Abstract
Vitamin E is a generic term for a group of lipid-soluble antioxidant compounds, the tocopherols and tocotrienols. While tocotrienols are considered as important vitamin E components in humans, with functions in health and disease, the protective functions of tocotrienols have never been investigated in plants, contrary to tocopherols. We took advantage of the strong accumulation of tocotrienols in leaves of double transgenic tobacco (Nicotiana tabacum) plants that coexpressed the yeast (Saccharomyces cerevisiae) prephenate dehydrogenase gene (PDH) and the Arabidopsis (Arabidopsis thaliana) hydroxyphenylpyruvate dioxygenase gene (HPPD) to study the antioxidant function of those compounds in vivo. In young leaves of wild-type and transgenic tobacco plants, the majority of vitamin E was stored in thylakoid membranes, while plastoglobules contained mainly δ-tocopherol, a very minor component of vitamin E in tobacco. However, the vitamin E composition of plastoglobules was observed to change substantially during leaf aging, with α-tocopherol becoming the major form. Tocotrienol accumulation in young transgenic HPPD-PDH leaves occurred without any significant perturbation of photosynthetic electron transport. Tocotrienols noticeably reinforced the tolerance of HPPD-PDH leaves to high light stress at chilling temperature, with photosystem II photoinhibition and lipid peroxidation being maintained at low levels relative to wild-type leaves. Very young leaves of wild-type tobacco plants turned yellow during chilling stress, because of the strongly reduced levels of chlorophylls and carotenoids, and this phenomenon was attenuated in transgenic HPPD-PDH plants. While sugars accumulated similarly in young wild-type and HPPD-PDH leaves exposed to chilling stress in high light, a substantial decrease in tocotrienols was observed in the transgenic leaves only, suggesting vitamin E consumption during oxygen radical scavenging. Our results demonstrate that tocotrienols can function in vivo as efficient antioxidants protecting membrane lipids from peroxidation.
The term “vitamin E” describes the biological activity of a group of structurally related compounds, the tocochromanols, in animals and humans. Vitamin E is considered to be an essential, lipid-soluble nutrient that functions as an antioxidant protecting polyunsaturated fatty acids against lipid peroxidation (Fukuzawa and Gebicky, 1983; Fryer, 1992; Brigelius-Flohe and Traber, 1999; Bramley et al., 2000). Natural vitamin E includes four tocopherols and four tocotrienols, which are synthesized exclusively by oxygenic photosynthetic organisms. Tocopherols consist of a chromanol ring and a 20-carbon tail derived from the aromatic compound homogentisate and phytyl diphosphate, respectively. Tocotrienols differ structurally from tocopherols by the presence of three trans-double bonds in the hydrocarbon tail, which derive from geranylgeranyl diphosphate.
The first step in vitamin E synthesis involves the production of homogentisate from hydroxyphenylpyruvate (HPP) in a complex enzymatic reaction involving HPP dioxygenase (HPPD; Fiedler et al., 1982; Garcia et al., 1997; DellaPenna and Pogson, 2006). HPPD has a key location in the tocochromanol pathway and is an important activity regulating tocochromanol fluxes in plants. However, overexpression of HPPD in Arabidopsis (Arabidopsis thaliana) or tobacco (Nicotiana tabacum) brought about modest increases in seed and leaf tocopherols (Tsegaye et al., 2002; Falk et al., 2003). This is due to the fact that the flux to HPP is an additional limiting step that is tightly regulated by feedback inhibition of arogenate dehydrogenase by its product Tyr (Rippert and Matringe, 2002). Rippert et al. (2004) succeeded in bypassing this feedback inhibition by expressing in tobacco a yeast (Saccharomyces cerevisiae) prephenate dehydrogenase (PDH) that catalyzes HPP production directly from prephenate. Although PDH expression alone had minor effects on tocochromanol levels, the simultaneous expression of yeast PDH and Arabidopsis HPPD in tobacco resulted in a strong increase (up to 8-fold) in leaves, indicating that both steps together are limiting for vitamin E synthesis in tobacco. However, a surprising result was that the increased tocochromanols in PDH-HPPD overexpressors were almost entirely because of tocotrienols, which are normally produced in tobacco seeds but not in tobacco leaves. Indeed, in plants, tocopherols and tocotrienols are distributed differently between organs, with tocotrienols being synthesized in seeds of some species, mainly monocotyledonous (Horvath et al., 2006), by a seed-specific geranylgeranyl homogentisate transferase (Cahoon et al., 2003). According to Rippert et al. (2004), it is possible that the high homogentisate flux in the double transgenic plants somehow altered the substrate specificity of homogentisate phytyl transferase so that it was able to use geranylgeranyl diphosphate as a cosubstrate, thus resulting in tocotrienol synthesis. Similarly, coexpression of a bacterial PDH and the Arabidopsis HPPD in soybean (Glycine max) was reported to cause a strong accumulation of tocotrienols in seeds (Karunanandaa et al., 2005). In Arabidopsis, expression of the barley (Hordeum vulgare) seed-specific homogentisate geranylgeranyl transferase under the control of the 35S promoter is another strategy that successfully led to tocotrienol accumulation in leaves (Cahoon et al., 2003).
α-Tocopherol is the most abundant form of vitamin E in nature, with the highest bioavailability in the human body (Traber and Sies, 1996). Vitamin E research, therefore, has mainly focused on this compound and has neglected the other vitamin E molecules. However, tocopherols and tocotrienols have similar antioxidant activities in vitro (Serbinova et al., 1991; Serbinova and Packer, 1994; Yoshida et al., 2003). Moreover, recent developments indicate that the members of the vitamin E family are not redundant with respect to their biological functions. For instance, in tobacco leaves, α-tocopherol and γ-tocopherol have been shown to provide protection against different stress conditions (Abbasi et al., 2007). In humans, tocotrienols have emerged as unsaturated vitamin E molecules with functions in health and disease that are clearly distinct from that of α-tocopherol (Theriault et al., 1999; Sen et al., 2006). While different functions of tocopherols have been identified and characterized in vascular plants and cyanobacteria (Trebst et al., 2002; Sattler et al., 2004; Havaux et al., 2005; Maeda et al., 2006; Munné-Bosch et al., 2007), the physiological role of tocotrienols is not yet documented in plants. Because of the considerable accumulation of tocotrienols in their vegetative organs, the HPPD-PDH double transgenic tobacco plants described above provide a unique material to investigate their antioxidative function in vivo. This study is centered on those tocopherol-biofortified plants, which are compared with the wild type under normal and photooxidative stress conditions. The results presented show that tocotrienols function in vivo as efficient photoprotectors and lipid antioxidants.
RESULTS
Tocopherol and Tocotrienol Levels and Distribution
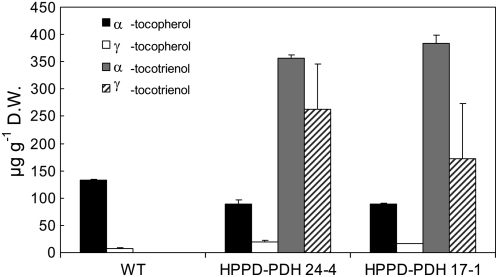
The major form of vitamin E in wild-type tobacco leaves was α-tocopherol (Fig. 1), but relatively small amounts of γ-tocopherol (Fig. 1) and traces of δ-tocopherol (data not shown) were also detected. As reported previously (Rippert et al., 2004), expression of yeast PDH in HPPD transgenic tobacco plants brought about a massive accumulation of tocotrienols in leaves (Fig. 1). Besides α-, γ-, and δ-tocopherol molecules, whose concentrations were close to the wild-type level, double transgenic leaves (lines 24-4 and 17-1) contained very high amounts of α- and γ-tocotrienols (Fig. 1) and traces of δ-tocotrienol (data not shown). A small increase in the γ:α-tocopherol ratio was observed, however, in HPPD-PDH transgenic leaves relative to wild-type leaves. The total vitamin E content (tocopherols + tocotrienols) of the double transgenic lines was increased by a factor of about 5 compared with the wild-type level, with tocotrienols representing about 85% of vitamin E. The tocotrienol level reached by transgenic leaves (approximately 0.6 mg g−1 dry weight) was comparable to that reported by Cahoon et al. (2003) in Arabidopsis plants overexpressing barley homogentisate geranylgeranyl transferase.
Figure 1.
Vitamin E content (tocopherols and tocotrienols) of wild-type and double transgenic HPPD-PDH tobacco leaves (lines 17-1 and 24-4) grown under control conditions (70 μmol m−2 s−1 and 25°C). D.W., Dry weight. Data are mean values of three separate experiments + sd.
Intact chloroplasts isolated from young developing tobacco leaves by centrifugation on a Percoll gradient were fractionated into three fractions by ultracentrifugation on a Suc gradient (Supplemental Fig. S1A), which correspond to the three lipid structures of the chloroplast: the thylakoid membranes, the envelope membranes, and the plastoglobules (Lichtenthaler, 1968). The green pellet at the bottom of the tube contained the thylakoid membranes, while the yellowish band in the upper part of the tube contained plastoglobules. The third band between the plastoglobules and the thylakoids was strongly enriched in envelope membranes. The purity of the preparations was controlled by Western blots of proteins specific to each fraction (Supplemental Fig. S1B). The major chlorophyll-binding antenna protein light-harvesting complex protein (LHCP) was found exclusively in the thylakoid fraction, as expected. ceQORH, a protein of the inner envelope membrane (Miras et al., 2002), was present in the envelope-enriched fraction only. None of those proteins was found in the plastoglobules, confirming the purity of this fraction. Tocopherol cyclase VTE1, a plastoglobule marker, was detected in the plastoglobules and also in the envelope-enriched fraction. Since the chloroplast envelope is supposed to be derived from tocopherol cyclase activity (Soll et al., 1985; Vidi et al., 2006), we speculate that the envelope fraction was contaminated by plastoglobules. Accordingly, when this fraction was washed and recentrifuged, VTE1 was not detected anymore in the resulting purified fraction, while ceQORH was still present (Supplemental Fig. S1C).
