Abstract
We demonstrate that binding of different IgE molecules (IgEs) to their receptor, FcεRI, induces a spectrum of activation events in the absence of a specific antigen and provide evidence that such activation reflects aggregation of FcεRI. Highly cytokinergic IgEs can efficiently induce production of cytokines and render mast cells resistant to apoptosis in an autocrine fashion, whereas poorly cytokinergic IgEs induce these effects inefficiently. Highly cytokinergic IgEs seem to induce more extensive FcεRI aggregation than do poorly cytokinergic IgEs, which leads to stronger mast cell activation and survival effects. These effects of both types of IgEs require Syk tyrosine kinase and can be inhibited by FcεRI disaggregation with monovalent hapten. In hybridoma-transplanted mice, mucosal mast cell numbers correlate with serum IgE levels. Therefore, survival effects of IgE could contribute to the pathogenesis of allergic disease.
Mast cells are major effector cells for immediate hypersensitivity and allergic diseases. Cross-linking of IgE bound to its high-affinity receptor, FcεRI, with multivalent antigen initiates the activation of mast cells by promoting the aggregation of FcεRI (1, 2). This FcεRI-dependent activation results in degranulation (secretion of preformed mediators that are stored in the cytoplasmic granules, such as vasoactive amines, neutral proteases, proteoglycans, etc.), the de novo synthesis of proinflammatory lipid mediators, and the synthesis and secretion of cytokines and chemokines. In addition to these IgE/antigen-induced activation events, IgE binding to FcεRI in the absence of a specific antigen induces the up-regulation of FcεRI surface expression in mast cells and basophils (3, 4) and the prolonged survival of mouse mast cells under growth factor-limiting conditions (5, 6). The enhanced surface expression of FcεRI by IgE has been shown to be caused by the stabilization and accumulation of FcεRI on the mast cell surface in the presence of continued basal levels of protein synthesis (7, 8).
Two studies on the survival effect of monomeric IgE (5, 6) suggest differences in the potential mechanisms: Kalesnikoff et al. (6) found that IgE binding induces secretion of a variety of cytokines that enhance cell survival by an autocrine mechanism. In support of this model, they also found tyrosine phosphorylation of the FcεRI β subunit and activation of Akt and mitogen-activated protein kinases (MAPKs) in IgE-treated mast cells. In contrast, Asai et al. (5) did not detect significant cytokine secretion or any signaling events, which are known to be elicited by IgE/antigen-induced FcεRI aggregation (9, 10), in IgE-treated mast cells (5).
We have addressed why the two studies detected different antiapoptotic mechanisms, and we have performed experiments to elucidate further the mechanisms responsible for these effects. Our results demonstrate that all of the various IgE molecules (IgEs) tested showed antiapoptotic effects on mast cells, but that the different IgEs exhibited a wide spectrum in their ability to induce the production and secretion of cytokines by mast cells. At one extreme, the most highly cytokinergic (HC) IgEs can induce strong antiapoptotic effects, in part by an autocrine mechanism. At the other end of the spectrum, poorly cytokinergic (PC) IgEs induce less robust survival effects, but without inducing detectable cytokine production. Importantly, several lines of evidence indicate that binding of either HC or PC IgEs can result in FcεRI aggregation in the absence of the antigen for which that IgE is known to have specificity, with more extensive FcεRI aggregation induced by HC than by PC IgEs. Furthermore, mast cell numbers were increased in some gastrointestinal mucosal tissues of mice harboring IgE-secreting hybridoma, consistent with a “survival effect” of high serum IgE levels.
Materials and Methods
IgE Preparations. Anti-dinitrophenyl (DNP) mouse IgE monoclonal antibodies [H1 DNP-ε-206 and H1 DNP-ε-26 (11)] were purified as described previously (12). Briefly, ascites recovered from CAF1/J mice harboring the IgE hybridoma were fractionated by ammonium sulfate precipitation. IgE-containing fractions were further purified by DEAE column chromatography. Alternatively, anti-DNP IgEs were affinity-purified by DNP/BSA column chromatography. IgEs eluted with DNP-phenol were extensively dialyzed against PBS. These preparations were ultracentrifuged to remove aggregates before use, and the supernatants were further purified by Sepharose gel filtration column chromatography in some experiments. Anti-trinitrophenyl (TNP) mouse IgE monoclonal antibodies (IgE-3, C48-2, and C38-2) and anti-dansyl mouse IgE monoclonal antibody (27-74) were purchased from BD Pharmingen. Anti-DNP IgE antibody SPE-7 was kindly provided by Gerry Krystal (The Terry Fox Laboratory, Vancouver) and also purchased from Sigma. Hybridoma cells secreting anti-DNP IgE SPE-7 (13) were kindly provided by Zelig Eshhar (Weizmann Institute of Science, Rehovot, Israel). Animal experiments were approved by the Animal Care Committee of the La Jolla Institute for Allergy and Immunology and performed in accordance with the guidelines of the National Institutes of Health.
