad de Madrid (CM). This work was supported by CM Grants S-0505/MAT/0303 (to M.G.M. and P.J.P.), Ministerio de Educación y Ciencia Grant BIO2006-00793 (to M.G.M.), CM Grant GR/MAT/0254/2004 (to P.J.P.), and an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular. M.G.M. is an associate member of the Centro de Biocomputación y Física de los Sistemas Complejos, Zaragoza, Spain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708017105/DC1.
References
- 1.Suttle CA. Nature. 2005;437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 2.Chiu W, Burnett R, Garcea RL, editors. Structural Biology of Viruses. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 3.Prigent M, Leroy M, Confalonieri F, Dutertre M, DuBow MS. Extremophiles. 2005;9:289–296. doi: 10.1007/s00792-005-0444-5. [DOI] [PubMed] [Google Scholar]
- 4.Hernando E, Llamas-Saiz AL, Foces-Foces C, McKenna R, Portman L, Agbandje-McKenna M, Almendral JM. Virology. 2000;267:299–309. doi: 10.1006/viro.1999.0123. [DOI] [PubMed] [Google Scholar]
- 5.Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. Nature. 2001;413:748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 6.Kindt J, Tzlil S, Ben-Shaul A, Gelbart WM. Proc Natl Acad Sci USA. 2001;98:13671–13674. doi: 10.1073/pnas.241486298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purohit PK, Kondev J, Phillips R. Proc Natl Acad Sci USA. 2003;100:3173–3178. doi: 10.1073/pnas.0737893100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordova A, Deserno M, Gelbart WM, Ben-Shaul A. Biophys J. 2003;85:70–74. doi: 10.1016/S0006-3495(03)74455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evilevitch A, Lavelle L, Knobler CM, Raspaud E, Gelbart WM. Proc Natl Acad Sci USA. 2003;100:9292–9295. doi: 10.1073/pnas.1233721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zandi R, Reguera D, Bruinsma RF, Gelbart WM, Rudnick J. Proc Natl Acad Sci USA. 2004;101:15556–15560. doi: 10.1073/pnas.0405844101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zandi R, Reguera D. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72 doi: 10.1103/PhysRevE.72.021917. 021917. [DOI] [PubMed] [Google Scholar]
- 12.van der Schoot P, Bruinsma R. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71 doi: 10.1103/PhysRevE.71.061928. 061928. [DOI] [PubMed] [Google Scholar]
- 13.Ivanovska I, Wuite G, Jönsson B, Evilevitch A. Proc Natl Acad Sci USA. 2007;104:9603–9608. doi: 10.1073/pnas.0703166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas T, Young M. Science. 2006;312:873–875. doi: 10.1126/science.1123223. [DOI] [PubMed] [Google Scholar]
- 15.Ivanovska IL, de Pablo PJ, Ibarra B, Sgalari G, MacKintosh FC, Carrascosa JL, Schmidt CF, Wuite GJL. Proc Natl Acad Sci USA. 2004;101:7600–7605. doi: 10.1073/pnas.0308198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel JP, Ivanovska IL, Gibbons MM, Klug WS, Knobler CM, Wuite GJL, Schmidt CF. Proc Natl Acad Sci USA. 2006;103:6184–6189. doi: 10.1073/pnas.0601744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrasco C, Carreira A, Schaap IAT, Serena PA, Gómez-Herrero J, Mateu MG, de Pablo PJ. Proc Natl Acad Sci USA. 2006;103:13706–13711. doi: 10.1073/pnas.0601881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kol N, Gladnikoff M, Barlam D, Shneck RZ, Rein A, Rousso I. Biophys J. 2006;91:767–774. doi: 10.1529/biophysj.105.079657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kol N, Tsvitov M, Barlam D, Shneck RZ, Kay MS, Rousso I. Biophys J. 2007;92:1777–1783. doi: 10.1529/biophysj.106.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llamas-Saiz AL, Agbandje-McKenna M, Wikoff WR, Bratton J, Tattersall P, Rossmann MG. Acta Crystallogr D. 1997;53:93–102. doi: 10.1107/S0907444996010566. [DOI] [PubMed] [Google Scholar]
- 21.Agbandje-McKenna M, Llamas-Saiz AL, Wang F, Tattersall P, Rossmann MG. Structure. 1998;6:1369–1381. doi: 10.1016/s0969-2126(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 22.Kontou M, Govindsamy L, Nam HJ, Bryant N, Llamas-Saiz AL, Foces-Foces C, Hernando E, Rubio MP, McKenna R, Almendral JM, Agbandje-McKenna M. J Virol. 2005;79:10931–10943. doi: 10.1128/JVI.79.17.10931-10943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Stauffacher C, Li Y, Schmidt T, Bomu W, Kamer G, Shanks M, Lomonosoff G, Johnson JE. Science. 1989;245:154–159. doi: 10.1126/science.2749253. [DOI] [PubMed] [Google Scholar]