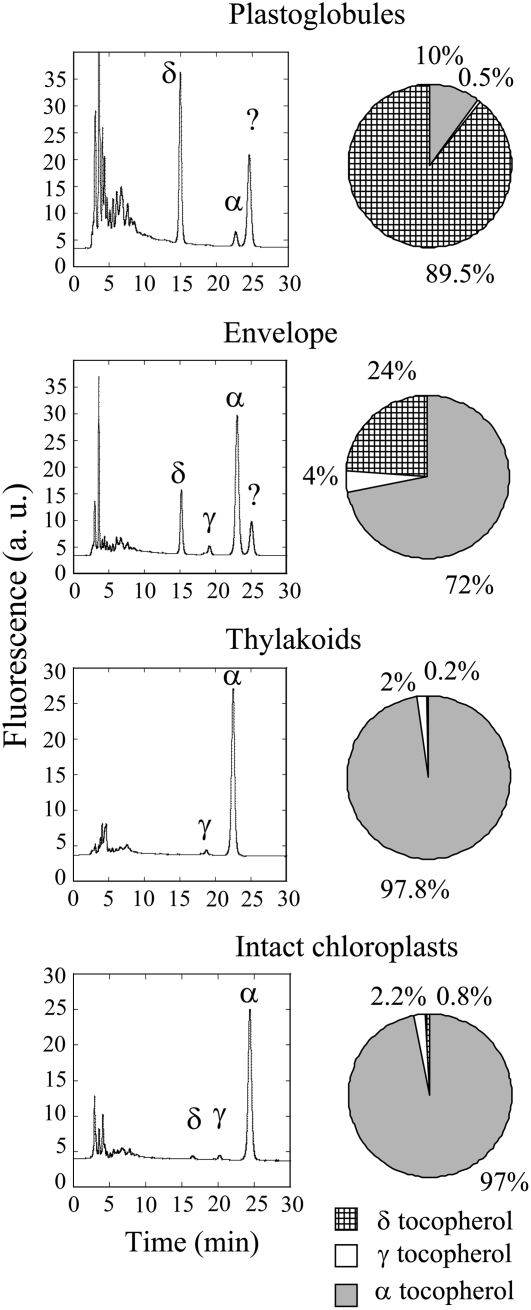
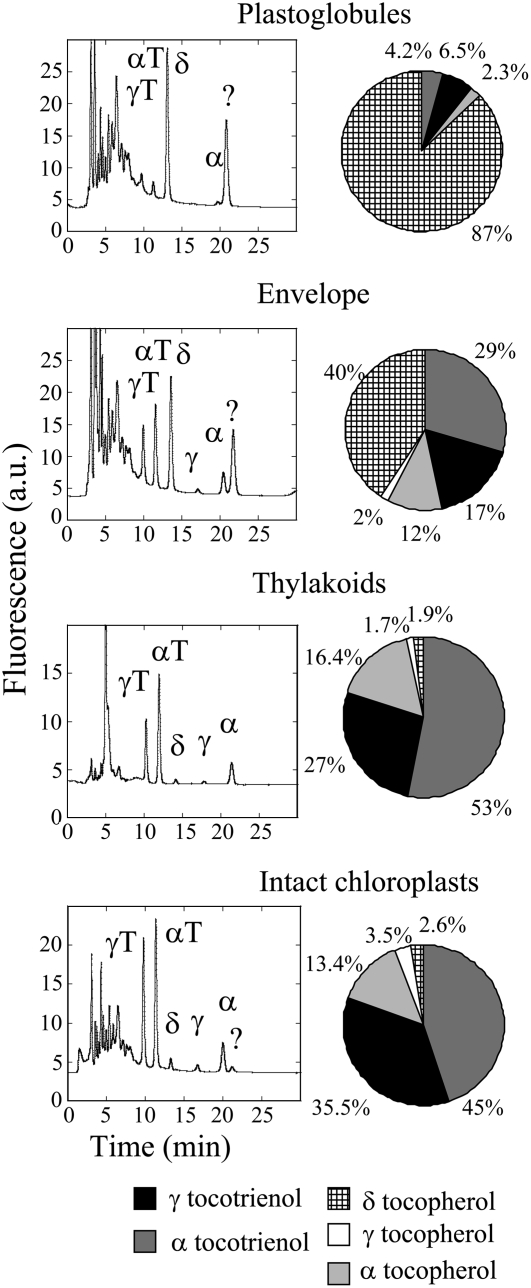
Total lipids of each chloroplast fraction were extracted with a chloroform-methanol mixture according to Folch et al. (1957), and the vitamin E content of each fraction was determined by HPLC (Fig. 2) using standards of the eight vitamin E compounds (α-, β-, γ-, and δ-tocopherols and -tocotrienols; Supplemental Fig. S2). Figure 2 (right) summarizes the quantification of the vitamin E distribution in each lipid fraction. Thylakoids of wild-type chloroplasts were enriched in α-tocopherol and contained also γ-tocopherol, with the α:γ-tocopherol ratio (approximately 15:1) being close to that measured in leaves or in intact chloroplasts (Fig. 2). δ-Tocopherol represented less than 1% of vitamin E in this fraction. In contrast, the plastoglobule fraction contained mainly δ-tocopherol (approximately 90%). The vitamin E content of the envelope-enriched lipid fraction appeared to be intermediate between that of plastoglobules and thylakoids, with a mixture of δ-tocopherol and α-tocopherol. The same pattern was observed in transgenic chloroplasts: the major form of vitamin E in plastoglobules was δ-tocopherol, whereas the vitamin E profile of the thylakoid membranes (i.e. α-tocopherol and the different forms of tocotrienols) was similar to that measured in leaves and intact chloroplasts (Fig. 3).
Figure 2.
Chromatograms of vitamin E in the different membrane fractions of wild-type chloroplasts, and vitamin E composition in plastoglobules, thylakoids, envelopes, and intact chloroplasts in wild-type plants. The experiments were repeated three times with similar results. The peak labeled ? corresponds to an unknown compound. α, γ, and δ represent α-, γ-, and δ-tocopherol, respectively. a.u., Arbitrary units.
Figure 3.
Chromatograms of vitamin E in the different membrane fractions of HPPD-PDH transgenic chloroplasts, and vitamin E composition in plastoglobules, thylakoids, envelopes, and intact chloroplasts in HPPD-PDH transgenic plants. αT and γT represent α-tocotrienol and γ-tocotrienol, respectively. See the legend of Figure 2 for the other symbols.
Photosynthetic Electron Transport
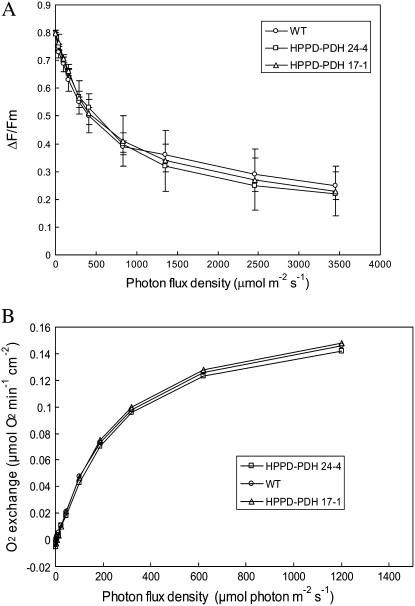
The quantum yield of PSII-mediated electron transport was measured by chlorophyll fluorometry at different photon flux densities (PFDs), and no significant difference was found between wild-type and transgenic plants (Fig. 4A). Neither the maximal photochemical efficiency of PSII (Fv/Fm; measured at PFD = 0 in Fig. 4A) nor the light saturation curve of the electron transport quantum yield were changed in tocotrienol-accumulating plants relative to wild-type plants. We also measured photosynthetic oxygen evolution at different PFDs using a Clark electrode (Fig. 4B). Again, transgenic leaves could not be distinguished from wild-type leaves. Therefore, we can conclude that massive accumulation of tocotrienols in thylakoid membranes of young HPPD-PDH leaves did not perturb the photochemical activity.
Figure 4.
Photosynthetic characteristics of wild-type and transgenic tobacco leaves (lines HPPD-PDH 17-1 and 24-4) grown under regular conditions. A, Quantum yield of PSII-mediated electron transport measured in attached leaves by the ΔF/Fm chlorophyll fluorescence parameter at different PFDs. Data are mean values of three separate experiments ± sd. B, Oxygen exchange by leaf discs (3.5 cm in diameter) at different PFDs. Each curve is from a representative experiment that was repeated at least three times with similar results.
