Abstract
Human immunodeficiency virus (HIV) type 1 infection requires functional interactions of the viral surface (gp120) glycoprotein with cell surface CD4 and a chemokine coreceptor (usually CCR5 or CXCR4) and of the viral transmembrane (gp41) glycoprotein with the target cell membrane. Extensive genetic variability, generally in gp120 and the gp41 ectodomain, can result in altered coreceptor use, fusion kinetics, and neutralization sensitivity. Here we describe an R5 HIV variant that, in contrast to its parental virus, infects T-cell lines expressing low levels of cell surface CCR5. This correlated with an ability to infect cells in the absence of CD4, increased sensitivity to a neutralizing antibody recognizing the coreceptor binding site of gp120, and increased resistance to the fusion inhibitor T-20. Surprisingly, these properties were determined by alterations in gp41, including the cytoplasmic tail, a region not previously shown to influence coreceptor use. These data indicate that HIV infection of cells with limiting levels of cell surface CCR5 can be facilitated by gp41 sequences that are not exposed on the envelope ectodomain yet induce allosteric changes in gp120 that facilitate exposure of the CCR5 binding site.
Human immunodeficiency virus type 1 (HIV-1) enters cells by membrane fusion mediated by its envelope (Env) glycoproteins (51). The Env proteins are synthesized as a 160-kDa precursor that is cleaved by a host protease to yield the surface gp120 (SU) and the transmembrane gp41 (TM) glycoprotein subunits. The functional Env structure is a trimer, with the gp120 subunits anchored on the virion surface by noncovalent interactions with the gp41 trimer. The gp120 binds first to CD4 and subsequently to a chemokine receptor/coreceptor (generally CCR5 or CXCR4). The gp41 then interacts with the target cell membrane through its N-terminal fusion domain, promoting lipid mixing and viral entry. An unusual feature of gp41 is its long cytoplasmic domain (CD) or tail of approximately 150 amino acids (aa), in contrast to the TM proteins of other retroviruses, such as avian and murine oncoretroviruses, which have a shorter CD (typically 20 to 30 aa).
The HIV gp41 CD region includes a number of domains, the exact functions of which are not well understood. The CD includes one or more palmitoylated cysteines, which may mediate localization of the Env to lipid rafts (4, 55). A tyrosine-based (YxxΦ) motif in the membrane-proximal region of the CD mediates binding to components of clathrin-associated adaptor complexes, which are involved in trafficking and endocytosis (3, 5, 7, 48, 56), and also targets Env to the basolateral membrane in polarized cells, resulting in basolateral budding (38, 49). The CD forms three highly conserved amphipathic α-helices, termed lentiviral lytic peptides (LLPs), that have been implicated in interactions that decrease the stability of lipid bilayers, causing pore formation and mediating T-cell death (11, 12, 22, 33, 42, 43, 62). The CD also contains two regions that closely resemble those found in calmodulin-activated enzymes that bind calmodulin and could potentially inhibit calmodulin-regulated proteins (32, 44, 59, 60). Since calmodulin helps regulate T-cell metabolism and function, these regions may modulate T-cell signal transduction pathways to facilitate infection.
To better understand the role of the CD in HIV infection, several investigators have introduced premature stop codons (18, 25, 66). The results do not provide a clear picture of CD function. Although the CD is dispensable for fusion, some truncations significantly reduce viral infectivity. Other point mutations and truncations of the CD, however, increase Env surface expression (36, 71) and incorporation into virions (9, 39, 69, 71), increasing the efficiency of entry. Interestingly, some truncations of CD, in combination with other env mutations, lead to CD4-independent infection (6, 68). A clear understanding of the role of the cytoplasmic tail is complicated further because truncations in the tail can have different biologic effects depending upon the target cell (46).
HIV-1 variants for which the initial step of CD4 binding is dispensable have been described (19, 20, 29, 30, 35). The gp120s of these viruses are thought to be in a “pretriggered” or partially triggered state in which the conserved coreceptor binding site is exposed and functional. Exposure of this site, however, results in an increased sensitivity to some neutralizing antibodies, such as 17b, that recognize epitopes induced by CD4 binding and overlapping the conserved coreceptor binding site. Such a pretriggered state of gp120 may facilitate conversion of the gp41 trimer to its three-stranded coiled-coil fusion intermediate, leading to more rapid fusion. Faster fusion kinetics would have a major impact on the efficiency of entry because fusion is likely rate limiting for entry (52).
Here we describe the isolation and characterization of HXBaLm1.2, a variant derived from an R5 (Ba-L) HIV chimera, and of HXBaLm1.2-derived molecular clones pHXBaLm1.2 and pHXBaLm6133. These variants infect cells expressing otherwise limiting levels of cell surface CCR5, in contrast to the parental virus. They also infect cells in the absence of CD4, are more sensitive to neutralization by soluble CD4 (sCD4) and an antibody against conserved epitopes that overlap the coreceptor binding site of gp120, and are less sensitive to inhibition by the fusion inhibitor T-20 (65) than is the parental virus. The genetic determinants for these properties are within the gp41, including the CD, a region not previously shown to influence coreceptor use. Our interpretation of the data is that the altered properties are due to changes in gp41 that induce allosteric alterations in either the tertiary or quarternary gp120 conformation that partly expose the chemokine receptor binding site, allowing more rapid or efficient fusion with the cell membrane.
(Much of this work was performed by Brian M. Taylor in partial satisfaction of his Ph.D. thesis requirements.)
MATERIALS AND METHODS
Cell lines.
COS-1 cells were from the ATCC. HOS cells expressing CD4 and CCR5, CXCR4, CCR1, CCR2b, or CCR3 were from the NIH AIDS Reagent Repository (Bethesda, MD). COS-1 and HOS cells were maintained in 90% Dulbecco's modified Eagle's medium and 10% heat-inactivated fetal bovine serum (FBS). The T-cell lines CEM-SS, H9, Sup-T1, and PM1 were obtained from the NIH AIDS Reagent Repository and were maintained in 90% RPMI 1640 and 10% heat-inactivated FBS. U87MG cells (a human astroglioma line), U87.CD4.CCR5 cells (expressing CD4 and CCR5) and U373/CD4/MAGI cells (63) (human astroglioma lines that express chemokine receptors and have a β-galactosidase [β-gal] indicator gene regulated by the HIV long terminal repeat), and Cf2Th/synCCR5 cells (an adherent canine thymocyte line expressing 0.5 to 1.0 ×106 human CCR5 molecules per cell) were from the NIH AIDS Reagent Repository. The JC10 and JC20 cell lines expressing defined levels of cell surface CCR5 (64) were a gift from David Kabat (Oregon Health Sciences University, Portland, OR). BC7/CCR5, a SupT1-derived T-cell line expressing CCR5 but not CD4 (40), was a gift from James Hoxie (University of Pennsylvania, Philadelphia, PA).
Reagents and antibodies.
The chemokines RANTES, macrophage inflammatory protein 1β (MIP-1β), and stromal cell-derived factor 1 (SDF-1) were purchased from R&D Systems (Minneapolis, MN). The CCR5-specific inhibitor TAK-779 (2) was obtained from the NIH AIDS Research and Reference Reagent Program. T-20 and −2 RANTES were gifts from Lai Xi Wang and Anthony DeVico, respectively, from the Institute of Human Virology (IHV), Baltimore, MD. The HIV-1 gp120 monoclonal antibody (MAb) immunoglobulin G1 (IgG1) b12 was obtained from the NIH AIDS Research and Reference Reagent Program. Monoclonal antibody 2G12 was purchased from Polymun Scientific Inc., Vienna, Austria. MAbs A32, ED47, C11, 19E, and 17B, as well as sCD4, were obtained from the μQUANT Core Facility, IHV. Human IgG was purchased from Sigma.
Infectious HIV-1 molecular clones.