Cell Culture and Stimulation. Femoral bone marrow cells derived from WT (129/SvJ or C57BL/6), FcεRIα knockout (14), btk knockout (15), and lyn knockout (16) mice were cultured in IL-3-containing medium for 4–6 weeks to generate >95% pure populations of bone marrow-derived cultured mast cells (BMCMC). Syk–/– BMCMC were generated from bone marrow cells of sublethally irradiated mice that had been reconstituted with syk–/– fetal liver cells (17). Cells were incubated with various concentrations of IgE for the indicated periods. For FcεRI cross-linking, cells incubated overnight with 0.5 μg/ml H1 DNP-ε-206 were stimulated with antigen, 0.1–100 ng/ml DNP21 human serum albumin (HSA) (a gift from Teruko Ishizaka, La Jolla Institute for Allergy and Immunology, San Diego), unless otherwise noted. For monovalent hapten inhibition, DNP-lysine (Sigma) or trinitrophenyl (TNP) glutamate (Research Organics) was used in conjunction with anti-DNP or anti-TNP IgEs, respectively.
Flow Cytometry. For measurement of FcεRI and c-Kit, BMCMC were incubated first with 10 μg/ml 2.4G2 monoclonal antibody (BD Pharmingen) at 4°C for 10 min, then with 5 μg/ml H1 DNP-ε-206 for 50 min, and then with FITC-conjugated anti-mouse IgE (BD Pharmingen) and phycoerythrin-conjugated anti-c-Kit monoclonal antibody (BD Pharmingen) for another 30 min. To monitor apoptosis, cells were incubated with 1 μg/ml FITC-labeled annexin V (Clontech) and 2.5 μg/ml propidium iodide (Clontech) at room temperature for 20 min in the dark. Flow cytometric analysis of the stained cells was performed with FACScan or FACSCalibur (Becton Dickinson) equipped with cellquest software.
Measurements of Histamine, Leukotrienes, and Cytokines. Amounts of histamine in BMCMC or in culture supernatants from BMCMC that had been incubated with IgE in the absence of IL-3 were measured as described (18). Leukotrienes C4/D4/E4 released into culture supernatants were quantified by radioimmunoassays by using a commercial kit (Amersham Pharmacia Biotech). Supernatants of BMCMC that had been incubated with IgE in the absence of IL-3 were measured by ELISA for IL-2, IL-6, and tumor necrosis factor (TNF)-α [BD Pharmingen or Endogen (Cambridge, MA)].
Immunoblotting Analysis and Antibodies. Mast cells were lysed in 1% Nonidet P-40-containing lysis buffer (20 mM Tris·HCl, pH 8.0/0.15 M NaCl/1 mM EDTA/1 mM sodium orthovanadate/1 mM phenylmethylsulfonyl fluoride/10 μg/ml aprotinin/10 μg/ml leupeptin/25 μM p-nitrophenyl p′-guanidinobenzoate/1 μM pepstatin/0.1% sodium azide). Cell lysates were analyzed by SDS/PAGE followed by immunoblotting. Antibodies used for probing were anti-phospho-p44/42 MAPK (Thr-202/Tyr-204), anti-phospho-p38 MAPK (Thr-180/Tyr-182), anti-phospho-Akt (Ser-473) (all from Cell Signaling Technology, Beverly, MA), and anti-phosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnology, Lake Placid, NY). Stripped membranes were reprobed with anti-extracellular signal-regulated kinase (ERK) (Zymed), anti-p38 (Santa Cruz Biotechnology), and anti-Akt (Santa Cruz Biotechnology), respectively. Proteins reactive with primary antibody were visualized with a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence reagents (NEN Life Science Products).
Transcription Assays. A luciferase reporter construct, the human TNF-α (-200)/Luc, was described previously (19). Mast cells (1.5 × 107) were transfected with 5–10 μg of reporter plasmid by electroporation at 400 V and 950 μF by using a Gene Pulser II apparatus (Bio-Rad). Transfected cells were incubated with or without 10 μg/ml IgE for 8 h before cell harvest. Cells were lysed in 0.2% Triton X-100 in 100 mM potassium phosphate buffer, pH 7.8/1 mM DTT. Luminescence of cleared lysates was measured after the addition of luciferin solution by using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego).
Time-Resolved Phosphorescence Anisotropy Measurements. IgEs were derivatized with erythrosin (Er) isothiocyanate (Molecular Probes) as previously described (20, 21). Before use, all dye-derivatized proteins were centrifuged at 130,000 × g for 10 min in an Airfuge (Beckman Instruments) to remove any protein aggregates formed during storage. RBL-2H3 rat mast cells were incubated with phosphorescent protein conjugates by using the indicated IgE concentration at 4°C for 1 h. Before phosphorescence measurements, cells were deoxygenated to eliminate phosphorescence quenching by O2. Experiments were performed by using methods previously described (20, 21) as adapted for RBL-2H3 cells. Phosphorescence from deoxygenated cell samples was excited by 532-nm pulses from a neodymium yttrium aluminum garnet (Nd:YAG) laser. Polarized phosphorescence parallel [I∥(t)] and perpendicular [I⊥(t)] to the excitation light was analyzed to yield a phosphorescence intensity function s(t) = [I∥(t)] + 2[I⊥(t)] and a phosphorescence anisotropy function r(t) = ([I∥(t)] – [I⊥(t)])/s(t). Anisotropy data were analyzed according to a single average exponential decay model r(t) = r∞ + (r0 – r∞)exp(–t/ϕ), which yielded the initial anisotropy value r0, the limiting anisotropy value r∞, and the rotational correlation time ϕ.