- 24.McKenna R, Xia D, Willingmann P, Ilag LL, Krishnaswamy S, Rossmann MG, Olson NH, Baker TS, Incardona NL. Nature. 1992;355:137–143. doi: 10.1038/355137a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher AJ, Johnson JE. Nature. 1993;361:176–179. doi: 10.1038/361176a0. [DOI] [PubMed] [Google Scholar]
- 26.Larson SB, Koszelak S, Day J, Greenwood A, Dodds JA, McPherson A. Nature. 1993;361:179–182. doi: 10.1038/361179a0. [DOI] [PubMed] [Google Scholar]
- 27.Tang L, Johnson KN, Ball A, Lin T, Yeager M, Johnson JE. Nat Struct Biol. 2001;8:77–83. doi: 10.1038/83089. [DOI] [PubMed] [Google Scholar]
- 28.Blink HHJ, Pleij CWA. Arch Virol. 2002;147:2261–2279. doi: 10.1007/s00705-002-0891-6. [DOI] [PubMed] [Google Scholar]
- 29.Reguera J, Grueso E, Carreira A, Sánchez-Martinez C, Almendral JM, Mateu MG. J Biol Chem. 2005;280:17969–17977. doi: 10.1074/jbc.M500867200. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham BC, Wells JA. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 31.Lau FT, Fersht AR. Biochemistry. 1989;28:6841–6847. doi: 10.1021/bi00443a010. [DOI] [PubMed] [Google Scholar]
- 32.Tsao J, Chapman MS, Agbandje M, Keller W, Smith K, Wu H, Luo M, Smith TJ, Rossmann MG, Compans RW, Parrish CR. Science. 1991;251:1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MS, Rossmann MG. Structure. 1995;3:151–162. doi: 10.1016/s0969-2126(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 34.Zlotnick A. J Mol Biol. 1994;241:59–67. doi: 10.1006/jmbi.1994.1473. [DOI] [PubMed] [Google Scholar]
- 35.Li T, Chen Z, Johnson JE, Thomas GJ., Jr Biochemistry. 1992;31:6673–6682. doi: 10.1021/bi00144a006. [DOI] [PubMed] [Google Scholar]
- 36.Da Poian AT, Johnson JE, Silva JL. J Biol Chem. 2002;277:47596–47602. doi: 10.1074/jbc.M209174200. [DOI] [PubMed] [Google Scholar]
- 37.Cotmore SF, D'Abramo AM, Ticknor CM, Tattersall P. Virology. 1999;254:169–181. doi: 10.1006/viro.1998.9520. [DOI] [PubMed] [Google Scholar]
- 38.Maroto B, Valle N, Saffrich R, Almendral JM. J Virol. 2004;78:10685–10694. doi: 10.1128/JVI.78.19.10685-10694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reguera J, Carreira A, Riolobos L, Almendral JM, Mateu MG. Proc Natl Acad Sci USA. 2004;101:2724–2729. doi: 10.1073/pnas.0307748101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valle N, Riolobos L, Almendral JM. In: Parvoviruses. Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR, editors. London: Arnold; 2005. pp. 291–304. [Google Scholar]
- 41.Farr GA, Cotmore SF, Tattersall P. J Virol. 2006;80:161–171. doi: 10.1128/JVI.80.1.161-171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carreira A, Menéndez M, Reguera J, Almendral JM, Mateu MG. J Biol Chem. 2004;279:6517–6525. doi: 10.1074/jbc.M307662200. [DOI] [PubMed] [Google Scholar]
- 43.Farr GA, Tattersall P. Virology. 2004;323:243–256. doi: 10.1016/j.virol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Carreira A, Mateu MG. J Mol Biol. 2006;360:1081–1093. doi: 10.1016/j.jmb.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Ramírez JC, Santarén JF, Almendral JM. Virology. 1995;206:57–68. doi: 10.1016/s0042-6822(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 46.Gardiner EM, Tattersall P. J Virol. 1988;62:2605–2613. doi: 10.1128/jvi.62.8.2605-2613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardo E, Ramírez JC, García J, Almendral JM. J Virol. 2002;76:7049–7059. doi: 10.1128/JVI.76.14.7049-7059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno-Herrero F, de Pablo PJ, Fernández-Sánchez R, Colchero J, Gómez-Herrero J, Baró AM. Appl Phys Lett. 2002;81:2620–2622. [Google Scholar]
- 49.Horcas I, Fernández R, Gómez-Rodriguez JM, Colchero J, Gómez-Herrero J, Baró AM. Rev Sci Instrum. 2007;78 doi: 10.1063/1.2432410. 013705–013708. [DOI] [PubMed] [Google Scholar]
- 50.Sader JE, Chon JWM, Mulvaney P. Rev Sci Instrum. 1999;70:3967–3969. [Google Scholar]
- 51.Sayle RA, Milner-White EJ. Trends Biochem Sci. 1995;20:374–376. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]
- 52.Vriend G. J Mol Graphics. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.