Levels of Antioxidants in HPPD-PDH Transgenic Tobacco Leaves
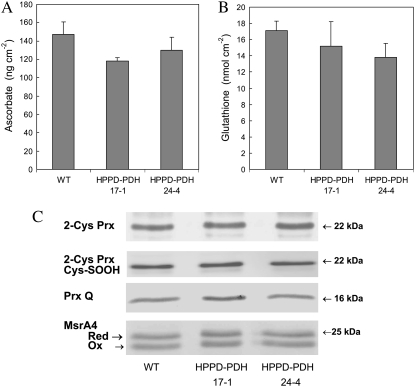
Engineering of soybean using Arabidopsis HPPD and bacterial PDH has been reported to cause large increases in tocotrienols in seeds but also massive accumulation of the precursor homogentisate (Karunanandaa et al., 2005). This was not the case in our transgenic plants: the homogentisate level, measured using HPLC (Garcia et al., 1997), was below the detection limit (<1 nmol) in both wild-type and HPPD-PDH leaves (data not shown). Similarly, the carotenoid level was not modified in the HPPD-PDH leaves (Rippert et al., 2004). We also measured two major antioxidant compounds, ascorbate and glutathione (Fig. 5, A and B), and the abundance of a number of antioxidant enzymes, peroxyredoxin Q (Prx Q), 2-Cys peroxyredoxin (2-Cys Prx), and Met sulfoxide reductase A4 (MsrA4; Fig. 5C). Prxs can detoxify organic peroxides (Dietz, 2003), while Msrs regenerate Met sulfoxide back to Met and are thus involved in protein repair during oxidative stress conditions (Rouhier et al., 2006). None of those antioxidant systems was significantly affected by tocotrienol accumulation. Moreover, the reduction levels of both ascorbate and glutathione were similar (approximately 90%) in wild-type and transgenic plants. Similarly, the ratio between reduced and oxidized MsrA4 (Vieira Dos Santos et al., 2005) and the amount of overoxidized Cys in 2-Cys Prx (i.e. the level of Cys sulfinic acid form; compare with Rey et al. [2007]) were unchanged in the transgenic plants. Thus, tocotrienol accumulation did not seem to significantly perturb the antioxidant machinery of the chloroplasts.
Figure 5.
Total ascorbate (A), total glutathione (B), and abundance of several antioxidant enzymes (Prx Q, 2-Cys Prx, and MsrA4) as determined by western blot (C). The redox state of 2-Cys Prx was also measured by western blot using a serum raised against sulfinylated and sulfonylated forms of 2-Cys Prx (Cys-SOOH). Soluble protein loadings were as follows: 2-Cys Prx, 5 μg; 2-Cys Prx Cys-SOOH, 15 μg; Prx Q, 25 μg; MsrA4, 20 μg. It was determined in separate experiments that those concentrations are in the linear range of the immunological reaction. WT, Wild type.
Tolerance to Photooxidative Stress and PSII Photoinhibition
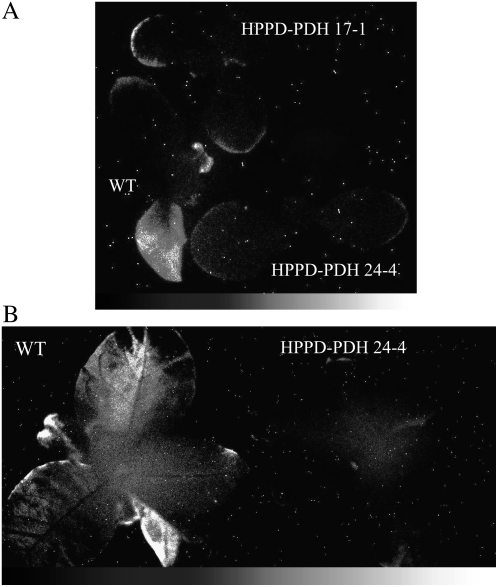
Tobacco plants grown under control conditions (25°C, 150 μmol photons m−2 s−1) were suddenly exposed to a higher PFD (700 μmol photons m−2 s−1) at low temperature (10°C). The combination of light and low temperature is very favorable for inducing photooxidative stress, especially in chilling-sensitive plants such as tobacco (Wise, 1995). After 4 d of such treatment, lipid peroxidation and oxidative stress were visualized by imaging leaf autoluminescence. The latter technique measures the faint light emitted by singlet oxygen and triplet carbonyls, the by-products of the slow spontaneous decomposition of lipid hydroperoxides and endoperoxides (Devaraj et al., 1997; Vavilin and Ducruet, 1998; Havaux et al., 2006). Deactivation of excited carbonyls and singlet oxygen produces photons in the blue and red spectral regions, respectively. The latter photon emissions, although very weak, can be recorded with a high-sensitivity, liquid nitrogen-cooled CCD camera (Havaux et al., 2006). This technique has been used to map lipid peroxidation and oxidative stress in various biological materials, including detached leaves (Flor-Henry et al., 2004), whole plants (Johnson et al., 2007; Collin et al., 2008), animals (Kobayashi et al., 1999), and humans (van Wijk et al., 2006). As shown in plants aged 25 or 40 d (Fig. 6, A and B, respectively), spontaneous photon emission by wild-type plants was noticeably enhanced relative to HPPD-PDH plants after high light stress at low temperature. Photon emission was greatest in the oldest wild-type leaves. In transgenic leaves, spontaneous photon emission was weak, being essentially restricted to the margins of some leaves.
Figure 6.
Autoluminescence imaging of oxidative stress in wild-type and transgenic tobacco leaves (lines HPPD-PDH 24-4 and 17-1). Plants aged 25 d (A) or 40 d (B) were exposed for 4 d to high light stress at low temperature (10°C, 700 μmol m−2 s−1, 14-h photoperiod). Spontaneous photon emission was imaged after a dark adaptation period of 2 h. For B, similar to the HPPD-PDH 24-4 transgenic line, leaves of the 17-1 line emitted very little light after the light stress treatment (data not shown). Randomly distributed white spots are the results of stray radiation in the range of gamma and cosmic rays.
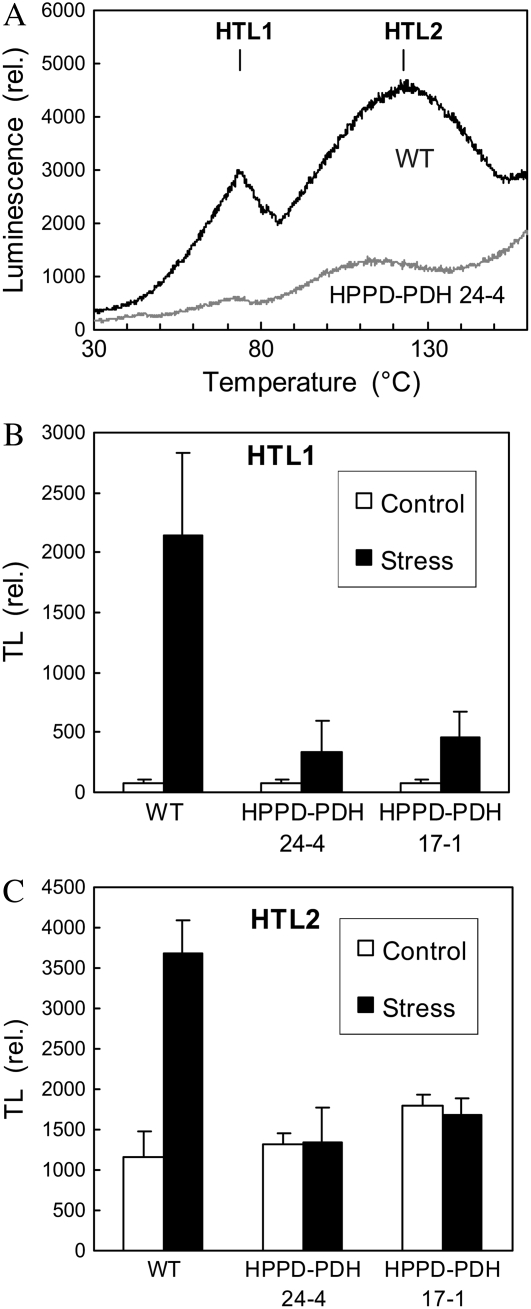
Thermoluminescence (TL) provides another convenient method for quantifying lipid peroxidation (Havaux, 2003). This method consists of slowly heating the leaf sample to 150°C to provoke the thermal decomposition of lipid hydroperoxides and hence to generate excited carbonyls. This leads to a luminescence burst at approximately 110°C to 130°C, as shown in Figure 7A (HTL2 peak) for tobacco plants exposed for 4 d to high light at low temperature. In tobacco, the HTL2 peak is preceded by another peak of lower intensity at approximately 75°C, as reported previously (Havaux et al., 2003). This first peak (HTL1) has been interpreted as a pseudoband resulting from competition between radiative thermolysis of peroxides and nonradiative hydrolysis below 100°C (Ducruet and Vavilin, 1999). High light at low temperature caused a strong increase in the amplitude of both TL bands in wild-type leaves (Fig. 7B). In striking contrast, the TL signal emitted by HPPD-PDH line 24-4 and 17-1 leaves was low: photostress induced a slight increase in the amplitude of the HTL1 band, and the HTL2 amplitude remained unchanged.
Figure 7.
TL measurements of lipid peroxidation in leaves of wild-type and transgenic tobacco plants exposed for 4 d to high light stress at low temperature (10°C, 700 μmol m−2 s−1, 14-h photoperiod). A, TL traces of wild-type and transgenic leaves, showing the two HTL peaks. B, Amplitude of the HTL1 and HTL2 peaks in wild-type and transgenic leaves before and after high light stress at low temperature. Data are mean values of three to five separate experiments + sd.
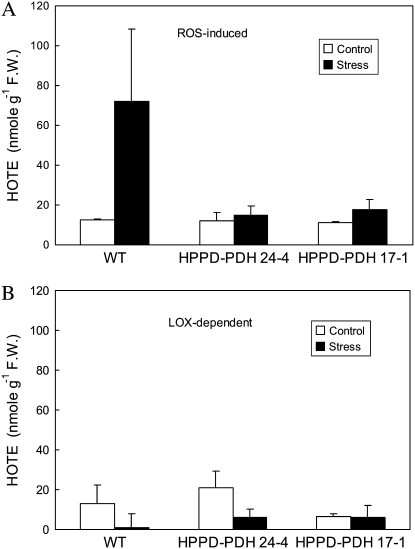
Lipid peroxidation was also analyzed by quantifying lipid hydroperoxides using HPLC. As shown in Figure 8A, the concentration of lipid hydroperoxides produced by the action of reactive oxygen species (ROS) increased in wild-type leaves after high light stress. This increase was not found in transgenic leaves, confirming their resistance to photooxidative stress. We also determined the level of lipid hydroperoxides catalyzed by lipoxygenases. We did not find that light stress caused the accumulation of lipoxygenase-dependent peroxides in wild-type or transgenic plants (Fig. 8B), indicating that lipoxygenase is not involved in the response of tobacco to high light at low temperature.