Hybridoma Transplantation. Hybridoma cells (2 × 106) were injected into the peritoneal cavity of 8- to 10-week-old CAF1/J female mice. Two weeks later, mice were killed, and various tissues were removed and fixed in Carnoy's fixative followed by 1% alcian blue/eosin Y staining. Mast cell numbers were counted by a scientist who was not informed of the sample identity.
Results and Discussion
Different IgEs Exhibit a Wide Spectrum of Cytokine-Producing Ability. To resolve the apparent discrepancies reported for the antiapoptotic mechanisms of monomeric IgEs (5, 6), we first examined the ability of various IgEs to induce cytokine production by mouse BMCMC. Consistent with our previous results (5), H1 DNP-ε-206 (three lots tested) at 5 μg/ml induced no detectable secretion of IL-6 (Fig. 1A), IL-2, or tumor necrosis factor (TNF)-α (data not shown) during a 6- to 8-h incubation. Similarly, monoclonal anti-dansyl antibody 27-74 and anti-trinitrophenyl (TNP) IgE antibodies IgE-3 and C48-2 exhibited little or no cytokine-producing ability. In contrast, substantial amounts of cytokines were secreted from BMCMC treated with H1 DNP-ε-26 (two lots tested), SPE-7 (three lots tested), and C38-2 IgE.
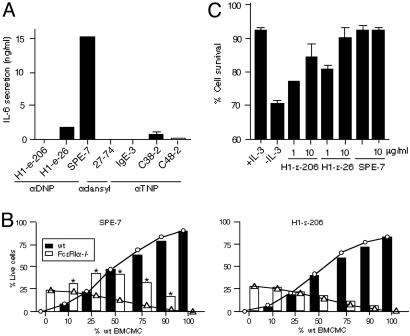
Fig. 1.
In vitro effects of HC and PC IgEs. (A) BMCMC were incubated with the indicated IgEs (5 μg/ml) for 8 h before measurement of IL-6 in culture supernatants. (B) Mixed cultures of WT and FcεRI α–/– BMCMC were incubated with 10 μg/ml SPE-7 or H1 DNP-ε-206 IgE for 3 days without IL-3 or other growth factors before flow cytometric analysis for FcεRI and annexin V. ○, Predicted survival values for WT cells; ▵, predicted survival values for FcεRI α–/– cells. These values are based on the assumption that each type of cell does not affect the survival of the other. Actual results are indicated by filled (WT) or open (FcεRI α–/–) bars. Asterisks indicate differences that are statistically significant (P < 0.05) from the predicted values. (C) WT BMCMC were incubated with or without IL-3, or with the indicated IgE without IL-3, for 3 days before flow cytometric analysis of cell survival.
We then devised a sensitive method to investigate whether the cytokines secreted from BMCMC treated with SPE-7 IgE could support survival of mast cells in an autocrine manner. FcεRI α–/– BMCMC (which cannot respond directly to effects of IgE mediated via FcεRI) and WT BMCMC were mixed at various ratios and incubated with 10 μg/ml SPE-7 or H1 DNP-ε-206 IgE in the absence of growth factors for 3 days. When WT BMCMC were included in the cultures with SPE-7, significantly increased survival of the mixed populations was observed compared with the survival expected if IgE enhanced the survival of WT, but not FcεRI α–/–, BMCMC (Fig. 1B Left). In contrast, H1 DNP-ε-206 IgE did not enhance the survival of FcεRI α–/– BMCMC in the mixed populations (Fig. 1B Right). These results are consistent with the hypothesis that the cytokine(s) secreted from SPE-7 IgE-treated, but not H1 DNP-ε-206 IgE-treated, WT BMCMC support the survival of both WT and FcεRI α–/– BMCMC. We designate those IgEs that can induce significant cytokine secretion, such as SPE-7, H1 DNP-ε-26, and C38-2 (Fig. 1 A), as HC. We designate those IgEs that do not induce significant amounts of cytokine secretion, including H1 DNP-ε-206, 27-74, IgE-3, and C48-2, as PC.
As shown in Fig. 1C, the antiapoptotic potency of H1 DNP-ε-206, H1 DNP-ε-26, and SPE-7 IgEs exhibited the same rank order as their ability to induce cytokine secretion (Fig. 1 A): SPE-7 > H1 DNP-ε-26 > H1 DNP-ε-206. However, even the least cytokinergic IgE (i.e., H1 DNP-ε-206) was able to enhance the survival of WT BMCMC on IL-3 withdrawal (Fig. 1C). The same rank order in the IgEs' ability to induce IL-6 secretion or enhance survival was observed in BMCMC that were tested in serum-free medium (data not shown).