The plasmids pHXB2-MCS and pHXB2-MCSΔenv were generously provided by Andrew Leigh Brown (University of Edinburgh, Scotland). pHXB2-MCS is a derivative of the infectious molecular clone pHXB2 with two cloning sites added by site-directed mutagenesis, a BstEII site 5 aa downstream of the gp160 signal sequence and an XbaI site 110 aa from the amino terminus of gp41. The addition of these sites does not alter the coding capacity of the clone. pHXB2-MCSΔenv is a derivative of pHXB2-MCS from which a NdeI-StuI fragment containing the V1 and V2 domains of gp120 was excised, and it does not produce viable virus. The proviral DNA sequences from pHXB2-MCS and pHXB2-MCSΔenv were excised with PacI, which cuts in the 5′and 3′ host cellular flanking regions, and spliced into the cloning vector pSP65gpt(PacI), containing a PacI site in place of the XbaI site of pSP65gpt. pHXBaL was constructed by substituting the coding regions for gp120 and the amino-terminal 110 aa of the gp41 coding regions of the R5 HIV-1(Ba-L) into the homologous region in pHXB2-MCS. This region was amplified from the plasmid pBaL-C3 using primers 5′-TGT-GGG-TCA-CCG-TCT-ATT-AT-3′ and 5′-CTC-ATC-TAG-AGA-TTT-ATT-ACT-CC-3′ and spliced into the BstEII/XbaI site of pHXB2-MCSΔenv.
To construct pHXBaLm1.2, a plasmid encoding a virus expressing the mutant Env proteins, the entire env gene from HXBaLm1.2 was PCR amplified using primers 5′-TGG-CAA-TGA-GAG-TGA-AGG-AG-3′ and 5′-CTA-AGA-TCT-ACA-GCT-GCC-TTG-TAA-3′ and spliced into the BstEII/Bpu1102 site of pHXB2-MCSΔenv. pHXBaLm1.2 includes the mutations in the gp120 of HXBaLm1.2 as well as its altered gp41 region. To make pHXBaLm3.4, the V3-V4 region of HXBaLm1.2 was PCR amplified and spliced into the BstEII/XbaI site of the parental plasmid, pHXBaL, using primers 5′-TGT-GGG-TCA-CCG-TCT-ATT-AT-3′ and 5′-CTT-ATC-TAG-AGA-TTT-ATT-ACT-CCA-ACT-AG-3′. pHXBaLm3.4 is identical to the parental virus except for the mutations in V3 and C3. To make pHXBaLm6133, the region of the gp41 of HXBaLm1.2 from heptad repeat 1 (HR1) to LLP1 was PCR amplified with primers 5′-GGC-AGT-CTA-GCA-GAA-GAA-GAG-GT-3′ and 5′-CTA-AGA-TCT-ACA-GCT-GCC-TTG-TAA-3′ and substituted for the reciprocal region in the parental vector, HXBaL. A polylinker that includes BstEII and XhoI sites was added to the vector, pcDNA3.1+/Zeo (Invitrogen). The BstEII/XhoI region from HXBaL was ligated into this vector to create a new vector, pcDNA/HXBaL. The PCR-amplified product from HXBaLm1.2 was spliced into the PshAI/Bpu1102 site of pcDNA/HXBaL. The BstEII/BlpI fragment from the resulting vector, pcDNA/BaLm6133, was cut and ligated into the same region in pHXBaL to yield pHXBaLm6133. pHXBaLm6133 contains the gp41 of the original mutant but lacks the gp120 mutations. All constructs were verified by DNA sequence analyses. To generate infectious virus from plasmids, 2 × 105 COS-1 cells were transfected with 2 μg of plasmid DNA using FuGene6 (Roche Diagnostics, Indianapolis, IN) under conditions specified by the manufacturer. At 72 h after transfection, supernatants were collected and stored at −80°C.
Infectivity assays.
Infectivity assays with HOS cells were performed in six-well plates containing 100,000 cells/well; 200 μl of transfected cell supernatant was added to 2 ml of medium. After 24 h, cells were washed three times with Dulbecco's phosphate-buffered saline (DPBS) and the medium replaced. Supernatant was collected every 3 days and tested for HIV-1 core antigen p24 by enzyme-linked immunosorbent assay (ELISA) (Perkin-Elmer Life Science, Inc., Boston, MA) per the directions of the manufacturer. Infected cells were detached, counted, and replated in 2 ml of fresh medium on six-well dishes at 200,000 cells per well. Stocks of resultant viruses were propagated in PM1 cells, and the 50% tissue culture infectious dose (TCID50) per milliliter of viruses in the supernatants determined by the μQUANT Core Facility, IHV, by limiting dilution on phytohemagglutinin-stimulated PBMCs using the Spearman-Karber method (25).
For infection of T-cell lines, 100,000 cells were treated with 50 TCID50s of virus in 1 ml of 90% RPMI-10% FBS, incubated for 3 h at 37°C, washed three times with DPBS, and resuspended in 1 ml of medium. The cultures were split at a ratio of 1:5 every 3 days and medium p24 antigen measured by ELISA. For chemokine inhibition assays, cells were incubated with 1 μg/ml of RANTES, SDF-1, MIP-1β, or no chemokine for 1 hour at 37°C. Virus was then added at a multiplicity of infection of 0.01 and incubated for 3 h. Cultures were then washed three times with DPBS, and new medium and chemokines were added. Cultures were monitored for p24 and chemokines added with each medium change.
Optiprep gradients.
Virions were prepared from supernatants from chronically infected PM1 cells by centrifugation to remove cellular debris (1,800 × g, 10 min) and pelleting the virus (141,000 × g, 2 h) through a 32% (wt/vol) sucrose cushion in a Beckman SW28 rotor. Virus was resuspended in 250 μl of PBS and centrifuged in an Optiprep (60% iodixanol [wt/vol]; Gibco Life Technologies) velocity gradient as described previously (17). Briefly, iodixanol gradients were prepared in PBS in 11 increments of 1.2%, ranging from 6 to 18%. Virions were layered on top of the gradient and centrifuged (200,000 × g, 1.5 h) in a Beckman SW41 Ti rotor. The bottom four fractions were collected and precipitated with 20% trichloroacetic acid, except that 50 μl from each pool of fractions was saved and used to infect PM1 cells. Acid-precipitated virions were resuspended in 50 μl PBS, with 1 μl used to determine p24 concentration prior to Western blot analyses.
Immunoblot analyses.
Samples from the Optiprep gradient were normalized by p24 concentration and electrophoresed on sodium dodecyl sulfate-polyacrylamide gels (Novex) in 4 to 20% Tris-glycine. An equivalent of 14 ng of p24 was loaded in each well. Envelope glycoprotein was detected by Western immunoblotting using a pool of gp120-specific antibodies (provided by George Lewis, IHV) and a secondary anti-mouse IgG, horseradish peroxidase-linked antibody (Cell Signaling Technology, Beverly, MA). Purified HIV-1 Ba-L gp120 protein (μQUANT Core Facility, IHV) was used as a positive control. p24 was measured with MAbs 13B6 and 13G4 (μQUANT Core Facility, IHV). Western blots were visualized by chemifluorescence using ECL-Plus (Amersham Biosciences Corp, Piscataway, NJ) and bands quantified densitometrically using ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA) and a Storm Fluor-Imager (Molecular Dynamics).
Doxycycline-regulated expression of CCR5.