Figure 8.
HPLC measurements of lipid peroxidation in leaves of wild-type and transgenic plants exposed for 4 d to high light at low temperature (10°C, 700 μmol m−2 s−1, 14-h photoperiod). A, Concentrations of lipid hydroperoxides formed by ROS. B, Concentrations of lipid hydroperoxides generated enzymatically by lipoxygenases (LOX). F.W., Fresh weight. Data are mean values of three separate experiments + sd.
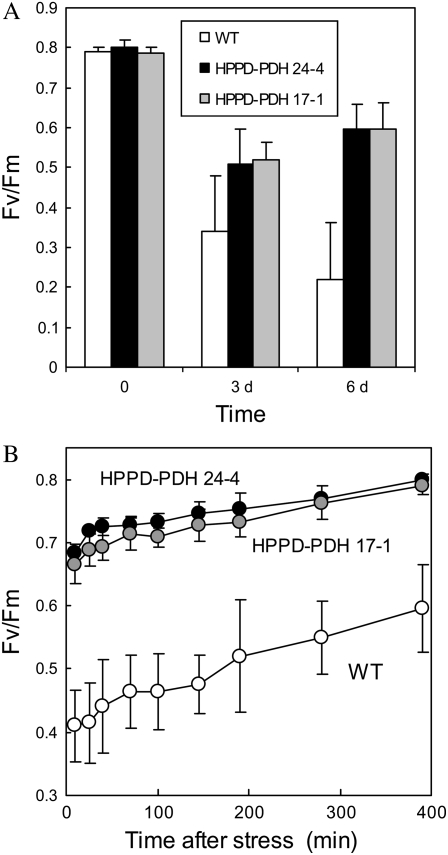
ROS-induced lipid peroxidation in tobacco plants was accompanied by a partial inactivation of the photosystems embedded in the thylakoid membranes. In the wild type, Fv/Fm dropped from approximately 0.8 to 0.32 after 3 d and to 0.22 after 6 d in high light at cold temperature (Fig. 9A). PSII was less affected in the transgenic plants, with Fv/Fm stabilizing at around 0.55 to 0.6. Moreover, PSII recovered fully (with Fv/Fm of approximately 0.8) in the HPPD-PDH transgenic leaves within 6 to 7 h after removal of the stress conditions (Fig. 9B). In wild-type leaves, PSII recovery was slow, and the inhibition was reversed only partially after 7 h of reacclimation to low light at 25°C (Fv/Fm of approximately 0.6); recovery was still incomplete (Fv/Fm = 0.73 ± 0.03) after 24 h in low light.
Figure 9.
A, Fv/Fm in wild-type and transgenic tobacco leaves before and after 3 or 6 d in high light at low temperature. Data are mean values of five separate experiments ± sd. B, Recovery of PSII photochemical activity after high light stress at low temperature. After 3 d in high light at low temperature, plants were transferred to low light at 25°C. Data are mean values of four or five measurements ± sd.
Acclimation of Young Leaves to High Light Stress at Low Temperature
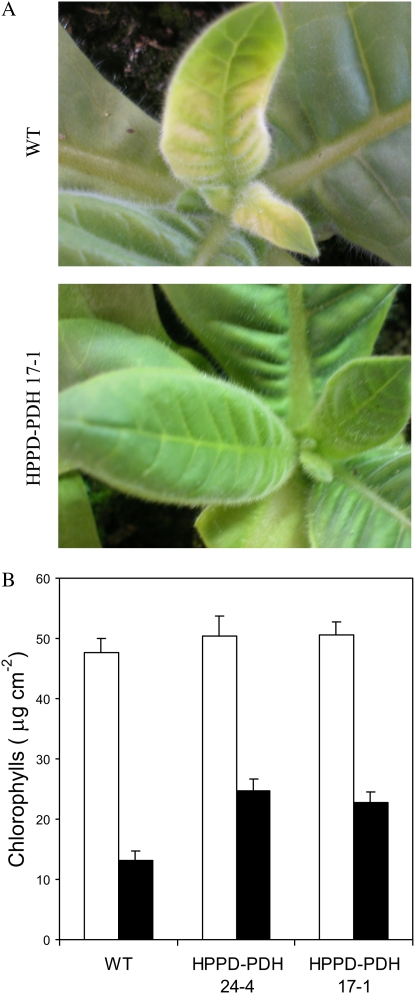
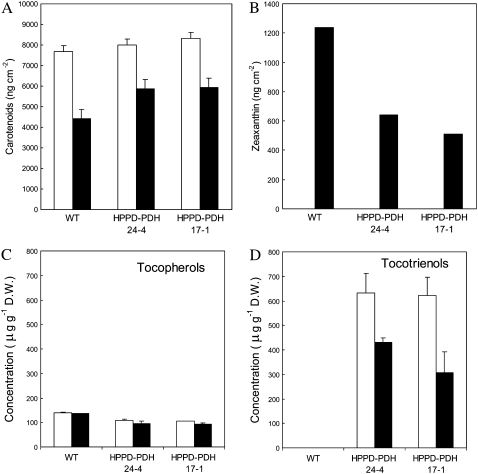
Young tobacco leaves have been shown to be more resistant to photooxidation than mature, well-developed leaves (Havaux et al., 2003). Under the stress conditions used in this study, young leaves did not exhibit symptoms of oxidative stress (Fig. 10A), even after long-term exposure (>15 d), and this is confirmed by the autoluminescence images shown in Figure 6. The intensity of spontaneous photon emission was most intense in the most developed leaves: autoluminescence was high in wild-type plants aged 40 d compared with wild-type plants aged 25 d, and in the latter plants the very young leaves at the center of the plants had a very low luminescence, hardly measurable by our imaging technique. These observations were corroborated by TL measurements that failed to show increases in HTL band amplitude in young leaves after light stress (data not shown). Although young leaves were photoresistant and were able to grow under the stress conditions, they were very pale (Fig. 10A), particularly in wild-type plants, due to a marked decrease in the chlorophyll concentration (Fig. 10B). Interestingly, young leaves of transgenic plants were much greener than wild-type leaves (Fig. 10A), and, as expected, this was associated with a higher chlorophyll content (Fig. 10B). The carotenoids followed the same trend: their concentration was reduced with light stress to a greater extent in young wild-type leaves relative to HPPD-PDH leaves (Fig. 11A), except for zeaxanthin, which accumulated in the wild type (Fig. 11B). Zeaxanthin synthesis is induced by conditions of excessive light energy (Demmig-Adams and Adams, 2000), suggesting that wild-type leaves sensed a higher level of light stress than HPPD-PDH leaves. We also determined the sugar level (starch and soluble sugars) after long-term stress, since vitamin E is known to be involved in photoassimilate transport (Russin et al., 1996; Hofius et al., 2004; Maeda et al., 2006). Stress conditions brought about a significant increase in the sugar concentration, and the effect was very pronounced for starch (Table I). However, we did not observe any difference between wild-type and transgenic plants. The vitamin E level of young wild-type leaves remained stable during acclimation to high light and low temperature (Fig. 11C). In contrast, a substantial decrease (approximately 30%) in vitamin E was observed in stressed HPPD-PDH leaves, mainly because of tocotrienol decrease (Fig. 11D). Nevertheless, the total vitamin E level remained higher in young transgenic leaves relative to wild-type leaves.
Figure 10.
Effects of long-term exposure (15 d) of young wild-type and transgenic tobacco leaves to high light at low temperature. A, Photographs of young, developing leaves (wild type and transgenic). B, Total chlorophyll content of the leaves before (white bars) and after (black bars) stress. Data are mean values of three separate experiments + sd.
Figure 11.
Concentration of total carotenoids (A), zeaxanthin (B), tocopherols (C), and tocotrienols (D) in young wild-type and transgenic tobacco leaves before (white bars) and after long-term exposure (15 d) to high light at low temperature (black bars). Data are mean values of three to five experiments + sd. Tocopherol and tocotrienol levels are semiquantitative and not adjusted for losses during extraction.
Table I.
Concentration of soluble sugars and starch (in mg g−1 fresh weight) in young leaves of wild-type and HPPD-PDH transgenic plants exposed for 15 d to high light stress at low temperature (10°C and 700 μmol m−2 s−1)
Leaf water content was not different in wild-type and HPPD-PDH plants. Data are mean values of three experiments ± sd.
| Material | Wild Type
|
HPPD-PDH 17-1
|
HPPD-PDH 24-4
|
|||
|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | Control | Stress | |
| Glc | 1.43 ± 0.17 | 2.25 ± 0.17 | 3.12 ± 0.53 | 3.59 ± 1.29 | 3.25 ± 0.10 | 4.27 ± 0.78 |
| Fru | 0.56 ± 0.07 | 0.78 ± 0.16 | 0.71 ± 0.06 | 0.86 ± 0.20 | 0.78 ± 0.20 | 1.34 ± 0.46 |
| Saccharose | 2.82 ± 0.37 | 5.61 ± 0.60 | 5.27 ± 0.45 | 6.56 ± 1.59 | 4.72 ± 1.97 | 5.57 ± 0.64 |
| Starch | 2.13 ± 0.80 | 27.9 ± 1.05 | 2.43 ± 0.67 | 21.01 ± 1.44 | 3.55 ± 0.74 | 29.16 ± 1.71 |
DISCUSSION
Tocotrienols Accumulated in the Thylakoid Membranes of Transgenic Tobacco Leaves and Did Not Perturb the Photochemical Activity of Chloroplasts
Tocotrienols are much less widespread in the plant kingdom than tocopherols (Horvath et al., 2006). In contrast to tocopherols, which are synthesized and accumulate in leaf plastids, tocotrienols are present in nongreen tissues, including seeds, fruits, and latex. However, plants can be genetically manipulated to synthesize and accumulate tocotrienols in leaves (Cahoon et al., 2003; Rippert et al., 2004). As shown here, high levels of tocotrienols can accumulate in modified tobacco leaves overexpressing yeast PDH and Arabidopsis HPPD, and this occurred without any significant perturbation of the leaf photochemical activity. Furthermore, tocotrienols had no effect on the chlorophyll and carotenoid contents of leaves (Rippert et al., 2004).