HC IgEs Efficiently Induce Biological Effects in Mast Cells. To investigate the molecular basis for the different biological effects of the various IgE species, we examined the effects of IgE binding on several known consequences of FcεRI aggregation (9, 10). SPE-7 IgE induced a dramatic increase in BMCMC histamine content (Fig. 2A), histamine release (Fig. 2 B and C), leukotriene release (Fig. 2D), FcεRI internalization (Fig. 2E), and DNA synthesis (Fig. 2F). In contrast, H1 DNP-ε-206 IgE failed to induce detectable histamine release, FcεRI internalization, or DNA synthesis, although it did induce a slight increase in histamine content and leukotriene release.
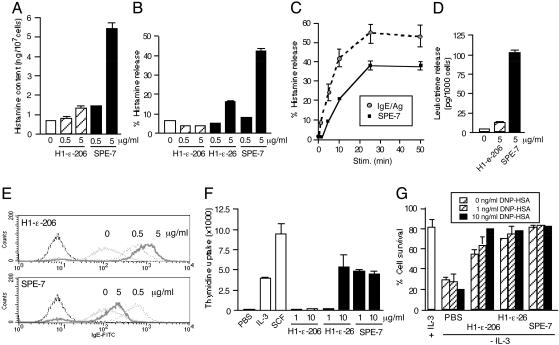
Fig. 2.
Biological outcomes of IgE binding to mast cells in the absence of added growth factors. (A) BMCMC were incubated with the indicated IgEs for 24 h before analysis of cellular content of histamine. (B) BMCMC were incubated with the indicated IgEs for 50 min before analysis of histamine released into culture supernatants. (C) Comparison of kinetics of histamine release induced by 5 μg/ml SPE-7 IgE and that induced from H1 DNP-ε-206 IgE-sensitized BMCMC after stimulation (Stim.) with 100 ng/ml DNP21 HSA. (D) Leukotriene release into culture supernatants from BMCMC incubated with the indicated IgEs for 50 min. (E) Internalization of FcεRI. BMCMC were incubated with the indicated concentrations of H1 DNP-ε-206 or SPE-7 IgE for 24 h before analysis of FcεRI expression. (F) DNA synthesis. BMCMC were incubated with the indicated IgEs for 24 h in the presence of [3H]thymidine for the last 6 h. (G) Effects of low-level cross-linking vs. IgE binding. BMCMC were incubated with the indicated IgEs (5 μg/ml) in the presence or absence of 1 or 10 ng/ml DNP21 HSA for 3 days before survival assays.
We also noticed subtle differences in the degranulation induced by SPE-7 binding alone vs. FcεRI aggregation elicited by sensitization with H1 DNP-ε-206 IgE followed by stimulation with the antigen DNP21 HSA (Fig. 2C). First, the initial rate of degranulation (during 5 min of stimulation) was approximately three times faster with IgE/antigen stimulation than with SPE-7 IgE binding. Second, there was a short lag time (from 1 to 5 min, depending on the experiment) before degranulation with SPE-7 IgE binding, but not with IgE/antigen stimulation.
These observations suggest that IgEs, especially HC IgEs, can induce mast cell activation by a mechanism very similar to that induced by IgE/antigen-dependent FcεRI aggregation. To test this possibility further, we examined the effects of low-level FcεRI aggregation as induced by the addition of low concentrations of known specific antigen on mast cell survival. Typically, FcεRI aggregation with IgE and antigen (Fig. 2C) includes a step of washing unbound IgE off the mast cells before the incubation with antigen. However (apparently depending on the details of the experiment), this protocol for inducing FcεRI aggregation has been reported to induce either enhanced or reduced mast cell proliferation (10). Therefore, in this experiment, we elected to add low concentrations (1–10 ng/ml) of the antigen DNP21 HSA to growth factor-depleted BMCMC cultures in the presence of 5 μg/ml IgE. As shown previously (6), survival induced by SPE-7 IgE was not further enhanced by the presence of the antigen. In contrast, the antigen did enhance the survival effects of H1 DNP-ε-206 and H1 DNP-ε-26 IgEs in a dose-dependent manner (Fig. 2G).
HC IgEs Efficiently Induce Intracellular Signaling Events. HC IgEs induced tyrosine phosphorylation of several proteins and activation of MAPKs (ERK1, ERK2, and p38) and Akt (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). In contrast, PC IgEs induced little or no activation of any of these events. HC IgEs also induced higher levels of transcriptional activity from the tumor necrosis factor (TNF)-α promoter than did PC IgEs (Fig. 7, which is published as supporting information on the PNAS web site).
As expected, incubation of BMCMC with low concentrations of DNP21 HSA in the presence of PC IgEs induced activation events such as tyrosine phosphorylation, MAPK activation, and cytokine production (data not shown). These results, together with those shown in Fig. 2, are consistent with the hypothesis that PC or HC IgEs, or PC IgEs plus low concentrations of antigen, all can induce FcεRI aggregation, but of varying magnitude and kinetics and, therefore, with varying biological consequences.
