Abstract
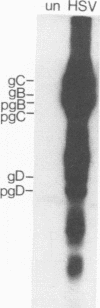
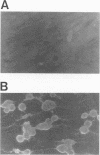
We examined the ability of human anti-herpes simplex virus (HSV) cytotoxic T lymphocytes (CTL) to lyse autologous human fibroblasts infected with HSV. In contrast to HSV-infected human Epstein-Barr virus-transformed B cells (LCL), which were lysed by HLA-restricted anti-HSV CTL, autologous fibroblasts infected with HSV were resistant to lysis. This resistance was not due to a lack of infectivity or production of HSV proteins since greater than 90% of the cells were infected and expressed abundant levels of viral proteins. HSV-infected human fibroblasts were also tested for susceptibility to lysis by alloantigen-specific CTL. Although allogeneic LCL and uninfected allogeneic fibroblasts were killed, human fibroblasts infected with HSV demonstrated a time-dependent resistance to lysis by alloantigen-specific CTL. HSV-infected human fibroblasts were not resistant to all forms of cell-mediated cytotoxicity since they were sensitive to antibody-dependent cellular cytotoxicity. Although one may suspect that the resistance of HSV-infected human fibroblasts to anti-HSV CTL and alloantigen-specific CTL-mediated lysis was due to a lack of major histocompatibility complex expression, Confer et al. (Proc. Natl. Acad. Sci. USA 87:3609-3613, 1990) previously demonstrated that incubation of human natural killer and lymphokine-activated killer cells with monolayers of human fibroblasts infected with HSV "disarmed" the killers in that they were unable to lyse sensitive target cells. We extend their results and show that incubation of anti-HSV CTL or alloantigen-specific CTL with uninfected fibroblasts did not affect their lytic activity, whereas CTL incubated with HSV-infected fibroblasts for 2 to 6 h rendered the CTL incapable of lysing their normally sensitive target cells. Indeed, human fibroblasts infected for merely 2 h with HSV were able to profoundly inhibit the cytotoxic activity of alloantigen-specific CTL. Thus, HSV-infected human fibroblasts are not inherently resistant to lysis by anti-HSV CTL or alloantigen-specific CTL, but rather contact of CTL with HSV-infected fibroblasts resulted in inactivation of the CTL. The inactivation of CTL appears to be HSV specific since incubation of alloantigen-specific CTL in sandwich assays with fibroblasts infected with HSV type 1 (HSV-1) or HSV-2 resulted in inactivation, whereas incubation of CTL with fibroblasts infected with adenovirus or vaccinia virus had no effect. Further, although incubation of alloantigen-specific CTL in sandwich assays with HSV-infected fibroblasts resulted in inhibition of CTL activity, exposure of CTL in Transwell cultures to cell-free supernatant from HSV-infected fibroblasts did not mediate this inhibitory effect.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer T., Moskophidis D., Rohrer U. H., Zinkernagel R. M., Hengartner H. In vitro selection of lymphocytic choriomeningitis virus escape mutants by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11047–11051. doi: 10.1073/pnas.88.24.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks T. A., Rouse B. T. Herpesviruses--immune escape artists? Clin Infect Dis. 1992 Apr;14(4):933–941. doi: 10.1093/clinids/14.4.933. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Morris S., Page J. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983 Oct;13(10):804–809. doi: 10.1002/eji.1830131005. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Vercellotti G. M., Kotasek D., Goodman J. L., Ochoa A., Jacob H. S. Herpes simplex virus-infected cells disarm killer lymphocytes. Proc Natl Acad Sci U S A. 1990 May;87(9):3609–3613. doi: 10.1073/pnas.87.9.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (1). N Engl J Med. 1986 Mar 13;314(11):686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P., Howell D. M., Pettera L., Tehrani S., Lopez C. Immediate-early gene expression is sufficient for induction of natural killer cell-mediated lysis of herpes simplex virus type 1-infected fibroblasts. J Virol. 1991 Jun;65(6):3151–3160. doi: 10.1128/jvi.65.6.3151-3160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Evans R., Kirkpatrick D., Lopez C. Heterogeneity of human NK cells: comparison of effectors that lyse HSV-1-infected fibroblasts and K562 erythroleukemia targets. J Immunol. 1983 Apr;130(4):1663–1667. [PubMed] [Google Scholar]