Inside chloroplasts, vitamin E is distributed between thylakoid membranes, envelope membranes, and plastoglobules (Lichtenthaler, 1969, 2007; Lichtenthaler et al., 1981; Vidi et al., 2006). However, only thylakoid vitamin E is expected to have a protective effect against peroxidative lipid destruction. It was thus of great importance to compare the distribution of vitamin E in wild-type and transgenic lines and to determine where tocotrienols, which are the major forms of vitamin E in transgenic lines, accumulate. In the transgenic lines, the pattern of vitamin E in the thylakoid membrane fraction was found to be identical to that of intact chloroplast and whole leaves, indicating that tocotrienol accumulation did occur in the thylakoid membranes. In contrast, plastoglobules were observed to contain mainly δ-tocopherol (approximately 90%), which was a very minor component of vitamin E (less than 1%) in whole leaves, intact chloroplast, and thylakoid membranes. The same situation was observed in chloroplasts prepared from young wild-type leaves, in which the major forms of vitamin E were α- and γ-tocopherols in thylakoid membranes (and in leaves), while the plastoglobule fraction contained mainly δ-tocopherol. Thus, in young tobacco leaves, in which plastoglobules are present in small amounts, vitamin E was not stored predominantly in plastoglobules. Although α-tocopherol (and tocotrienols in the transgenic lines) was found in the envelope-enriched fraction, envelope membranes are assumed to represent around 5% to 6% of chloroplast membrane lipids (M. Block, personal communication) and therefore constitute a minor site of vitamin E storage relative to thylakoid membranes. For each fraction, vitamin E was normalized to the lipid content (data not shown), so that the distribution of vitamin E within the chloroplast could be estimated: we found 89%, 8%, and 3% for the thylakoids, the envelopes, and the plastoglobules, respectively.
This thylakoid localization fits with the function of vitamin E in plant leaves, which protects, in collaboration with the xanthophyll cycle (Havaux et al., 2005) and other antioxidant systems (Kanwischer et al., 2005), the thylakoid membrane lipid phase from photooxidative damage. This finding is in apparent contradiction to a recent work by Vidi et al. (2006), who identified the plastoglobules as a major site of vitamin E accumulation in Arabidopsis chloroplasts. It is unlikely that the difference was due to the purification procedure, since the protocols used in both studies were very similar, except that we did not perform ultracentrifugation prior to the Suc gradient. This is to reduce plastoglobule losses and to improve vitamin E quantification in this fraction, since chloroplasts of our plants contain very low amounts of plastoglobules. In order to exclude the possibility that the contradictory results obtained in tobacco in this study and in Arabidopsis by Vidi et al. (2006) are due to the species used as starting material, we prepared plastoglobules from young Arabidopsis leaves. Again, we found δ-tocopherol as the major tocopherol and very little α-tocopherol in this fraction (data not shown). Plastoglobules are known to fluctuate during chloroplast development. They enlarge during thylakoid disassembly in senescing chloroplasts (Lichtenthaler, 1968; Tuquet and Newman, 1980; Ghosh et al., 2001) and under stress conditions (Locy et al., 1996; Eymery and Rey, 1999; Bondada and Syvertsen, 2003; Lichtenthaler, 2007). It is thus likely that the discrepancy in terms of plastoglobule composition between the two studies resulted from differences in the age and growth conditions of the plants used as experimental material. Accordingly, when old and senescing leaves were used in our preparations, plastoglobules were more abundant and their vitamin E content was modified relative to that of young leaves, with α-tocopherol being the major vitamin E form (71%; Supplemental Fig. S3), as found by Vidi et al. (2006). However, they still contained δ-tocopherol (23%).
In good agreement with Vidi et al. (2006) and Austin et al. (2006), VTE1 was immunodetected in the plastoglobules of tobacco, confirming the occurrence of an active vitamin E synthesis at this level. Plastoglobules thus appear to have a dual function with respect to vitamin E: they actively participate to vitamin E synthesis via the tocopherol cyclase VTE1 and, under certain conditions, they also participate in vitamin E storage. In the study by Vidi et al. (2006), it seems likely that the second role of plastoglobules (i.e. vitamin E storage) was predominant. In contrast, the storage function of plastoglobules was very limited in the young tobacco leaves examined here. The partition of vitamin E biosynthesis between the chloroplast envelope and plastoglobules, and the accumulation of vitamin E in thylakoid membranes and, under certain conditions, in plastoglobules, highlight the very complex trafficking of tocopherols inside the chloroplast. However, it is clear from our data that there are conditions in which vitamin E is located predominantly in the thylakoid membranes.
The presence of δ-tocopherol as a major vitamin E component of plastoglobules is intriguing. This observation is in agreement with the localization of VTE1 in plastoglobules (Austin et al., 2006; Vidi et al., 2006; this study), since δ-tocopherol is one of two possible products of the cyclase reaction (Porfirova et al., 2002; Sattler et al., 2003). However, this finding also suggests that, in vivo, 2-methyl-6-phytyl-1,4-benzoquinone (MPBQ), rather than 2,3-dimethyl-5-phytyl-1,4-benzoquinone (DMPBQ), could be the substrate of the tobacco tocopherol cyclase. As a corollary, the substrate of VTE3 (MPBQ methyltransferase) should be δ-tocopherol instead of MPBQ, leading to the formation of γ-tocopherol. However, these conclusions are difficult to reconcile with the biochemical characterization of Arabidopsis VTE1 and VTE3. In vitro, VTE1 presents a strong selectivity for DMPBQ (Porfirova et al., 2002; Sattler et al., 2003), and VTE3 was unable to methylate δ-tocopherol (Cheng et al., 2003). Moreover, DMPBQ, and not MPBQ, accumulated in the Arabidopsis vte1 mutant deficient in tocopherol cyclase (Maeda et al., 2006). Similarly, the massive accumulations of tocotrienol in transgenic HPPD-PDH tobacco leaves (Rippert et al., 2004) and Arabidopsis seeds (Karunanandaa et al., 2005) were also difficult to reconcile with the biochemical characterization of homogentisate phytyltransferase (HPT; Collakova and DellaPenna, 2001). One could argue that VTE1, VTE3, and HPT are all integral membrane proteins operating in a lipid environment and that such conditions are probably difficult to reproduce in vitro. Moreover, analysis of the tocopherol composition of Arabidopsis mutants deficient in VTE3 activity confirmed that, in planta, Arabidopsis VTE1 can utilize MPBQ, since δ- and β-tocopherols were found to accumulate in these mutants (Cheng et al., 2003). As far as the Arabidopsis vte1 mutant is concerned, it is possible that the pressure of the metabolic flux when the tocopherol synthesis is completely blocked by the lack of VTE1 can “force” the synthesis of DMPBQ from MPBQ by a methyltransferase, causing accumulation of the latter compound. The presence of δ-tocopherol in tobacco (and Arabidopsis) plastoglobules is an intriguing observation that cannot be fully understood in the light of the available literature, hence raising questions about vitamin E synthesis in plants. This aspect is beyond the scope of this article and will be studied further in the future.
Tocotrienols Function in Vivo as Efficient Antioxidants and Photoprotectors in Thylakoid Membranes
In vitro, tocotrienols have been shown to be better antioxidants than α-tocopherol (Serbinova et al., 1991; Suzuki et al., 1993). In animal and human cells, tocotrienols were reported to work in vivo as antioxidants at least as efficiently as tocopherols (Sen et al., 2006). Besides, tocotrienols possess powerful neuroprotective, anticancer, and cholesterol-lowering properties, which do not seem to be shared with α-tocopherol (Theriault et al., 1999; Sen et al., 2006). Thus, tocotrienols are considered to be major protective molecules in animal and human cells, with important bioactivities. In contrast, the antioxidant function of tocotrienols is not documented in plants. This is probably due to the fact that tocotrienol distribution is restricted essentially to seeds. Possibly, this peculiar distribution of tocotrienols may have hampered the study of their antioxidative activity, contrary to tocopherols, which are present in large quantities in leaves. The role of tocopherols in the protection of leaves against high light stress at low temperature (Havaux et al., 2005) and salt or sorbitol stress (Abbasi et al., 2007) was recently demonstrated in tocopherol-deficient mutants. Similarly, tocopherols or their immediate precursors were observed to limit lipid peroxidation during germination in the light and during the early photoautotrophic growth of young seedlings (Sattler et al., 2004).