Evidence That Binding of IgE Induces Aggregation of FcεRI. The above data collectively suggest that FcεRI bound by IgE, particularly HC IgE, is aggregated in a manner that is similar to FcεRI aggregation induced by IgE and antigen. To address this issue more directly, we measured time-resolved phosphorescence anisotropies for Er-conjugated HC and PC IgEs bound to FcεRI on mast cell surfaces. Phosphorescence anisotropy quantifies, in the nano- to microsecond range, motions of phosphorescent dye-labeled membrane proteins, thus assessing the size and rigidity of the membrane structures containing these proteins. Table 1 compares the phosphorescence anisotropy parameters of Er-conjugated SPE-7 IgE with those of H1 DNP-ε-206 IgE. The initial anisotropy r0 represents the degree of orientational order exhibited by all excited-state Er chromophores immediately, i.e., <1 μs, after the excitation laser pulse. Thus, r0 reflects the average anisotropy of all molecules in the sample. The fact that the anisotropies of both Er IgEs increased over time from their initial values (r0) at time 0 to larger limiting anisotropy values (r∞) later may suggest that these IgEs induce spontaneous receptor aggregation. Rising anisotropies typically occur when chromophores with long phosphorescence lifetimes also exhibit large anisotropies. Substantial receptor aggregation would be expected to produce this effect, because Ers on IgEs within the largest receptor aggregates would also have the least accessibility to solution oxygen and, hence, the longest phosphorescence lifetimes. Another important finding is that the initial anisotropy (r0) for the HC IgE was substantially higher than that for the PC IgE. This difference indicates that the average HC IgE molecule is more rigid on the submicrosecond time scale than is the PC IgE, as might be a consequence of more extensive spontaneous receptor aggregation by FcεRI-binding HC IgE.
Table 1. Comparison of FcεRI-bound Er-IgE phosphorescence anisotropy decay at 4°C for the HC Er-SPE-7 vs. PC Er-H1 DNP-ε-206 IgEs.
| IgE | r0 | r∞ | Rotational correlation time, μs | n |
|---|---|---|---|---|
| Er-SPE-7 | 0.033 ± 0.006 | 0.045 ± 0.008 | 27 ± 10 | 11 |
| Er-H1 DNP-ε-206 | 0.016 ± 0.004 | 0.041 ± 0.013 | 52 ± 13 | 6 |
The labeling concentration for each IgE shown was 25 nM. r0, Initial anisotropy; r∞, limiting or long-time anisotropy.
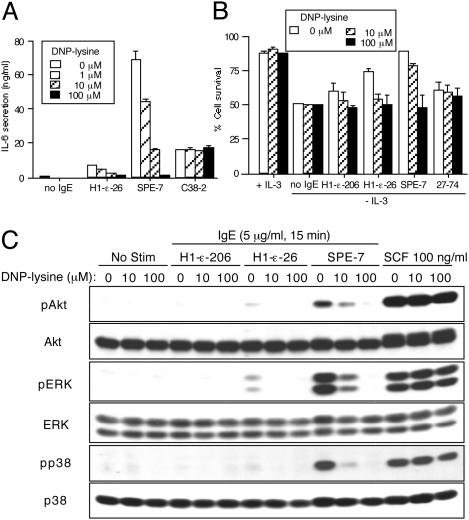
IgE-Mediated Survival and Cytokine-Producing Effects Are Inhibited by Monovalent Hapten. We next investigated the effect of monovalent hapten, which can disengage IgE/antigen-mediated aggregation of FcεRI (22), on IgE-induced cytokine production and survival. Growth factor-depleted BMCMC were incubated with SPE-7 or H1 DNP-ε-26 IgE in the presence of the hapten DNP-lysine. Hapten inhibited IgE-induced IL-6 secretion in a dose-dependent manner (Fig. 3A), with an IC50 of ≈2 μM. In contrast, IL-6 secretion induced by stem cell factor (23) was not affected by DNP-lysine (data not shown). DNP-lysine also inhibited IgE-induced survival (Fig. 3B). In control experiments, DNP-lysine influenced neither IL-6 secretion induced by C38-2 (Fig. 3A) nor the survival effect elicited by 27-74 (Fig. 3B). Using HC IgE with a different specificity, C38-2, and a corresponding hapten, trinitrophenyl (TNP) glutamate, we again showed that hapten inhibited IgE-induced IL-6 production and survival in a dose-dependent manner (data not shown). Hapten also inhibited HC IgE-induced, but not stem cell factor-induced, activation of MAPKs and Akt (Fig. 3C), as well as FcεRI internalization (data not shown), but it did not affect the ability of IgE to bind to FcεRI (data not shown). DNP-lysine treatment markedly reduced both initial and limiting anisotropies for both SPE-7 and H1 DNP-ε-206 IgEs (data not shown). These results indicate that monovalent hapten-bound IgE cannot assume a ligand/receptor configuration that allows FcεRI aggregation.
Fig. 3.