U87MG cells were sequentially transfected with three plasmids (pcDNA-CD4, pBI-EGFP-CCR5, and pEF1prtTA) using FuGene6 (Roche) and stable clones selected to create the U87.DOX.CCR5 cell line. pcDNA-CD4 was constructed by inserting a HindIII-BamHI fragment from the plasmid plck-CD4 A+, containing human CD4 cDNA (8) (a gift from Harris Goldstein, Albert Einstein College of Medicine, Bronx, NY), into the cognate sites of pcDNA 3.1+/Zeo (Invitrogen). pBI-EGFP-CCR5 was constructed by inserting a polylinker containing BclI and SalI sites into a plasmid containing a bidirectional tetracycline-inducible promoter (pBI-EGFP; Clontech) and then ligating the BamHI/SalI fragment from pBABE.CCR-5, obtained from the NIH AIDS Research and Reference Reagent Program, to create pBI-EGFP-CCR5. pEF1prtTA, encoding the transactivator for the tetracycline-inducible promoter (26), was a gift from P. B. Fisher (Columbia University, New York, NY). As a negative control, U87MG cells were transfected with pcDNA-CD4 alone. Cells were maintained in Dulbecco's modified Eagle's medium-10% FBS supplemented with 250 μg/ml zeocin and 250 μg/ml geneticin to maintain the CD4 and “reverse” Tet repressor genes, respectively. Variable levels of CCR5 expression were induced by addition of different concentrations (0.1 to 1,000 ng/ml) of doxycycline (Sigma) to the culture medium. Infectivity assays in U87.DOX.CCR5 cells were performed in six-well plates containing 50,000 cells per well. The cells were treated with the indicated amount of doxycycline and incubated at 37°C overnight to induce CCR5 expression. The next day, 100 TCID50s of virus was added and the culture incubated at 37°C. After 3 h, cells were thrice washed with DPBS, and 2 ml of culture medium and antibiotics were added. Supernatants were collected every 3 days and tested for medium p24 by ELISA.
Determination of absolute surface levels of CCR5 and CD4.
A total of 100,000 cells were washed twice and resuspended in DPBS plus 2% heat-inactivated FBS, 2% heat-inactivated human AB serum (Sigma), and 0.1% sodium azide (wash/stain buffer) and then incubated for 15 min at room temperature with purified anti-CD16 and anti-CD32 (both from BD Pharmingen) diluted 1:20 in wash/stain buffer to block nonspecific binding. Then, either phycoerythrin (PE)-conjugated anti-human CD195 and allophycocyanin-conjugated anti-human CD4 or PE-conjugated anti-human CD4 (all from BD Pharmingen) alone was added and the cells incubated at room temperature for 30 min, washed twice in wash/stain buffer, and fixed in 1% paraformaldehyde. A minimum of 50,000 events were acquired with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with FlowJo batch analysis software (Treestar, San Carlos, CA). Quantibrite PE flow cytometry beads (Becton Dickinson) containing a known number of PE molecules per bead were used to prepare a calibration curve to quantify the number of CD4 and CCR5 receptors per cell. The calibration curve was used to estimate the number of molecules of PE-labeled reagent bound per cell from the median fluorescence intensity of the samples.
Inhibition of HIV-1 infection.
PM1 cells were cultured in RPMI 1640 medium with 10% heat-inactivated FBS, and 100,000 cells were resuspended in 100-μl aliquots of TAK-779, MIP-1β, or −2 RANTES serially diluted in culture medium and incubated for 1 h at 37°C. Next, 100-μl aliquots of culture medium containing 50 TCID50s of virus were added. The cultures were incubated at 37°C for 3 h, washed three times with PBS, and resuspended in fresh culture medium containing the appropriate concentration of inhibitor. For studies with T-20 (which binds to the virus, not the target cell), 50-μl aliquots of serially diluted inhibitor were combined with 50-μl aliquots of culture medium containing 50 TCID50s of virus for 1 h at 37°C. The mixtures of T-20 with virus were combined with PM1 cells (100,000 cells resuspended in 100 μl of culture medium) for 3 h at 37°C. The cultures were washed three times with PBS and resuspended in fresh culture medium containing the appropriate concentration of inhibitor. Three days later, half the medium was replaced with the corresponding medium or medium plus inhibitor. Tests under all conditions were carried out in triplicate. HIV-1 replication was quantified by medium p24 at days 3 and 6 postinfection. The percent inhibition of HIV-1 was calculated from p24 concentrations obtained in the presence of the inhibitors divided by the p24 concentration in the absence of any inhibitors.
Antibody neutralization assays.
A total of 5 × 103 U373/CD4/MAGI cells expressing CCR5 were added to wells in a 96-well microtiter plate and incubated overnight at 37°C in 100 μl of complete medium. The medium was removed and replaced with 50 μl of fresh medium plus 50 μl of medium containing 50 TCID50s of virus preincubated for 1 h at 37°C with the indicated antibodies. Following incubation at 37°C for 18 h, the cells were washed in PBS and 100 μl of fresh medium with antibodies. The cultures were incubated at 37°C for 4 days and treated with a β-gal chemiluminescent reagent (Galactostar; Tropix, Bedford, MA) according to the manufacturer's protocol. Infection was quantified by chemiluminescence using a Victor2 (EG&G Wallac, Gaithersburg, MD) fluorescence plate reader. β-gal expression from the long terminal repeat is dependent upon infection and expression of Tat. Values were corrected by subtracting the background value determined in the absence of virus. The percent infection was calculated by dividing the corrected relative light units for each experimental well by the corrected relative light units for control wells containing only cells and virus. Inhibition data were analyzed by linear regression to calculate the protein concentrations that gave 50% reductions in infection using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Tests under all conditions were carried out in triplicate.
Selection of a CD4-independent HIV-1.
The method of Kolchinsky et al. (35) was modified to isolate a CD4-independent HIV(Ba-L) variant (BaL10001) from U87MG cell mixtures expressing CCR5 plus CD4 or CCR5 alone. A heterogeneous population of HIV(Ba-L) (from the μQUANT Facility, IHV) was used to infect a 10:1 mixture of U87.CCR5 and U87.CD4.CCR5 cells. At day 7 postinfection, virus-containing supernatants were collected and used to infect a 20:1 mixture of U87.CCR5 and U87.CD4.CCR5 cells. This process was repeated every 7 days at increasing ratios of CD4− to CD4+ cells of 50:1, 100:1, 200:1, 500:1, and 1,000:1.
To verify CD4 independence, viruses were tested on two CD4+ T-cell lines. A total of 100,000 BC7/CCR5 cells were infected with 50 TCID50s of virus in 1 ml of 90% RPMI-10% FBS. The cultures were incubated for 3 h at 37°C, washed three times with DPBS, and resuspended in 1 ml of medium. The cultures were split at a ratio of 1:5 every three days, and supernatant p24 was measured by ELISA. Cf2TH/synCCR5 cells were plated at 100,000 cells in a 12-well plate and incubated at 37°C overnight. The next day, 50 TCID50s of virus was added and the culture incubated at 37°C. After 3 h, the cells were washed three times with DPBS and 1 ml of culture medium replaced. Cultures were monitored by p24 as described above.
RESULTS
Generation of a variant R5 HIV able to infect T-cell lines.
HXBaL is a chimeric infectious molecular clone that encodes most of the R5 HIV-1(Ba-L) gp120 and the first 110 aa of HIV-1(Ba-L) gp41 as well as the first five amino-terminal amino acids of gp120 and the 237 carboxy-terminal amino acids of gp41 of the X4 HIV-1(HXB2). All other genes are HXB2 derived. To assess the coreceptor usage of HXBaL, an infection assay was performed using HOS.CD4 cells that express either CCR5 or CXCR4. Surface coreceptor expression was verified by flow cytometry (data not shown). As determined by extracellular p24 Gag protein, HXBaL infected cells expressing CCR5, but not CXCR4, as did an R5 control virus, uncloned HIV-1(Ba-L) (data not shown). HXB2-MCS, the infectious molecular clone from which HXBaL was constructed, infected cells expressing either CXCR4 or CCR5, confirming an X4 phenotype (HOS.CD4.CCR5 cells are leaky for CXCR4 expression).