Because they accumulate tocotrienols in their leaves, our HPPD-PDH transgenic plants provided a unique opportunity to study in vivo the function of tocotrienols (e.g. under light-induced oxidative stress). When wild-type tobacco plants were exposed to high light and low temperature, they suffered from photooxidative stress and extensive lipid peroxidation, as measured by a variety of methods (HPLC, TL, and autoluminescence imaging). Compared with wild-type leaves, tocotrienol-accumulating plants were more phototolerant, showing no or very little lipid peroxidation and PSII photodamage. The differential phototolerance of wild-type and HPPD-PDH transgenic leaves provides clear evidence for the antioxidative potency of tocotrienols against peroxidation of membrane lipids. It is very unlikely that the increased phototolerance of the HPPD-PDH leaves was indirectly due to secondary changes in other antioxidant systems. No significant change in antioxidant molecules, such as ascorbate, glutathione, and carotenoids, or in antioxidant enzymes, such as Prx and Msr, was detected in the tocotrienol-accumulating transgenic leaves. We can also exclude the notion that the small changes in the α-tocopherol content and in the γ:α-tocopherol ratio were involved in the increased resistance of the HPPD-PDH plants to oxidative stress. Indeed, the Arabidopsis vte1 mutant, which lacks tocopherols (Porfirova et al., 2002), and the vte4 mutant, in which α-tocopherol is almost completely substituted by γ-tocopherol, did not exhibit a significant increase (or decrease) in phototolerance (Porfirova et al., 2002; Bergmüller et al., 2003; Havaux et al., 2005).
Moreover, tocotrienol accumulation enhanced the ability of young transgenic leaves to acclimate to high light stress in the long term. It has been shown in various plant species, including tobacco, that young, developing leaves are more tolerant to photooxidative stress than mature, well-developed leaves (Carlsson et al., 1996; Havaux et al., 2003). Although young tobacco leaves were able to tolerate the stress conditions used in this study, their pigmentation was strongly perturbed, leading to pale green/yellow leaves. This finding is consistent with previous studies on other thermophilic species, which have shown that chloroplast biogenesis is impaired by chilling stress (Nie and Baker, 1991; Yoshida et al., 1996). This phenomenon was attenuated in HPPD-PDH double transgenic leaves, which were greener and contained more chlorophyll than young wild-type leaves. Thus, chloroplast biogenesis was less perturbed by chilling stress when tocotrienols were able to accumulate in the chloroplasts.
The origin of leaf chlorosis under chilling stress is elusive. One possible cause is the accumulation of photoassimilates (Strand et al., 1997), a phenomenon that is known to repress photosynthetic gene expression (Sheen, 1990) and to cause the down-regulation of chlorophyll level (Braun et al., 2006). Importantly, tocopherol deficiency has been reported to lead to cold-induced blockage of photoassimilate transport in Arabidopsis (Maeda et al., 2006). Although sugars and starch did accumulate in young tobacco leaves exposed to chilling stress in high light, these accumulations were similar in wild-type and HPPD-PDH transgenic leaves.
Leaf chlorosis could also result from chilling-induced changes in the physical properties of the thylakoid membranes. A primary effect of chilling stress in thermophilic species is believed to be a decrease in membrane fluidity associated with the transition of membrane lipids from a flexible liquid crystalline to a solid gel phase (Murata et al., 1975; Wada et al., 1990; Nishida and Murata, 1996). In tobacco, phase separation was measured to occur below around 10°C (Terzaghi et al., 1989). Chloroplast biogenesis and photosystem assembly are strongly influenced by thylakoid membrane lipid composition and fluidity (McCourt et al., 1987; Jarvis et al., 2000; Routaboul et al., 2000; Babiychuk et al., 2003), and, consequently, chilling-induced phase transition could be involved in the pale-green phenotype of young chilled leaves. Interestingly, in vitro experiments with artificial lipid membranes incorporated with vitamin E constituents have shown that both tocopherol and tocotrienol affect the structure and dynamics of membranes (Stillwell et al., 1992; Suzuki et al., 1993). Particularly, they disrupt molecular packing with gel state membranes, resulting in additional acyl chain motion. Therefore, the accumulation of high amounts of vitamin E in thylakoid membranes of HPPD-PDH plants could possibly affect membrane dynamics and physical properties and, hence, could mitigate chilling-induced impairment of chloroplast biogenesis.
Rather surprisingly, the tocotrienol level decreased substantially during long-term acclimation of HPPD-PDH leaves to high light at low temperature. One of the functions of the vitamin E constituents is to scavenge oxygen radicals (Fryer, 1992). During this process, the molecule donates its phenolic hydrogen atom to the oxygen radical and becomes a chromanoxyl radical. The latter radical can be reconverted to vitamin E by the reducing power of ascorbate (Munné-Bosch and Alegre, 2002) or by reaction with carotenoids (Böhm et al., 1997). It has been estimated that one α-tocopherol molecule is capable of protecting up to 220 molecules of polyunsaturated fatty acid before being consumed (Fukuzawa et al., 1982). During chilling stress in high light, α-tocopherol was found to be the first antioxidant that was degraded in cucumber (Cucumis sativus) leaves (Wise and Naylor, 1987). The loss of tocotrienols in stressed transgenic leaves thus could suggest either that they are less stable than tocopherols or that the strongly increased tocotrienol-carotenoid and tocotrienol-ascorbate ratios rendered the tocotrienol-recycling process less efficient. As a corollary, the consumption of tocotrienols suggests that, although no obvious symptom of photooxidation was found, a chronic production of ROS took place in young tobacco leaves growing under the stress conditions. Active scavenging of the produced ROS by tocotrienols may have participated to the acclimation of HPPD-PDH leaves to chilling stress in high light. Alternatively, the specific decrease in tocotrienol content could be explained by the fact that the promoter controlling the expression of the two transgenes, in contrast to endogenous tocopherol promoters, is not dependent on environmental conditions (Rippert et al., 2004).
To sum up, we have shown, for the first time in planta to our knowledge, that tocotrienols can protect membrane lipids from oxidation. Although this study was performed on plants that artificially accumulate high amounts of tocotrienols in leaf plastids, we assume that tocotrienols exert a similar function in other biological tissues, such as those in which tocotrienols usually accumulate (e.g. plant seeds). The antioxidative function of tocotrienols shown here in tobacco leaves confers an interesting agronomic trait to tocotrienol-accumulating plants. Moreover, tocotrienols have a number of pharmaceutical properties, ranging from the prevention of cholesterol accumulation to antiproliferation action in cancer cells (Theriault et al., 1999). Consequently, tocotrienol-biofortified plants are potentially helpful, not only for agriculture in stressful environments but also for human health.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tobacco plants (Nicotiana tabacum var PBD6) were grown on compost in a phytotron under controlled conditions of light (150 μmol photons m−2 s−1, 14 h d−1) and temperature (25°C/20°C, day/night). Two transgenic lines (named HPPD-PDH 24-4 and HPPD-PDH 17-1) that coexpress the yeast (Saccharomyces cerevisiae) PDH gene and the Arabidopsis (Arabidopsis thaliana) HPPD gene were used in this study. This double transformation increases the HPP and homogentisate fluxes and causes a massive accumulation of tocotrienols (Rippert et al., 2004). Photooxidative stress was imposed by transferring plants to a growth chamber at 10°C/10°C (day/night) and under a high PFD of 700 μmol photons m−2 s−1 (14 h d−1). PFDs were measured with a Li-Cor quantum meter (Li-185B/Li-190SB).
Chlorophylls, Carotenoids, and Vitamin E
Photosynthetic pigments extracted from leaf discs in methanol were separated and quantified by HPLC, as reported previously (Havaux et al., 2005). Vitamin E was analyzed from freeze-dried leaves (approximately 150 mg) ground in liquid nitrogen and extracted three times with 2 mL of hexane in dim light in the presence of argon. The pooled supernatants were evaporated with argon and dissolved in methanol bubbled with argon. The methanolic extracts were stored at −80°C before analysis. Total lipids from chloroplast membrane fractions were extracted according to Folch et al. (1957), resuspended in hexane, and centrifuged, and the resulting supernatant was dried under argon and dissolved in methanol. Tocopherols and tocotrienols were separated by HPLC as described elsewhere (Rippert et al., 2004). No internal standard was used during extraction. Consequently, since the vitamin E level was not adjusted for recovery, the data should be considered semiquantitative.
Purification of Intact Tobacco Chloroplasts
Chloroplasts were purified from tobacco leaves according to Douce and Joyard (1982), with the following modifications. Crude chloroplasts were obtained from 400 to 500 g of young tobacco leaves and purified by isopycnic centrifugation on 50% preformed Percoll gradients (40,000g for 55 min). Leaves were ground three times for 2 s, and the filtrate was centrifuged at 2,070g for 2 min. After resuspension, chloroplasts were loaded on top of the preformed Percoll gradients, and the gradients were centrifuged at 13,300g for 10 min. Intact chloroplasts were collected from the gradients, diluted three to four times, and centrifuged at 2,070g for 2 min. All operations were carried out at 0°C to 5°C.
Purification of Plastoglobules, Envelope Membranes, and Thylakoid Membranes from Tobacco Chloroplasts
Purified intact chloroplasts were lysed in hypotonic medium in the presence of protease inhibitors (10 mm MOPS-NaOH, pH 7.8, 4 mm MgCl2, 1 mm phenylmethylsulfonyl fluoride, 1 mm benzamidine, and 0.5 mm ɛ-amino caproic acid). Plastoglobules and envelope membranes were purified from the lysate by centrifugation at 70,000g for 4 h (SW41-Ti rotor; Beckman) on Suc gradients (0.93, 0.6, and 0.3 m Suc; Teyssier et al., 1996). Plastoglobules were collected at the 0/0.3 m interface. Envelope membranes were collected at the 0.6/0.93 m interface, diluted three to four times in 10 mm MOPS-NaOH, pH 7.8, buffer containing protease inhibitors, and concentrated by centrifugation at 110,000g for 1 h. Thylakoids were collected at the bottom of the centrifuge tube of the Suc gradient. Chloroplast fractions were stored in liquid nitrogen in 10 mm MOPS-NaOH, pH 7.8, in the presence of protease inhibitors.