Inhibition of IgE-induced mast cell cytokine secretion, survival, and signaling by monovalent hapten. (A) BMCMC were incubated with the indicated IgEs (5 μg/ml) in the presence of the indicated concentrations of hapten for 8 h before measurement of IL-6. (B) BMCMC were incubated with the indicated IgEs (5 μg/ml) in the presence of the indicated concentrations of hapten, but without IL-3, for 3 days before survival assays. (C) BMCMC were incubated with the indicated IgEs (5 μg/ml) in the presence of the indicated concentrations of DNP-lysine for 15 min. For comparison, cells were stimulated with 100 ng/ml stem cell factor (SCF) for 15 min. Cell lysates were subjected to immunoblotting with anti-phospho-ERK (pERK), anti-phospho-p38 (pp38), or anti-phospho-Akt (pAkt). No Stim, incubation with PBS.
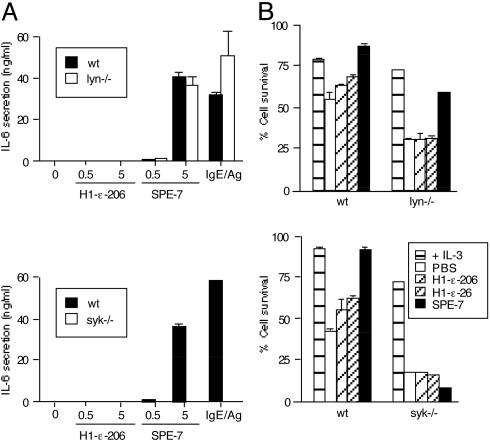
IgE-Mediated Survival and Cytokine-Producing Effects Depend on Syk. Degranulation and other activation events elicited by FcεRI aggregation with IgE and antigen are totally abrogated by deficiency of the protein tyrosine kinase Syk (24, 25). Strikingly, syk–/– BMCMC failed to produce and secrete cytokines or to exhibit antiapoptotic effects in response to either HC or PC IgEs (Fig. 4). In contrast, syk–/– BMCMC secreted IL-6 on stimulation with Escherichia coli lipopolysaccharides (ref. 23 and data not shown). Both PC and HC IgEs rendered btk–/– BMCMC resistant to growth factor-depletion-induced apoptosis (data not shown), whereas HC, but not PC, IgEs rendered lyn–/– BMCMC resistant (Fig. 4B). Moreover, HC IgEs induced levels of IL-6 secretion in lyn–/– (Fig. 4A) and btk–/– (data not shown) BMCMC that were very similar to those in WT cells. All of these mutant and WT BMCMC express comparable levels of FcεRI and c-Kit on their cell surfaces (data not shown). Therefore, these results demonstrate that Syk, but not Lyn or Btk, is absolutely required for IgE-mediated survival effects and HC IgE-mediated cytokine production/secretion.
Fig. 4.
Syk is required for HC IgE-induced cytokine production and IgE-induced survival. (A) Lyn–/– or syk–/– BMCMC and control (WT) cells were incubated with the indicated IgEs for 8 h before measurement of IL-6. For comparison, cells were sensitized overnight with 0.5 μg/ml 206 IgE and stimulated with 100 ng/ml DNP21 HSA for 8 h. (B) Lyn–/– or syk–/– BMCMC were incubated with the indicated IgEs (5 μg/ml) but without IL-3 for 3 days before survival assays.
IgE Levels Correlate with Mast Cell Numbers in Some Mucosal Tissues of Hybridoma-Transplanted Mice. To analyze IgE survival effects in more pathophysiological settings, we first examined whether PC IgE can interfere with the survival effect induced by HC IgE or vice versa. To this end, BMCMC were incubated with a 10 μg/ml IgE solution composed of H1 DNP-ε-206 and SPE-7 IgEs at various ratios. Survival rates correlated positively with the amount of HC SPE-7 IgE in the IgE mixtures (Fig. 7). These results indicate that the survival effect induced by HC IgE can be observed in the presence of much higher concentrations of PC IgE. Second, a mixture of several monoclonal IgEs exhibited a survival effect that was approximately an average of those of the individual IgEs (Fig. 7).
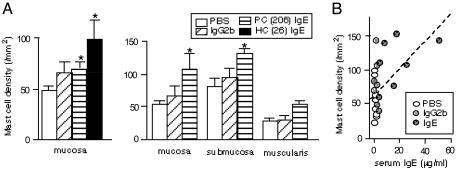
Third, we tested whether IgE binding can influence mast cell numbers in vivo. We transplanted IgE- or IgG2b-secreting hybridomas into the peritoneal cavity of CAF1/J mice, and mast cells in various tissues were enumerated two weeks after transplantation. Mast cell numbers in the stomach mucosa of the mice harboring H1 DNP-ε-206 or H1 DNP-ε-26 IgE hybridomas (11) were higher than in those harboring an anti-DNP IgG2b hybridoma or in those injected with PBS (Fig. 5A), and mice harboring H1 DNP-ε-26 IgE hybridoma cells had more stomach mucosal mast cells than those harboring H1 DNP-ε-206 IgE hybridoma cells. Similar findings were observed in the mucosa of the jejunum. However, in two of three experiments, there were no significant differences in the mast cell numbers in other tissues in which mast cell turnover is not as rapid as in the gastrointestinal mucosa (26), such as back or ear skin or the muscularis propria of the stomach. Importantly, there was a significant correlation between mast cell numbers in the mucosa of the stomach or jejunum and levels of serum IgE, but not IgG2b (Fig. 5B and data not shown). These experiments do not define the mechanism(s) linking elevated levels of circulating IgE with increased numbers of mucosal mast cells in vivo. However, the data are consistent with the hypothesis that IgE can enhance mast cell development and/or survival in vivo.