CCR5 antagonists are the most recent class of drugs for treating HIV infection. One concern is that the use of such compounds might lead to the generation of more virulent HIV variants that are able to use CXCR4 as a coreceptor. We wanted to determine the kinds of genetic changes that would result in a switch in coreceptor usage from CCR5 to CXCR4. To this end, a library of mutated HIV-1(Ba-L) env genes was generated from a 1.75-kb env fragment encoding gp120 and the N-terminal region of gp41by PCR-based in vitro saturation mutagenesis (53) and ligated into pHXB2-MCSΔenv, which lacks a functional env gene. The resultant plasmid library was expanded in Escherichia coli. DNA sequence analyses of the V3 regions of four individual clones indicated a point mutation frequency of 0.6% (four mutations in 655 aa). Four libraries of mutagenized env genes were transfected into COS-1 cells. The supernatants were collected at 72 h and extracellular p24 measured to monitor virus production. Concentrations of p24 ranged from 57 to 1,120 ng/ml.
HOS cells expressing CD4 and either CCR5 or CXCR4 were infected by viruses from the four libraries and monitored for virus expression. In no case did we observe virus from cells expressing CXCR4. Virus was detected, however, from one culture of cells expressing CCR5 at 31 days postinfection. Since HOS.CD4.CCR5 cells do express CXCR4 at low levels, we wondered whether the emergent virus, designated HXBaLm1.2, had adapted to use CXCR4. Indeed, HXBaLm1.2 infected the T-cell line CEM-SS, which is often considered to be negative for cell surface CCR5 (not shown). To determine whether HXBaLm1.2 was using CXCR4 as a coreceptor, CEM-SS cells were pretreated with 1 μg/ml RANTES or SDF-1 or with no chemokines. HXBaLm1.2 infected SDF-1-treated CEM cells and weakly infected untreated cells, but RANTES blocked infection (not shown). In contrast, infection of CEM-SS cells by HXB2, the X4 control virus, was blocked by SDF-1, but not by RANTES. These results suggested that HXBaLm1.2 was not using CXCR4 as a coreceptor. HXBaL (the parental clone) did not detectably infect CEM-SS cells under any conditions, indicating that HXBaLm1.2 had an altered cell host range. HXBaLm1.2 from supernatants of CEM-SS cells treated with SDF-1 was serially passaged in CEM-SS cells not treated with SDF-1. The serially passaged virus was highly infectious (500 ng/ml supernatant p24) for CEM-SS cells in the absence or presence of added SDF-1 (not shown).
To determine whether HXBaLm1.2 could infect other T-cell lines, we compared its infectivity for CEM-SS, H9, and SupT-1 cells, all of which are often considered to not express surface CCR5. HXBaLm1.2 infected all three cell lines, in contrast to R5 viruses Ba-L and HXBaL (the parental cloned virus) (Fig. 1A and B and data not shown). As expected, HXB2-MCS also infected all three cell lines. These data confirmed an alteration in cell host range.
FIG. 1.
HXBaLm1.2 infects T-cell lines. CEM-SS cells (A) or H9 cells (B) were infected with the indicated viruses or not infected (control). H9 cells (C) or Sup-T1 cells (D) were infected with the indicated viruses without added chemokines or in the presence of CCR5 ligands (MIP-1β or RANTES) or the CXCR4 ligand SDF. p24 antigen in supernatants was measured by ELISA on the indicated days after infection. Results are from representative experiments. Error bars show standard deviations determined on replicate wells.
Chemokine blocking studies were performed to confirm that HXBaLm1.2 remained R5 dependent. H9 and SupT-1 cells were treated with 1 μg/ml SDF-1, RANTES, MIP-1β, or no chemokine and challenged with either HXBaLm1.2 or the parental virus, HXBaL (Fig. 1C and D). The R5 ligands RANTES and MIP-1β blocked infection by HXBaLm1.2, while the X4 ligand SDF-1 had no effect. The parental virus did not infect H9 or SupT-1 cells, whether treated or not. Consistent with the idea that HXBaLm1.2 was infecting these T-cell lines through CCR5, we could detect CCR5 mRNA by reverse transcription-PCR of RNA from each cell line (data not shown). CCR5 surface protein expression was confirmed by flow cytometry (see below).
The range of coreceptors that HXBaLm1.2 could use was tested using HOS-CD4 cell lines expressing CCR1, CCR2b, CCR3, CCR4, CCR5, or CXCR4. In repeated experiments, HXBaLm1.2 only infected HOS-CD4 cells expressing CCR5 (data not shown). These results, taken together with the chemokine blocking experiments, confirm that although HXBaLm1.2 is able to infect most T-cell lines, it remains strictly R5 dependent.
Sequence analysis of HXBaLm1.2.
To identify mutations in HXBaLm1.2, we sequenced env from cDNA prepared from infected cultures of CEM-SS and H9 cells and from virions pelleted from culture supernatants. Identical sequences were obtained from all sources. There were three point mutations in gp120, His to Arg at aa 313 in the V3 loop, Ile to Val at aa 349, and Lys to Arg at aa 352. In addition, the comparison of HXBaLm1.2 with the parental virus, HXBaL, revealed an unexpected change. The original construct contained only first 108 aa of the Ba-L gp41. HXBaLm1.2, however, contained an additional Ba-L-related substitution, encoding approximately an additional140 aa of the Ba-L gp41, in place of the HXB2-derived sequence of the parental virus (Fig. 2). Both HXBaLm1.2 and the parental virus have the fusion domain and HR1 of Ba-L. However, HXBaLm1.2 also has the HR2, the TM domain, and 60 aa of the membrane-proximal CD of Ba-L rather than that of HXB2, which the parental virus has. The reason for this change is not clear, but it may have occurred by recombination with Ba-L, which we speculate could have been present as a contaminant at some point during the long time period before viral production became evident. Figure 2 compares variant and parental gp41 sequences.
FIG. 2.
HXBaLm1.2 gp41 DNA sequence. Shown is a comparison of the gp41-coding regions of HXBaLm1.2 and its T-cell-line-competent derived clone HXBaLm6133 with those of the parental virus and wild-type HIV-1(Ba-L). Functional domains are outlined with boxes. FD, fusion domain. Arrows denote predicted sites of palmitoylation. Dashes indicate amino acid identity; letters indicate differences. The numbers above the alignment are the amino acid residue numbers based on HIV-1(Ba-L) gp160.
Identifying genetic determinants for altered tropism.
To identify which mutations in HXBaLm1.2 determine the altered tropism, smaller segments of env were subcloned and inserted back into the parental vector, pHXBaL. The constructs included pHXBaLm1.2 (which contains all the mutations), pHXBaLm3.4 (which contains only the three mutations in V3 and C3), and pHXBaLm6133 (which contains only the alteration in gp41) (Fig. 3). The clones were tested for their ability to infect T-cell lines. As summarized in the bottom panel of Fig. 3, all the mutant viruses productively infected PM1 cells, indicating that they were replication competent. HXBaLm1.2 retained the ability to infect CEM-SS, H9, and Sup-T1 cells. HXBaLm3.4, with only the gp120 mutations, did not infect these T-cell lines. In contrast, HXBaLm6133, which contains only the altered gp41, infected all three cell lines efficiently (consistently reaching supernatant p24 levels of >0.1 ng/ml). Thus, the alterations in gp41 determine the altered host of HXBaLm1.2.
FIG. 3.
T-cell line competence is determined by gp41. The top panel shows a linear representation of env genes of HXBaLm1.2 and derived viruses. Shaded boxes denote HXB2 sequence; white boxes denote Ba-L sequence. gp120 and gp41 are not to scale. FD, fusion domain. The bottom panel shows the ability of these viruses to replicate on the indicated cell lines. (A) Surface expression levels of CCR5 and CD4 on the indicated T-cell lines were determined by flow cytometry using Quantibrite PE microbeads as described in Materials and Methods. (B) Relative levels of infection were judged by medium p24 at 6 days after infection. Infections were performed in triplicate. −, p24 not detectable; +, 0. 050 to 0.500 ng/ml p24; ++, 0.501 to 0.750 ng/ml p24; +++, ≥0.750 ng/ml p24.