SDS-PAGE and Western-Blot Analyses of Chloroplast Fractions
SDS-PAGE analyses were performed as described by Chua (1980). For western-blot analyses, gels were transferred to a nitrocellulose membrane (BA85; Schleicher and Schuell). To analyze the purity of the membrane fractions, we used antibodies directed against the plastoglobule protein VTE1, the envelope protein ceQORH, and the thylakoid protein LHCP. Western blots were revealed with ECS+ on a Typhoon 9400 phosphor imager (GE Healthcare) according to the manufacturer's recommendations.
The procedure used for the western-blot analysis of antioxidant proteins (Prx Q, 2-Cys Prx, and MsrA4) was described in detail in previous publications (Vieira Dos Santos et al., 2005; Rey et al., 2007; Collin et al., 2008). Briefly, tobacco leaves were ground in liquid nitrogen and the powder was resuspended in 50 mm β-mercaptoethanol, 1 mm PMSF, and 50 mm Tris-HCl, pH 8. After centrifugation (30,000g, 20 min, 4°C) and precipitation of soluble proteins using acetone, the protein content was determined using a method based on bicinchoninic acid (BC Assay Reagent; Interchim). For western-blot analysis, polyclonal antibodies raised against Arabidopsis plastidic 2-Cys Prx, poplar (Populus spp.) Prx Q, and poplar MsrA4 were used diluted 1:10,000, 1:1,000, and 1:1,000, respectively. The abundance of overoxidized 2-Cys Prx was investigated using a serum raised against Cys sulfinic and sulfonic acid forms of the protein (LabFrontier), as described by Rey et al. (2007).
Lipid Hydroperoxides
Lipid peroxidation was assessed by HPLC analysis of hydroxy fatty acids recovered from plant tissue after NaBH4 reduction and saponification of total lipids. Leaves were frozen in liquid N2 and stored at −20°C before extraction. Extraction was carried out according to the previously described procedure (Montillet et al., 2004). An aliquot of the extract (50 μL) was submitted to straight phase HPLC (Waters, Millipore) using a Zorbax rx-SIL column (4.6 × 250 mm, 5-μm particle size; Hewlett-Packard), isocratic elution with 70:30:0.25 (v/v/v) hexane:diethyl ether:acetic acid at a flow rate of 1.5 mL min−1, and UV detection at 234 nm. ROS-induced lipid peroxidation was evaluated from the levels of the different hydroxyoctadecatrienoic acid (HOTE) isomers as described previously using 15-hydroxy-11,13(Z,E)eicosadienoic acid as an internal standard (Montillet et al., 2004). Lipoxygenase-induced lipid peroxidation was estimated from the level of 13-HOTE after subtraction of racemic 13-HOTE (attributable to ROS-mediated lipid peroxidation), as explained by Montillet et al. (2004).
TL and Autoluminescence Imaging
TL measurements were performed with a custom-built apparatus that has been described (Havaux, 2003). The leaf samples (two discs of 8 mm in diameter) were slowly heated from 25°C to 150°C at a rate of 6°C min−1. Leaf temperature was measured with a tiny K-type thermocouple, and luminescence emission was measured with a photomultiplier tube, the current of which was amplified by a transimpedance amplifier. Both leaf temperature and TL were recorded by a computer using a DaqPad-1200 data acquisition system (National Instruments). The TL band peaking at around 110°C to 130°C (HTL2) corresponds to the thermal decomposition of lipid hydroperoxides yielding light-emitting triplet excited carbonyls (Devaraj et al., 1997; Vavilin and Ducruet, 1998; Havaux, 2003). The HTL1 band peaking at approximately 75°C is a pseudoband resulting from a competition between radiative thermolysis of peroxides and nonradiative hydrolysis below 100°C, which is dependent on leaf hydration (Ducruet and Vavilin, 1999).
Spontaneous photon emission associated with lipid peroxidation was also imaged at room temperature with a high-sensitivity, liquid nitrogen-cooled CCD camera, as described in detail elsewhere (Havaux et al., 2006). Plants were adapted to darkness for 2 h before recording photon emission. The integration time was 20 min, and on-CCD binning of 2 × 2 was used to increase detection sensitivity (the resulting resolution was 650 × 670 pixels).
Photosynthetic Electron Transport
Chlorophyll fluorescence emission from the upper surface of attached leaves was measured with a PAM-2000 fluorometer (Walz), as described previously (Havaux et al., 2003, 2005). The maximal quantum yield of PSII photochemistry was measured in dark-adapted samples by (Fm − Fo)/Fm = Fv/Fm, where Fo is the fluorescence level induced by a dim red light modulated at 600 Hz and Fm is the maximal level induced by an 800-ms pulse of intense white light. The quantum yield of PSII-mediated electron transport was measured in illuminated leaves by the ΔF/Fm′ ratio, where Fm′ is the maximal fluorescence level and ΔF is the difference between Fm′ and the steady-state fluorescence level Fs. Leaves were illuminated with white light produced by a Schott KL1500 light source equipped with a light guide.
Photosynthetic oxygen evolution by leaf discs (3.2 cm in diameter) was measured with a Clark-type oxygen electrode (Hansatech LD2/2), as described elsewhere (Walker, 1987). A carbonate/bicarbonate buffer was used to generate CO2 inside the electrode. White light was produced by a Hansatech LS2 light source.
Carbohydrate Analyses
Soluble sugar (Glc, Fru, and saccharose) and starch contents of leaves were quantified as described. Five hundred milligrams of leaf tissue was ground three times in 0.5 n NaOH and centrifuged at 11,000g for 10 min. The cleared supernatants were pooled and neutralized to pH 7.5 with 6 n HCl and 1 m Tris-HCl (pH 7.5). An aliquot of 100 μL was used for saccharose hydrolysis by β-fructosidase in 100 mm acetate buffer (pH 4.6), and an aliquot of 200 μL was used for starch hydrolysis in 100 mm acetate buffer (pH 4.6) by amyloglucosidase at 35°C. Soluble sugars were quantified by a coupled enzymatic assay using hexokinase, phosphoglucosisomerase, and Glc-6-P dehydrogenase, by following the formation of NADPH at 340 nm (Bergmeyer and Brent, 1974).
Soluble Antioxidants
Ascorbate extracted from leaf discs in 4.5% metaphosphoric acid was measured by HPLC as described previously (Havaux et al., 2005). Reduction of ascorbate by 10 mm Tris-(2-carboxyethyl)phosphine hydrochloride allowed total ascorbate content to be determined. Glutathione was measured using the HPLC method described elsewhere (Collin et al. 2008). Similar to total ascorbate, total glutathione was measured after Tris-(2-carboxyethyl)phosphine hydrochloride treatment.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF047834 (Arabidopsis HPPD) and Z36035 (yeast PDH).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Separation of plastoglobules, envelopes, and thylakoids of wild-type chloroplasts by ultracentrifugation on a Suc gradient, and western blots of VTE1, CeQORH, and LHCP in each fraction.
Supplemental Figure S2. HPLC separation of the tocochromanol standards.
Supplemental Figure S3. Chromatograms of vitamin E, and vitamin E composition in plastoglobules purified from intact chloroplasts of old and senescing leaves of wild-type plants.
Supplementary Material
Acknowledgments
We thank the members of the GRAP Laboratory (Commissariat à l'Energie Atomique, Cadarache, France) for their help in growing the plants and Françoise Eymery (Commissariat à l'Energie Atomique, Cadarache) for valuable assistance in preparing protein extracts. The VTE1 antibody was received from Peter Doërmann (Max Planck Institute, Golm, Germany), and the Prx Q and MsrA4 antibodies were kind gifts from Nicolas Rouhier (Nancy University, Nancy, France); both are gratefully thanked. Arabidopsis plastoglobule fractions were prepared by Daniel Salvi (Commissariat à l'Energie Atomique, Grenoble, France). We also thank Professors H. Lichtenthaler (Universität Karlsruhe, Karlsruhe, Germany) and Christian Triantaphylidès (Commissariat à l'Energie Atomique, Cadarache) for useful discussions.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Michel Havaux (michel.havaux@cea.fr).
The online version of this article contains Web-only data.