Fig. 5.
Effects of IgE on mast cell numbers in vivo. Hybridoma cells secreting H1 DNP-ε-206 or H1 DNP-ε-26 IgE and hybridoma cells secreting anti-DNP IgG2b or PBS were inoculated i.p. into CAF1/J mice. Mice were killed 2 weeks later. Serum IgE and IgG2b levels were measured by ELISA, and mast cells were counted in various tissues. (A) Data for the stomach are shown for two of three separate experiments performed. (Left) n = 6–8. (Right) n = 4. Asterisks indicate statistical significance; P < 0.05 vs. values in PBS-injected mice. (B) Correlation coefficients for mast cell numbers in the stomach mucosa and serum Ig from the experiment shown on the right in A were 0.534 (P = 0.0077) for serum IgE and 0.243 (P = 0.2675) for serum IgG2b.
Conclusions
We have demonstrated that binding of various IgEs by mast cells can induce a spectrum of activation events in the absence of antigen for which the IgE is known to have specificity. HC IgEs can promote mast cell survival more strongly than PC IgEs, presumably in part by inducing secretion of cytokines, whereas PC IgEs also can enhance mast cell survival, but less strongly and by an apparently cytokine-independent mechanism. However, the simplest (albeit not the only) explanation for all of our data is that PC and HC IgEs can induce a spectrum of FcεRI aggregation, associated with a corresponding spectrum of effects on mast cell signaling, survival, FcεRI internalization, and mediator/cytokine release. Whether “monomeric” IgE can enhance mast cell survival and/or mediator secretion by a mechanism that involves FcεRI aggregation (as our data strongly suggest) or by some other process, the structural features that account for the functional differences between HC and PC IgEs remain to be identified.
Potentially related to this issue, a recent study (27) showed that the SPE-7 antibody can adopt different antigen binding site conformations before antigen binding and that binding of different antigens can induce isomerization of the binding site, leading to high-affinity complexes with a deep or narrow binding site. Given this and related studies (28) and the results of our experiments with monovalent hapten (Fig. 3), one could hypothesize that IgEs can induce FcεRI aggregation in the absence of the antigen for which those IgEs are known to have specificity when the Fab fragment of an FcεRI-bound IgE molecule interacts with a neighboring FcεRI-bound IgE directly or indirectly via a third molecule whose epitopes are recognized by neighboring IgEs. For example, in the case of anti-DNP IgE, a cross-reactive epitope within IgE or another molecule might be bound by anti-DNP IgE. Regardless of the nature of the mechanism(s) by which IgE might enhance mast cell survival and/or mediator secretion in the absence of the antigens for which that IgE is known to have specificity, our findings offer a perspective on the well established and highly positive correlation between serum levels of IgE and the prevalence of asthma and other allergic diseases (29). In addition to enhancing antigen-dependent mast cell activation and mediator/cytokine secretion by increasing surface expression of FcεRI (3, 4), high levels of IgE also may promote mast cell activation and survival independently of the presence of antigens for which the IgE is known to have specificity. One may even speculate that the severity of allergic disorders in some individuals may reflect a predisposition to secrete one type of IgE (HC) over others (PC).
Supplementary Material
Acknowledgments
We thank Robert McHenry for excellent technical help; Zhen-Sheng Wang for histological analysis; Gerry Krystal and Zelig Eshhar for providing valuable reagents; and Marc Daeron, David Holowka, Howard Katz, Salaheddine Mecheri, and Carl Ware for helpful advice during the course of this study. This work was supported in part by National Institutes of Health grants (to T.K., F.-T.L., C.A.L., B.G.B., and S.J.G.) and a National Science Foundation grant (to B.G.B.). This is Publication 551 from the La Jolla Institute for Allergy and Immunology.
Abbreviations: MAPK, mitogen-activated protein kinase; IgEs, IgE molecules; HC, highly cytokinergic; PC, poorly cytokinergic; DNP, dinitrophenyl; BMCMC, bone marrow-derived cultured mast cells; HSA, human serum albumin; ERK, extracellular signal-regulated kinase; Er, erythrosin.