HXBaLm1.2 infects cells with low surface CCR5 expression.
R5 viruses do not infect most T-cell lines. Since HXBaLm1.2 remains R5 dependent yet can infect CEM-SS, H9, and Sup-T1 cells, we wanted to confirm that these cells express CCR5 on their surface and determine their expression levels. As shown in the bottom panel of Fig. 3, CCR5 could be detected on T-cell lines by flow cytometry using Quantibrite PE beads and the mean number of molecules of CD4 and CCR5 per cell estimated. PM1, CEM-SS, and H9 cells expressed similar amounts of cell surface CCR5, even though only PM-1 cells are permissive for the parental virus. These results confirm previous findings by Lee et al. (37) that T-cell lines express surface CCR5, and they are in approximate agreement with the reported expression levels except for that of CCR5 by Sup-T1 cells. Differences in Sup-T1 sublines could explain this discrepancy. The absolute levels of CCR5 expressed on these cell lines thus do not appear to be the sole determinants for permissivity for the mutants, nor are they explained by absolute CD4 levels. Other factors, such as production of inhibitory chemokines, the degree of CD4-CCR5 colocalization, or posttranslational modifications of CCR5 or CD4, may account for these differences.
To test whether HXBaLm1.2 has a reduced dependence on high CCR5 levels, we constructed a cell line with regulated CCR5 expression. U87 cells stably expressing CD4 were transfected with the plasmid pBI-EGFP-CCR5, which coexpresses CCR5 and enhanced green fluorescent protein from a tetracycline-sensitive bidirectional promoter. Increasing the doxycycline concentration increases expression of both enhanced green fluorescent protein and CCR5. As determined by flow cytometry using Quantibrite PE beads, increasing doxycycline from 0.1 to 1,000 ng/ml increased CCR5 surface expression from 5,000 to 80,000 molecules/cell (Fig. 4A). CCR5 expression was leaky; the lower limit of expression was 4,500 molecules of CCR5 per cell. All cells expressed equivalent levels of CD4. Viruses were tested for the ability to infect U87.DOX.CCR5 cells expressing different levels of CCR5. A reduction from 80,000 CCR5 molecules/cell to 16,000 molecules/cell or lower caused a 50 to 60% decrease in the infectivity of Ba-L and the parental virus HXBaL (Fig. 4B). In contrast, there was no reduction of infection of cells expressing even the lowest levels of CCR5 per cell by HXBaLm1.2 or HXBaLm6133. None of the viruses could infect control cells that expressed no CCR5 (not shown).
FIG. 4.
HXBaLm1.2 infects cells expressing low levels of CCR5. (A) Surface expression of CD4 and CCR5 on U87.DOX.CCR5 cells was measured as a function of doxycycline (Dox) concentration by flow cytometry using Quantibrite PE microbeads as described in Materials and Methods. Also shown are the mean channel fluorescence and percent positive cells for CCR5. (B) Infection of U87.DOX.CCR5 cells was measured as a function of cell surface CCR5 expression. Medium p24 antigen was measured at day 3 after infection. Results are from a representative experiment. Standard deviations were determined from triplicate wells. (C) Infection of JC cells expressing different CCR5 levels was measured by p24 at 7 and 10 days after infection. Results are from a representative experiment. Standard deviations were determined from triplicate wells.
To confirm that HXBaLm1.2 and HXBaLm6133 could infect cells expressing too few CCR5 molecules to support infection with the parental virus, infections were also carried out in JC.10 and JC.20 cells. JC.10 cells express only 2,000 molecules of CCR5/cell, and JC.20 cells express fewer than 700 molecules/cell (64). We confirmed these numbers using Quantibrite PE beads. All viruses tested were able to infect JC.10 cells (Fig. 4C). However, only HXBaLm1.2 and HXBaLm6133 were able to infect JC.20 cells (Fig. 4D), confirming that HXBaLm1.2 can infect cells expressing reduced levels of CCR5.
HXBaLm1.2 has wild-type levels of virion gp120.
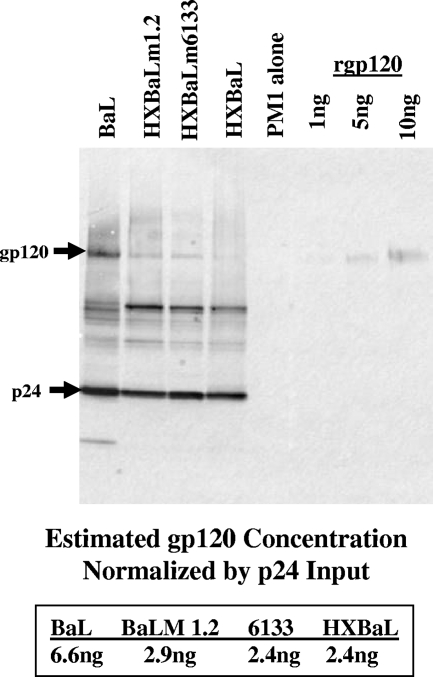
The only differences between HXBaLm6133 and the parental viruses Ba-L and HXBaL are in the CD and the region of HR2 to just upstream from the LLP2/3 region (including the TM domain), respectively. In light of previous studies showing that the CD of simian immunodeficiency virus (SIV) gp41 can affect the density of virion gp120 (36, 57, 71), we thought it possible that the mutant viruses could infect cells with low levels of CCR5 because of an increase in virion gp120, resulting in a higher probability of gp120-CCR5 interactions. To examine this possibility, virions were purified from chronically infected PM1 cells and analyzed by Western immunoblotting. We adapted a protocol that uses velocity rather than density gradients to isolate highly purified HIV-1 virions without disrupting the infectivity of the virus (17). Viral protein inputs were normalized for p24. Densitometric analyses showed no consistent differences in gp120 content that could explain the increased infectivity of HXBaLm1.2 and HXBaLm 6133 (Fig. 5). Indeed, Ba-L generally contained the largest amount of gp120, and we conclude that the reduced dependence on CCR5 levels for entry is not due to elevated levels of gp120.
FIG. 5.
HXBaLm1.2 virions do not incorporate excess gp120. Virions were purified by sucrose cushion and Optiprep (6 to 18% iodixanol) velocity gradient. Protein profiles were analyzed by immunoblotting with anti-gp120 and anti-p24 antibodies. PM1, uninfected negative control; rgp120, purified recombinant gp120 protein. Bands were quantified by densitometric analysis. Shown is the ratio of gp120 to p24 expression for the indicated viruses.
HXBaLm1.2 is not more resistant to CCR5 blocking agents.
HIV-1 envelope gp120s can differ in their affinity for CCR5. We wondered whether the alteration in gp41 caused an increased affinity of gp120 for CCR5, thereby making the viruses less sensitive to entry inhibitors. To test this possibility, PM1 cells were infected with mutant or parental virus in the presence of serially diluted concentrations of the CCR5 binding inhibitor TAK-779 or the CCR5 ligands −2 RANTES and MIP-1β. As seen in Fig. 6A and B (and data not shown), HXBaLm1.2 had sensitivities similar to those of the parental virus HXBaL, suggesting that the mutant gp120 does not have an increased affinity for CCR5.
FIG. 6.
HXBaLm1.2 is resistant to a fusion inhibitor but not entry inhibitors. PM1 cells were infected by HXBaL or HXBaLm1.2 and infection measured 3 days later by medium p24 levels as described in Materials and Methods. Cells were treated with the indicated concentrations of TAK-779 (A), −2 RANTES (B), or T-20 (C) as described in Materials and Methods. Results are from representative experiments. Error bars show standard deviations determined from triplicate wells.