- Hickling J. K., Borysiewicz L. K., Sissons J. G. Varicella-zoster virus-specific cytotoxic T lymphocytes (Tc): detection and frequency analysis of HLA class I-restricted Tc in human peripheral blood. J Virol. 1987 Nov;61(11):3463–3469. doi: 10.1128/jvi.61.11.3463-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel-Berrey G. A., Shenoy A. M., Brahmi Z. Receptor modulation and early signal transduction events in cytotoxic T lymphocytes inactivated by sensitive target cells. J Immunol. 1991 Nov 1;147(9):3237–3243. [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Trinchieri G., Bandyopadhyay S., Starr S. E. Natural killer cell-mediated lysis of herpes simplex virus-infected fibroblasts: inability to detect soluble factors that contribute to lysis. Cell Immunol. 1990 May;127(2):221–229. doi: 10.1016/0008-8749(90)90127-d. [DOI] [PubMed] [Google Scholar]
- Pass R. F., Whitley R. J., Whelchel J. D., Diethelm A. G., Reynolds D. W., Alford C. A. Identification of patients with increased risk of infection with herpes simplex virus after renal transplantation. J Infect Dis. 1979 Oct;140(4):487–492. doi: 10.1093/infdis/140.4.487. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Pien F. D., Smith T. F., Anderson C. F., Webel M. L., Taswell H. F. Herpesviruses in renal transplant patients. Transplantation. 1973 Nov;16(5):489–495. doi: 10.1097/00007890-197311000-00014. [DOI] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Masur H., Rook A. H., Armstrong G., Frederick W. R., Epstein J., Manischewitz J. F., Macher A. M., Jackson L., Ames J. Herpesvirus infections in the acquired immune deficiency syndrome. JAMA. 1984 Jul 6;252(1):72–77. [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr Immune suppression by herpesviruses. Annu Rev Med. 1990;41:331–338. doi: 10.1146/annurev.me.41.020190.001555. [DOI] [PubMed] [Google Scholar]
- Sadat-Sowti B., Debré P., Idziorek T., Guillon J. M., Hadida F., Okzenhendler E., Katlama C., Mayaud C., Autran B. A lectin-binding soluble factor released by CD8+CD57+ lymphocytes from AIDS patients inhibits T cell cytotoxicity. Eur J Immunol. 1991 Mar;21(3):737–741. doi: 10.1002/eji.1830210329. [DOI] [PubMed] [Google Scholar]
- Schmid D. S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988 May 15;140(10):3610–3616. [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Human T-cell cultures from virus-sensitized donors can mediate virus-specific and HLA-restricted cell lysis. Nature. 1980 Aug 14;286(5774):718–720. doi: 10.1038/286718a0. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Lopez C., Hammer G. S., Brown A. E., Kornfeld S. J., Gold J., Hassett J., Hirschman S. Z., Cunningham-Rundles C., Adelsberg B. R. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981 Dec 10;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- Torpey D. J., 3rd, Lindsley M. D., Rinaldo C. R., Jr HLA-restricted lysis of herpes simplex virus-infected monocytes and macrophages mediated by CD4+ and CD8+ T lymphocytes. J Immunol. 1989 Feb 15;142(4):1325–1332. [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Kobayashi Y. Differential in vitro activation of CD4+CD8- and CD8+CD4- herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989 Sep 15;143(6):2051–2057. [PubMed] [Google Scholar]
- Yasukawa M., Shiroguchi T., Kobayashi Y. HLA-restricted T lymphocyte-mediated cytotoxicity against herpes simplex virus-infected cells in humans. Infect Immun. 1983 Apr;40(1):190–197. doi: 10.1128/iai.40.1.190-197.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. III. Analysis of viral glycoproteins recognized by CTL clones by using recombinant herpes simplex viruses. J Immunol. 1985 Apr;134(4):2679–2682. [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]