References
- 1.Metzger, H. (1992) Immunol. Rev. 125 37–48. [DOI] [PubMed] [Google Scholar]
- 2.Turner, H. & Kinet, J. P. (1999) Nature 402 B24–B30. [DOI] [PubMed] [Google Scholar]
- 3.Hsu, C. & MacGlashan, D., Jr. (1996) Immunol. Lett. 52 129–134. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi, M., Lantz, C. S., Oettgen, H. C., Katona, I. M., Fleming, T., Miyajima, I., Kinet, J. P. & Galli, S. J. (1997) J. Exp. Med. 185 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asai, K., Kitaura, J., Kawakami, Y., Yamagata, N., Tsai, M., Carbone, D. P., Liu, F. T., Galli, S. J. & Kawakami, T. (2001) Immunity 14 791–800. [DOI] [PubMed] [Google Scholar]
- 6.Kalesnikoff, J., Huber, M., Lam, V., Damen, J. E., Zhang, J., Siraganian, R. P. & Krystal, G. (2001) Immunity 14 801–811. [DOI] [PubMed] [Google Scholar]
- 7.Borkowski, T. A., Jouvin, M. H., Lin, S. Y. & Kinet, J. P. (2001) J. Immunol. 167 1290–1296. [DOI] [PubMed] [Google Scholar]
- 8.Kubo, S., Matsuoka, K., Taya, C., Kitamura, F., Takai, T., Yonekawa, H. & Karasuyama, H. (2001) J. Immunol. 167 3427–3434. [DOI] [PubMed] [Google Scholar]
- 9.Kinet, J. P. (1999) Annu. Rev. Immunol. 17 931–972. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami, T. & Galli, S. J. (2002) Nat. Rev. Immunol. 2 773–786. [DOI] [PubMed] [Google Scholar]
- 11.Liu, F. T., Bohn, J. W., Ferry, E. L., Yamamoto, H., Molinaro, C. A., Sherman, L. A., Klinman, N. R. & Katz, D. H. (1980) J. Immunol. 124 2728–2737. [PubMed] [Google Scholar]
- 12.Ishizaka, K. (1985) Methods Enzymol. 116 76–94. [DOI] [PubMed] [Google Scholar]
- 13.Eshhar, Z., Ofarim, M. & Waks, T. (1980) J. Immunol. 124 775–780. [PubMed] [Google Scholar]
- 14.Mayr, S. I., Zuberi, R. I., Zhang, M., de Sousa-Hitzler, J., Ngo, K., Kuwabara, Y., Yu, L., Fung-Leung, W. P. & Liu, F. T. (2002) J. Immunol. 169 2061–2068. [DOI] [PubMed] [Google Scholar]
- 15.Khan, W. N., Alt, F. W., Gerstein, R. M., Malynn, B. A., Larsson, I., Rathbun, G., Davidson, L., Muller, S., Kantor, A. B., Herzenberg, L. A., et al. (1995) Immunity 3 283–299. [DOI] [PubMed] [Google Scholar]
- 16.Chan, V. W., Meng, F., Soriano, P., DeFranco, A. L. & Lowell, C. A. (1997) Immunity 7 69–81. [DOI] [PubMed] [Google Scholar]
- 17.Turner, M., Mee, P. J., Costello, P. S., Williams, O., Price, A. A., Duddy, L. P., Furlong, M. T., Geahlen, R. L. & Tybulewicz, V. L. (1995) Nature 378 298–302. [DOI] [PubMed] [Google Scholar]
- 18.Siraganian, R. P. (1974) Anal. Biochem. 57 383–394. [DOI] [PubMed] [Google Scholar]
- 19.Hata, D., Kitaura, J., Hartman, S. E., Kawakami, Y., Yokota, T. & Kawakami, T. (1998) J. Biol. Chem. 273 10979–10987. [DOI] [PubMed] [Google Scholar]
- 20.Song, J., Hagen, G. M., Roess, D. A., Pecht, I. & Barisas, B. G. (2002) Biochemistry 41 881–889. [DOI] [PubMed] [Google Scholar]
- 21.Song, J., Hagen, G., Smith, S., Roess, D., Pecht, I. & Barisas, B. (2002) Mol. Immunol. 38 1315–1321. [DOI] [PubMed] [Google Scholar]
- 22.Pribluda, V. S. & Metzger, H. (1987) J. Biol. Chem. 262 11449–11454. [PubMed] [Google Scholar]
- 23.Gagari, E., Tsai, M., Lantz, C. S., Fox, L. G. & Galli, S. J. (1997) Blood 89 2654–2663. [PubMed] [Google Scholar]
- 24.Zhang, J., Berenstein, E. H., Evans, R. L. & Siraganian, R. P. (1996) J. Exp. Med. 184 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello, P. S., Turner, M., Walters, A. E., Cunningham, C. N., Bauer, P. H., Downward, J. & Tybulewicz, V. L. (1996) Oncogene 13 2595–2605. [PubMed] [Google Scholar]
- 26.Walker, B. E. (1961) Nature 192 980–981. [DOI] [PubMed] [Google Scholar]
- 27.James, L. C., Roversi, P. & Tawfik, D. S. (2003) Science 299 1362–1367. [DOI] [PubMed] [Google Scholar]
- 28.Foote, J. (2003) Science 299 1327–1328. [DOI] [PubMed] [Google Scholar]
- 29.Burrows, B., Martinez, F. D., Halonen, M., Barbee, R. A. & Cline, M. G. (1989) N. Engl. J. Med. 320 271–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.