HXBaLm1.2 is more resistant to a fusion inhibitor.
Truncations of the gp41 CD can decrease sensitivity to the fusion entry inhibitor T-20 (Enfuvirtide) (1, 68). We therefore asked whether the altered HXBaLm1.2 gp41 would have a similar effect. Infectivity assays were performed with PM1 cells in the presence of increasing T-20 concentrations. HXBaLm1.2 was much less sensitive to T-20 than was the parental virus (Fig. 6C). HXBaLm6133, which lacks the gp120 mutations of HXBaLm1.2 but has the altered gp41, was likewise less sensitive than the parental virus, indicating that the phenotype is determined by the gp41. The gp41 of HXBaLm1.2 contains an additional Ba-L-derived region compared to the parental virus. However, this alone cannot explain the increase in T-20 resistance, as pure Ba-L virus was even more sensitive to T-20 than was HXBaL. The decreased sensitivity to T-20 suggests that the gp41 alteration is causing accelerated fusion kinetics or increased fusion efficiency, determined partially by the Ba-L-related substitutions within HR2 and the transmembrane region and partially by the HXB2-related substitutions in the CD (Fig. 2 and 3).
Infection of T-cell lines is dependent upon CCR5.
Inhibition of infection of SupT1 and H9 cells by RANTES and MIP-1β (Fig. 1) strongly suggested that infection of these T-cell lines depended on CCR5 as a coreceptor. To confirm this, we carried out infection of both cell lines in the presence of 500 pM and 500 nM TAK-779, a specific CCR5 antagonist. As shown in Fig. 7, infection was strongly inhibited by TAK-779, confirming a dependence upon CCR5.
FIG. 7.
Infection of T-cell lines is CCR5 dependent. T-cell lines were infected with HXB2 or HXBaLm6133 as described in Materials and Methods in the absence or presence of 500 pM or 500 nM TAK-779. Six days after infection, virus production was determined by ELISA of medium p24. (A) SupT1 cells were infected with BaLm6133; (B) SupT1 cells were infected with HXB2; (C) H9 cells were infected with BaLm6133; (D) H9 cells were infected with HXB2. All samples were run in triplicate; error bars show standard deviations.
HXBaLm1.2 is CD4 independent.
HIV variants that are able to infect in the absence of CD4 have been described (19, 20, 29, 30, 35). Several studies have reported that truncations of the HIV or SIV gp41 CD can cause such a CD4-independent viral phenotype (6, 68). We tested the ability of HXBaLm1.2 to infect CD4-negative cells. For a positive control, we selected a culture of HIV-1(Ba-L) with a decreasing ratio of CD4+ to CD4− T cells and obtained a variant, BaL10001, that could productively infect CD4− cells (not shown). BaL10001 has six mutations in the V1/V2 region, two in the C2 region, and three in the CD compared with published sequences of the parental virus Ba-L (not shown). To assess CD4 independence, infections were carried out in Cf2th/synCCR5 canine thymus cells and BC7/CCR5 cells, a CD4-CCR5+ derivative of SupT1. HXBaLm1.2 and its derivative HXBaLm6133 infected both cell types, similarly to BaL10001 but in contrast to the parental virus and to Ba-L itself (Fig. 8A and B). Thus, the changes in gp41 result in both a more efficient utilization of CCR5 and the ability to infect cells lacking CD4.
FIG. 8.
HXBaLm1.2 infects cells not expressing CD4. Cells were infected with HXBaL, HXBaLm1.2, HXBaLm6133, or BaL10001 [derived from HIV(BaL) by passage on CD4− cells cocultured with progressively decreasing levels of CD4+ cells as described in Materials and Methods]. Infection was monitored by medium p24 at the indicated times after infection. Target cells were BC7/CCR5 CD4-negative cells (A) or Cf2th/synCCR5 CD4-negative cells (B). Results are from representative experiments. Error bars show standard deviations determined from triplicate wells.
HXBaLm1.2 has an altered neutralization profile.
Several studies have shown CD4-independent viruses to be highly sensitive to sCD4 and to neutralizing antibodies that recognize binding sites exposed after CD4 binding (15, 20, 21, 29, 34). If the coreceptor binding site in HXBaLm1.2 gp120 was indeed more exposed, HXBaLm1.2 might be similarly more neutralization sensitive. U373/CD4/MAGI cells expressing CCR5 were used as target cells. Infection was quantified after 4 days by chemiluminescent detection of β-gal. HXBaL and HXBaLm6133 were compared for neutralization sensitivity to several MAbs and sCD4. HXBaL was neutralized by 2G12, a broadly neutralizing MAb that binds a carbohydrate-dependent epitope on gp120; by the 17B antibody, which recognizes conserved, discontinuous structures on the HIV-1 gp120 that are exposed upon CD4 binding; and by sCD4. HXBaLm6133 was neutralized more efficiently by 17B and sCD4 (Fig. 9A and B), similar to neutralization data reported for other CD4-independent viruses. In contrast, HXBaLm6133 was neutralized less efficiently by 2G12 (Fig. 9C). The data confirm a conformational change in gp120.
FIG. 9.
HXBaLm1.2 is more sensitive to sCD4 and the neutralizing MAb 17b. U373/CD4/MAGI cells expressing CCR5 were infected with HXBaL or HXBaLm6133. Virus was treated with sCD4 (A), 17B (B), 2G12 (C), or control IgG (D) as described in Materials and Methods. β-gal activity was measured at 4 days postinfection. Results are normalized for values from control samples with no antibodies or sCD4, and IC50 were determined. Results are from representative experiments. Error bars show standard deviations determined from triplicate wells. The table shows the calculated IC50, with the 95% confidence intervals for CD4, 17B, and 2G12 indicated in parentheses.
DISCUSSION
We characterized a variant of an R5 HXB2/Ba-L chimera (HXBaLm1.2) that is able to grow in T-cell lines to better understand how structural changes in Env can lead to alterations in functional interactions with its coreceptors. HXBaLm1.2 and its clonal derivative HXBaLm6133 were able to infect cells expressing <700 molecules of surface CCR5/cell, in contrast to the parental viruses. Several lines of evidence suggested that the CCR5 binding site of gp120 of the mutant viruses is partially exposed without prior binding to CD4, thereby reducing the threshold level of CCR5 required to trigger fusion. First, the variant viruses could infect cells expressing CCR5 and no CD4, while the parental viruses were strictly CD4 dependent. Second, the variants were more sensitive than the parental viruses to neutralization by sCD4 or a MAb against gp120 sites exposed after binding to CD4.
A substitution within the coding region for gp41 was sufficient to cause the observed phenotypic changes. The phenotype clearly requires the interaction of several regions within the gp41, however. The gp41 of HXBaLm1.2 differs from those of both the parental virus HXBaL and Ba-L itself, neither of which is CD4 independent or able to infect cells expressing limiting CCR5. The gp41 differs from the HXBaL gp41 in residues 619 to 757, which are derived from Ba-L rather than HXB2. This encompasses HR2 in the ectodomain and the TM region. Residue 758 to the carboxy terminus, including the CD, is derived from HXB2 rather than from Ba-L. Thus, both changes are necessary, suggesting that the two regions act cooperatively to affect gp120 and allow infection of cells expressing low surface CCR5 levels.
