Abstract
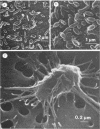
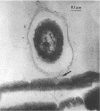
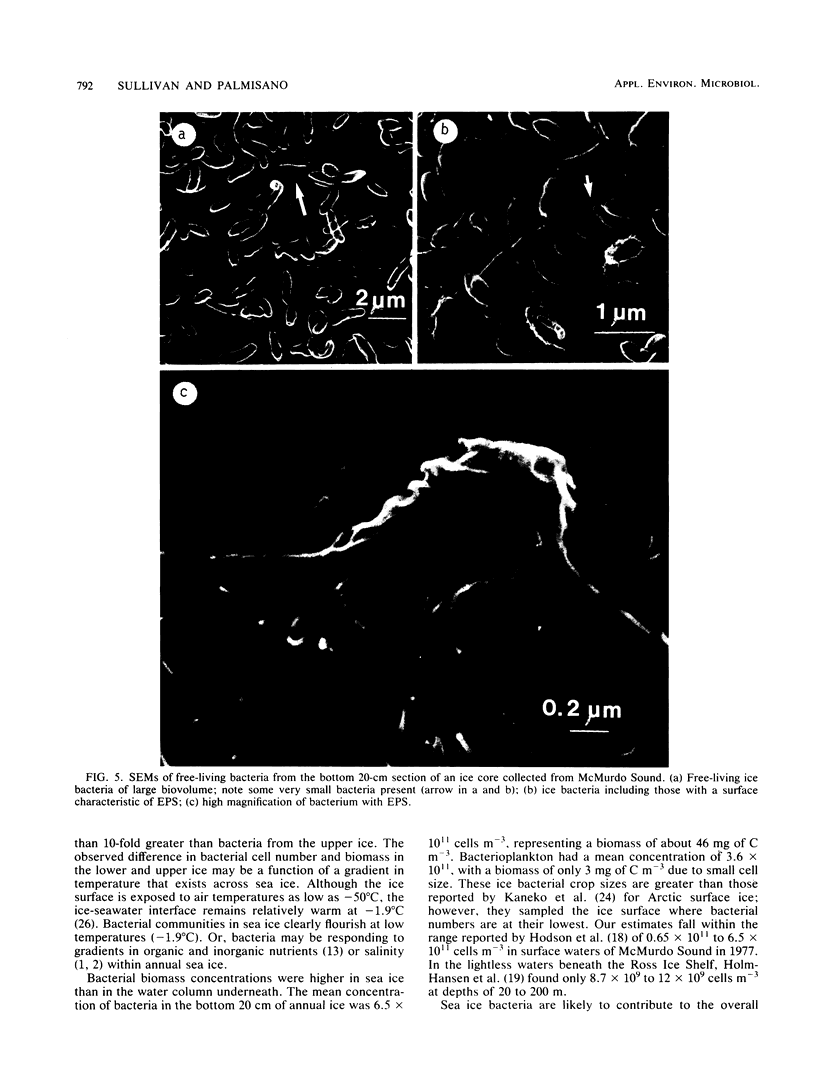
An abundant and diverse bacterial community was found within brine channels of annual sea ice and at the ice-seawater interface in McMurdo Sound, Antarctica, in 1980. The mean bacterial standing crop was 1.4 × 1011 cells m−2 (9.8 mg of C m−2); bacterial concentrations as high as 1.02 × 1012 cells m−3 were observed in ice core melt water. Vertical profiles of ice cores 1.3 to 2.5 m long showed that 47% of the bacterial numbers and 93% of the bacterial biomass were located in the bottom 20 cm of sea ice. Ice bacterial biomass concentration was more than 10 times higher than bacterioplankton from the water column. Scanning electron micrographs showed a variety of morphologically distinct cell types, including coccoid, rod, fusiform, filamentous, and prosthecate forms; dividing cells were commonly observed. Approximately 70% of the ice bacteria were free-living, whereas 30% were attached to either living algal cells or detritus. Interactions between ice bacteria and microalgae were suggested by a positive correlation between bacterial numbers and chlorophyll a content of the ice. Scanning and transmission electron microscopy revealed a close physical association between epibacteria and a dominant ice alga of the genus Amphiprora. We propose that sea ice microbial communities are not only sources of primary production but also sources of secondary microbial production in polar ecosystems. Furthermore, we propose that a detrital food web may be associated with polar sea ice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrici A. T., Johnson D. E. Studies of Freshwater Bacteria: II. Stalked Bacteria, a New Order of Schizomycetes. J Bacteriol. 1935 Jul;30(1):61–93. doi: 10.1128/jb.30.1.61-93.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H., Ito K., Fukushima H. Diatoms and the ecological conditions of their growth in sea ice in the arctic ocean. Science. 1966 May 20;152(3725):1089–1090. doi: 10.1126/science.152.3725.1089. [DOI] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T., Bont J. A., Jonge K. Prosthecobacter fusiformis nov. gen. et sp., the fusiform caulobacter. Antonie Van Leeuwenhoek. 1976;42(3):333–342. doi: 10.1007/BF00394132. [DOI] [PubMed] [Google Scholar]
- Tippit D. H., Pickett-Heaps J. D. Mitosis in the pennate diatom Surirella ovalis. J Cell Biol. 1977 Jun;73(3):705–727. doi: 10.1083/jcb.73.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]