The ability to infect cells expressing limiting CCR5 does not appear to be due to an increased affinity for CCR5, since both mutants and the parental viruses are equally sensitive to the CCR5 blockers TAK-779, MIP-1β, and −2 RANTES. Furthermore, although several studies have shown that some gp41 mutations increase gp120 density on virions (9, 39, 69, 71), this is not the case for HXBaLm1.2. This suggests that the changes in gp41 cause alterations of the tertiary or quarternary structure of gp120. As judged by 50% inhibitory concentrations (IC50), HXBaLm6133 is approximately sevenfold more sensitive than the parental virus to neutralization by MAb 17B (which recognizes sites on gp120 exposed after binding to CD4), suggesting that these sites are more exposed on HXBaLm1.2 gp120. Greater exposure of similar epitopes in the absence of CD4 has been reported for several CD4-independent HIV variants, compared to their CD4-dependent counterparts (20, 21, 29, 34). Primary HIV-1 variants selected in vitro that can enter cells expressing reduced levels of CD4 are also neutralized more readily by sCD4 (34), similar to HXBaLm1.2.
HXBaLm1.2 has a sharply decreased sensitivity to the fusion inhibitor T-20, a peptide based on the gp41 HR2 sequence that blocks the association of HR2 with HR1 during formation of the gp41 six-helix bundle (41, 65). In other studies, T-20 resistance of CD4-independent viruses has been attributed to more rapid fusion kinetics leading to decreased duration of exposure of the gp41 fusion intermediate (1, 52), making this likely to also be the case for HXBaLm1.2 and HXBaLm6133. These results are thus consistent with the increased sensitivity to neutralization by the 17B antibody and sCD4, to suggest preexposure of the coreceptor binding site.
Lentiviral envelope transmembrane glycoproteins, including HIV-1 and SIV gp41, have an unusually long CD. There is not a clear understanding of the functions of this domain, but alterations in the CD can affect Env levels on the cell surface and incorporation into viral particles (9, 23, 25, 39, 52, 66, 71). Truncations of the CD can also affect fusogenicity and dependence on CD4 for viral entry (6, 68). Similarly, alterations in the HXBaLm1.2 gp41 allow CD4-independent infection, but to our knowledge, the observation that determinants in the CD can influence coreceptor use is novel. Several previous variants that can infect cells expressing low CCR5 levels have been selected in vitro. Using the R5 entry inhibitor AD101, Trkola et al. (61) selected a variant of a primary R5 isolate that was able to use low CCR5 levels for entry. Dejucq et al. (16) described a JRCSF-C3 variant selected by in vitro passage in peripheral blood mononuclear cells that can infect the T-cell lines Molt4 and Sup-T1, which have low cell surface CCR5. In both instances, determinants for the altered tropism were within gp120, altering its conformation to allow more efficient utilization of CCR5. Variants that can use low CCR5 levels also occur in vivo; determinants in env of a brain-derived isolate apparently increase affinity for CCR5 and reduce dependence on CCR5 and CD4 (27).
In a study of the energetics of HIV gp120-CD4 binding, Myszka et al. demonstrated that there is considerable conformational flexibility within gp120 (47). However, once bound to CD4, gp120 gains conformational rigidity, leading to a metastable association with gp41 (28, 45) and a predisposition toward fusion (10, 24). Thus, we speculate that the gp41 changes described here influence gp120 to adapt a more stable conformation in which preexposure of the coreceptor binding site results in more rapid formation of the six-helix bundle in gp41, resulting in more efficient membrane fusion.
Several lines of evidence underscore the importance of CCR5 in HIV-1 transmission and indicate that CCR5 may be a promising target for therapeutic intervention in HIV infection. First, primary infection with HIV-1 principally involves R5 viruses (54, 58, 70). Second, individuals who are homozygous for a mutant CCR5 gene containing a deletion (Δ32) in the coding region are strongly resistant to infection (50, 67), and those who do become infected display lower rates of disease progression (14, 31). Finally, cross-sectional studies have correlated a natural capacity to produce high levels of MIP-1α and MIP-1β with better clinical outcome in HIV infection (13). However, therapies that target CCR5 put HIV under selective pressures. Although our isolation of a CD4-independent variant of HIV-1 that uses low levels of CCR5 for entry occurred in vitro, it is likely that similar adaptations will occur in vivo, especially under adaptive pressure from entry or fusion inhibitors, and could affect their efficacy. An ability to infect cells expressing low levels of CCR5 in vivo would expand the cellular host range in infected people and could make the virus more pathogenic.
Acknowledgments
Part of this work was supported by NIH grants R01 HL59796 and R01 AI060481-03 to A.D.
We thank Gregory Melikian for a critical reading of the manuscript.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Abrahamyan, L. G., S. R. Mkrtchyan, J. Binley, M. Lu, G. B. Melikyan, and F. S. Cohen. 2005. The cytoplasmic tail slows the folding of human immunodeficiency virus type 1 Env from a late prebundle configuration into the six-helix bundle. J. Virol. 79106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 965698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 731350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya, J., P. J. Peters, and P. R. Clapham. 2004. Human immunodeficiency virus type 1 envelope glycoproteins that lack cytoplasmic domain cysteines: impact on association with membrane lipid rafts and incorporation onto budding virus particles. J. Virol. 785500-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 27315773-15778. [DOI] [PubMed] [Google Scholar]
- 6.Bonavia, A., B. T. Bullock, K. M. Gisselman, B. J. Margulies, and J. E. Clements. 2005. A single amino acid change and truncated TM are sufficient for simian immunodeficiency virus to enter cells using CCR5 in a CD4-independent pathway. Virology 34112-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers, K., A. Pelchen-Matthews, S. Honing, P. J. Vance, L. Creary, B. S. Haggarty, J. Romano, W. Ballensiefen, J. A. Hoxie, and M. Marsh. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1661-674. [DOI] [PubMed] [Google Scholar]
- 8.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 9414637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celma, C. C., J. M. Manrique, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Domains in the simian immunodeficiency virus gp41 cytoplasmic tail required for envelope incorporation into particles. Virology 283253-261. [DOI] [PubMed] [Google Scholar]
- 10.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93681-684. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. S., S. F. Lee, and C. T. Wang. 2001. Cellular membrane-binding ability of the C-terminal cytoplasmic domain of human immunodeficiency virus type 1 envelope transmembrane protein gp41. J. Virol. 759925-9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chernomordik, L., A. N. Chanturiya, E. Suss-Toby, E. Nora, and J. Zimmerberg. 1994. An amphipathic peptide from the C-terminal region of the human immunodeficiency virus envelope glycoprotein causes pore formation in membranes. J. Virol. 687115-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocchi, F., A. L. DeVico, R. Yarchoan, R. Redfield, F. Cleghorn, W. A. Blattner, A. Garzino-Demo, S. Colombini-Hatch, D. Margolis, and R. C. Gallo. 2000. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proc. Natl. Acad. Sci. USA 9713812-13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 2731856-1862. [DOI] [PubMed] [Google Scholar]
- 15.Decker, J. M., F. Bibollet-Ruche, X. Wei, S. Wang, D. N. Levy, W. Wang, E. Delaporte, M. Peeters, C. A. Derdeyn, S. Allen, E. Hunter, M. S. Saag, J. A. Hoxie, B. H. Hahn, P. D. Kwong, J. E. Robinson, and G. M. Shaw. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 2011407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejucq, N., G. Simmons, and P. R. Clapham. 1999. Expanded tropism of primary human immunodeficiency virus type 1 R5 strains to CD4(+) T-cell lines determined by the capacity to exploit low concentrations of CCR5. J. Virol. 737842-7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dettenhofer, M., and X. F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 731460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 666616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumonceaux, J., S. Nisole, C. Chanel, L. Quivet, A. Amara, F. Baleux, P. Briand, and U. Hazan. 1998. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J. Virol. 72512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 755230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 762683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg, D., and M. Wesson. 1990. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein 41. Biopolymers 29171-177. [DOI] [PubMed] [Google Scholar]
- 23.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 691984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5276-279. [DOI] [PubMed] [Google Scholar]
- 25.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 663306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalkrishnan, R. V., K. A. Christiansen, N. I. Goldstein, R. A. DePinho, and P. B. Fisher. 1999. Use of the human EF-1alpha promoter for expression can significantly increase success in establishing stable cell lines with consistent expression: a study using the tetracycline-inducible system in human cancer cells. Nucleic Acids Res. 274775-4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorry, P. R., J. Taylor, G. H. Holm, A. Mehle, T. Morgan, M. Cayabyab, M. Farzan, H. Wang, J. E. Bell, K. Kunstman, J. P. Moore, S. M. Wolinsky, and D. Gabuzda. 2002. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J. Virol. 766277-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart, T. K., R. Kirsh, H. Ellens, R. W. Sweet, D. M. Lambert, S. R. Petteway, J. Leary, and P. J. Bugelski. 1991. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 882189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman, T. L., C. C. LaBranche, W. Zhang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 966359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoxie, J. A., C. C. LaBranche, M. J. Endres, J. D. Turner, J. F. Berson, R. W. Doms, and T. J. Matthews. 1998. CD4-independent utilization of the CXCR4 chemokine receptor by HIV-1 and HIV-2. J. Reprod. Immunol. 41197-211. [DOI] [PubMed] [Google Scholar]
- 31.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 21240-1243. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa, H., M. Sasaki, S. Noda, and Y. Koga. 1998. Apoptosis induction by the binding of the carboxyl terminus of human immunodeficiency virus type 1 gp160 to calmodulin. J. Virol. 726574-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliger, Y., and Y. Shai. 1997. A leucine zipper-like sequence from the cytoplasmic tail of the HIV-1 envelope glycoprotein binds and perturbs lipid bilayers. Biochemistry 365157-5169. [DOI] [PubMed] [Google Scholar]
- 34.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 752041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolchinsky, P., T. Mirzabekov, M. Farzan, E. Kiprilov, M. Cayabyab, L. J. Mooney, H. Choe, and J. Sodroski. 1999. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J. Virol. 738120-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, M. Marsh, and J. A. Hoxie. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 695217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 965215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manrique, J. M., C. C. Celma, J. L. Affranchino, E. Hunter, and S. A. Gonzalez. 2001. Small variations in the length of the cytoplasmic domain of the simian immunodeficiency virus transmembrane protein drastically affect envelope incorporation and virus entry. AIDS Res. Hum. Retroviruses 171615-1624. [DOI] [PubMed] [Google Scholar]
- 40.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 753903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, M. A., M. W. Cloyd, J. Liebmann, C. R. Rinaldo, Jr., K. R. Islam, S. Z. Wang, T. A. Mietzner, and R. C. Montelaro. 1993. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology 19689-100. [DOI] [PubMed] [Google Scholar]
- 43.Miller, M. A., R. F. Garry, J. M. Jaynes, and R. C. Montelaro. 1991. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res. Hum. Retroviruses 7511-519. [DOI] [PubMed] [Google Scholar]
- 44.Miller, M. A., T. A. Mietzner, M. W. Cloyd, W. G. Robey, and R. C. Montelaro. 1993. Identification of a calmodulin-binding and inhibitory peptide domain in the HIV-1 transmembrane glycoprotein. AIDS Res. Hum. Retroviruses 91057-1066. [DOI] [PubMed] [Google Scholar]
- 45.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 2501139-1142. [DOI] [PubMed] [Google Scholar]
- 46.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myszka, D. G., R. W. Sweet, P. Hensley, M. Brigham-Burke, P. D. Kwong, W. A. Hendrickson, R. Wyatt, J. Sodroski, and M. L. Doyle. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 979026-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238305-315. [DOI] [PubMed] [Google Scholar]
- 49.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 883987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philpott, S., H. Burger, T. Charbonneau, R. Grimson, S. H. Vermund, A. Visosky, S. Nachman, A. Kovacs, P. Tropper, H. Frey, and B. Weiser. 1999. CCR5 genotype and resistance to vertical transmission of HIV-1. J. Acquir. Immune. Defic. Syndr. 21189-193. [DOI] [PubMed] [Google Scholar]
- 51.Pierson, T. C., and R. W. Doms. 2003. HIV-1 entry and its inhibition. Curr. Top. Microbiol. Immunol. 2811-27. [DOI] [PubMed] [Google Scholar]
- 52.Platt, E. J., J. P. Durnin, and D. Kabat. 2005. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J. Virol. 794347-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice, G. C., D. V. Goeddel, G. Cachianes, J. Woronicz, E. Y. Chen, S. R. Williams, and D. W. Leung. 1992. Random PCR mutagenesis screening of secreted proteins by direct expression in mammalian cells. Proc. Natl. Acad. Sci. USA 895467-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos, M. T., J. M. Lange, R. E. de Goede, R. A. Coutinho, P. T. Schellekens, F. Miedema, and M. Tersmette. 1992. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 165427-432. [DOI] [PubMed] [Google Scholar]
- 55.Rousso, I., M. B. Mixon, B. K. Chen, and P. S. Kim. 2000. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc. Natl. Acad. Sci. USA 9713523-13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155473-488. [PubMed] [Google Scholar]
- 57.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarlatti, G., E. Tresoldi, A. Bjorndal, R. Fredriksson, C. Colognesi, H. K. Deng, M. S. Malnati, A. Plebani, A. G. Siccardi, D. R. Littman, E. M. Fenyo, and P. Lusso. 1997. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 31259-1265. [DOI] [PubMed] [Google Scholar]
- 59.Srinivas, S. K., R. V. Srinivas, G. M. Anantharamaiah, R. W. Compans, and J. P. Segrest. 1993. Cytosolic domain of the human immunodeficiency virus envelope glycoproteins binds to calmodulin and inhibits calmodulin-regulated proteins. J. Biol. Chem. 26822895-22899. [PubMed] [Google Scholar]
- 60.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retroviruses 13263-269. [DOI] [PubMed] [Google Scholar]
- 61.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venable, R. M., R. W. Pastor, B. R. Brooks, and F. W. Carson. 1989. Theoretically determined three-dimensional structures for amphipathic segments of the HIV-1 gp41 envelope protein. AIDS Res. Hum. Retroviruses 57-22. [DOI] [PubMed] [Google Scholar]
- 63.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233193-198. [DOI] [PubMed] [Google Scholar]
- 64.Walter, B. L., K. Wehrly, R. Swanstrom, E. Platt, D. Kabat, and B. Chesebro. 2005. Role of low CD4 levels in the influence of human immunodeficiency virus type 1 envelope V1 and V2 regions on entry and spread in macrophages. J. Virol. 794828-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wild, C., T. Greenwell, D. Shugars, L. Rimsky-Clarke, and T. Matthews. 1995. The inhibitory activity of an HIV type 1 peptide correlates with its ability to interact with a leucine zipper structure. AIDS Res. Hum. Retroviruses 11323-325. [DOI] [PubMed] [Google Scholar]
- 66.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189167-177. [DOI] [PubMed] [Google Scholar]
- 67.Wilkinson, D. A., E. A. Operskalski, M. P. Busch, J. W. Mosley, and R. A. Koup. 1998. A 32-bp deletion within the CCR5 locus protects against transmission of parenterally acquired human immunodeficiency virus but does not affect progression to AIDS-defining illness. J. Infect. Dis. 1781163-1166. [DOI] [PubMed] [Google Scholar]
- 68.Wyss, S., A. S. Dimitrov, F. Baribaud, T. G. Edwards, R. Blumenthal, and J. A. Hoxie. 2005. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J. Virol. 7912231-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuste, E., J. D. Reeves, R. W. Doms, and R. C. Desrosiers. 2004. Modulation of Env content in virions of simian immunodeficiency virus: correlation with cell surface expression and virion infectivity. J. Virol. 786775-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. Holmes, A. J. Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 673345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J. Virol. 672824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]