References
- Abbasi A-R, Hajirezaei M, Hofius D, Sonnewald U, Voll L (2007) Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol 143 1720–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin II Jr, Frost E, Vidi P-A, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk E, Müller F, Eubel H, Braun H-P, Frentzen M, Kushnir S (2003) Arabidopsis phosphatidylglycerolphosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J 33 899–909 [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Brent E (1974) Sucrose. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Vol 3. Academic Press, New York, pp 1176–1179
- Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in g-tocopherol methytransferase. Plant Mol Biol 52 1181–1190 [DOI] [PubMed] [Google Scholar]
- Böhm F, Edge R, Land EJ, McGarvey DJ, Truscott TG (1997) Carotenoids enhance vitamin E antioxidant efficiency. J Am Chem Soc 119 621–622 [Google Scholar]
- Bondada BR, Syvertsen JP (2003) Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiol 23 553–559 [DOI] [PubMed] [Google Scholar]
- Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner K-H (2000) Vitamin E. J Sci Food Agric 80 913–938 [Google Scholar]
- Braun DM, Ma Y, Inada N, Muszynski MG, Baker RF (2006) Tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiol 142 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Traber MG (1999) Vitamin E: function and metabolism. FASEB J 13 1145–1155 [PubMed] [Google Scholar]
- Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21 1082–1087 [DOI] [PubMed] [Google Scholar]
- Carlsson AS, Wallin G, Sandelius AS (1996) Species- and age-dependent sensitivity to ozone in young plants of pea, wheat and spinach: effects in acyl lipid and pigment content and metabolism. Physiol Plant 98 271–280 [Google Scholar]
- Cheng Z, Sattler S, Maeda H, Sakuragi Y, Bryant DA, Dellapenna D (2003) Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15 2343–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua NH (1980) Electrophoresis analysis of chloroplast proteins. Methods Enzymol 69 434–436 [Google Scholar]
- Collakova E, DellaPenna D (2001) Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 127 1113–1124 [PMC free article] [PubMed] [Google Scholar]
- Collin VC, Eymery F, Genty B, Rey P, Havaux M (2008) Vitamin E is essential for the tolerance of Arabidopsis to metal-induced oxidative stress. Plant Cell Environ 31 244–257 [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Pogson B (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57 711–738 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW III (2000) Harvesting sunlight safely. Nature 403 371–374 [DOI] [PubMed] [Google Scholar]
- Devaraj B, Usa M, Inaba H (1997) Biophotons: ultraweak light emission from living systems. Curr Opin Solid State Mater Sci 2 188–193 [Google Scholar]
- Dietz KJ (2003) Plant peroxiredoxins. Annu Rev Plant Biol 54 93–107 [DOI] [PubMed] [Google Scholar]
- Douce R, Joyard J (1982) Purification of the chloroplast envelope. In M Eldman, R Hallick, NH Chua, eds, Methods in Chloroplast Molecular Biology. Elsevier North Holland, Amsterdam, pp 239–256
- Ducruet JM, Vavilin D (1999) Chlorophyll high-temperature thermoluminescence emission as an indicator of oxidative stress: perturbating effects of oxygen and leaf water content. Free Radic Res (Suppl) 31 S187–S192 [DOI] [PubMed] [Google Scholar]
- Eymery F, Rey P (1999) Immunocytolocalization of CDSP 32 and CDSP 34, two chloroplastic drought-induced stress proteins in Solanum tuberosum plants. Plant Physiol Biochem 37 305–312 [Google Scholar]
- Falk J, Andersen G, Kernebeck B, Krupinska K (2003) Constitutive overexpression of barley 4-hydrophenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Lett 540 35–40 [DOI] [PubMed] [Google Scholar]
- Fiedler E, Soll J, Schultz G (1982) The formation of homogentisate in the biosynthesis of tocopherol and plastoquinone in spinach chloroplasts. Planta 155 511–515 [DOI] [PubMed] [Google Scholar]
- Flor-Henry M, McCabe TC, de Bruxelles GL, Roberts MR (2004) Use of a highly sensitive two-dimensional luminescence imaging system to monitor endogenous bioluminescence in plant leaves. BMC Plant Biol 4: 19 [DOI] [PMC free article] [PubMed]
- Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226 497–509 [PubMed] [Google Scholar]
- Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15 381–392 [Google Scholar]
- Fukuzawa K, Gebicky JM (1983) Oxidation of α-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch Biochem Biophys 226 242–251 [DOI] [PubMed] [Google Scholar]
- Fukuzawa K, Tokumura A, Ouchi S, Tsukatani H (1982) Antioxidant activities of tocopherols on iron(2+)-ascorbate-induced lipid peroxidation in lecithin liposome. Lipids 17 511–513 [DOI] [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Lenne C, Rolland A, Sailland A, Matringe M (1997) Subcellular localization and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterisation of the corresponding cDNA. Biochem J 325 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Mahoney SR, Penterman JN, Peirson D, Dumbroff EB (2001) Ultrastructural and biochemical changes in chloroplasts during Brassica napus senescence. Plant Physiol Biochem 39 777–784 [Google Scholar]
- Havaux M (2003) Spontaneous and thermoinduced photon emission: new methods to detect and quantify oxidative stress in plants. Trends Plant Sci 8 409–413 [DOI] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17 3451–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Lutz C, Grimm B (2003) Chloroplast membrane photostability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol 132 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Triantaphylidès C, Genty B (2006) Autoluminescence imaging: a non invasive tool for mapping oxidative stress. Trends Plant Sci 11 480–484 [DOI] [PubMed] [Google Scholar]
- Hofius D, Hajirezaei M-R, Geiger M, Tschiersch H, Melzer M, Sonnewald U (2004) RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol 135 1256–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Wessjohann L, Bigirimana J, Jansen M, Guisez Y, Caubergs R, Horemans N (2006) Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 67 1185–1195 [DOI] [PubMed] [Google Scholar]
- Jarvis P, Dörmann P, Peto CA, Lutes J, Benning C, Chory J (2000) Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc Natl Acad Sci USA 97 8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Havaux M, Triantaphylides C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photo-oxidative stress by a lipid-protective, anti-oxidant mechanism. J Biol Chem 282 22605–22618 [DOI] [PubMed] [Google Scholar]
- Kanwischer M, Porfirova S, Bergmüller E, Dörmann P (2005) Alterations in tocopherol cyclase activity in transgenic and mutant plants affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol 137 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanandaa B, Qi Q, Hao M, Baszis SR, Jensen PK, Wong YH, Jiang J, Venkatramesh M, Gruys KJ, Moshiri F, et al (2005) Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab Eng 7 384–400 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takeda M, Ito K, Kato H, Inaba H (1999) In vivo imaging of spontaneous ultraweak photon emission from a rat's brain correlated with cerebral energy metabolism and oxidative stress. Neurosci Res 34 103–113 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1968) Plastoglobuli and the fine structure of plastids. Endeavor 27 144–149 [Google Scholar]
- Lichtenthaler HK (1969) Localisation and functional concentrations of lipoquinones in chloroplasts. In H Metzner, ed, Photosynthesis Research, Vol I. International Union of Biological Sciences, Tübingen, Germany, pp 304–314
- Lichtenthaler HK (2007) Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth Res 92 163–179 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Prenzel U, Douce R, Joyard J (1981) Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim Biophys Acta 641 99–105 [DOI] [PubMed] [Google Scholar]
- Locy RD, Chang C-C, Nielsen BL, Singh NK (1996) Photosynthesis in salt-adapted heterotrophic tobacco cells and regenerated plants. Plant Physiol 110 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Song W, Sage TL, DellaPenna D (2006) Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18 2710–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P, Kunst L, Browse J, Somerville CR (1987) The effects of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol 84 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras S, Salvi D, Ferro M, Grunwald D, Garin J, Joyard J, Rolland N (2002) Non-canonical transit peptide for import into the chloroplast. J Biol Chem 277 47770–47778 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Cacas JL, Garnier L, Montané MH, Douki T, Bessoule JJ, Polkowska-Kowalczyk L, Maciejewska U, Agnel JP, Vial A, et al (2004) The upstream oxylipin profile of Arabidopsis thaliana: a tool to scan for oxidative stresses. Plant J 40 439–451 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2002) Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett 524 145–148 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Weiler EW, Alegre L, Müller M, Düchting P, Falk J (2007) α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225 681–691 [DOI] [PubMed] [Google Scholar]
- Murata N, Troughton JH, Fork DC (1975) Relationships between the transition of the physical phase of membrane lipids and photosynthetic parameters in Anacystis nidulans and lettuce and spinach chloroplasts. Plant Physiol 56 508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Baker NR (1991) Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiol 95 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47 541–568 [DOI] [PubMed] [Google Scholar]
- Porfirova S, Bergmuller E, Tropf S, Lemke R, Dörmann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99 12495–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey P, Becuwe N, Barrault MB, Rumeau D, Havaux M, Biteau B, Toledano MB (2007) The Arabidopsis thaliana sulfiredoxin is a plastidic cysteine-sulfinic acid reductase involved in the photooxidative stress response. Plant J 49 505–514 [DOI] [PubMed] [Google Scholar]
- Rippert P, Matringe M (2002) Purification and kinetic analysis of the two recombinant arogenate dehydrogenase isoforms of Arabidopsis. Eur J Biochem 269 4753–4761 [DOI] [PubMed] [Google Scholar]
- Rippert P, Scimemi C, Dubald M, Matringe M (2004) Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol 134 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N, Vieira Dos Santos C, Tarrago L, Rey P (2006) Plant methionine sulfoxide reductase A and B multigenic families. Photosynth Res 89 247–262 [DOI] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J (2000) Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol 124 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin WA, Evert RE, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in a sucrose export defective1 maize mutant. Plant Cell 8 645–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and cyanobacteria: evolutionary implications for tocopherol synthesis and function. Plant Physiol 132 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16 1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S (2006) Tocotrienols: vitamin E beyond tocopherols. Life Sci 78 2088–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbinova E, Kagan V, Han D, Packer L (1991) Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med 10 263–275 [DOI] [PubMed] [Google Scholar]
- Serbinova EA, Packer L (1994) Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol 234 354–366 [DOI] [PubMed] [Google Scholar]
- Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Schultz G, Joyard J, Douce R, Block M (1985) Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys 238 290–299 [DOI] [PubMed] [Google Scholar]
- Stillwell W, Ehringer W, Wassall SR (1992) Interaction of α-tocopherol with fatty acids in membranes and ethanol. Biochim Biophys Acta 1105 237–244 [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry V, Gustafsson P, Gardestrom P (1997) Development of Arabidopsis leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J 12 605–614 [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L (1993) Structural and dynamic membrane properties of α-tocopherol and α-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry 32 10692–10699 [DOI] [PubMed] [Google Scholar]
- Terzaghi WB, Fork DC, Berry JA, Field CB (1989) Low and high temperature limits to PSII. Plant Physiol 91 1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier E, Block MA, Douce R, Joyard J (1996) Is E37, a major polypeptide of the inner membrane from plastid envelope, an S-adenosyl methionine-dependent methyltransferase? Plant J 10 903–912 [DOI] [PubMed] [Google Scholar]
- Theriault A, Chao J-T, Wang Q, Gapor A, Adeli K (1999) Tocotrienol: a review of its therapeutic potential. Clin Biochem 32 309–319 [DOI] [PubMed] [Google Scholar]
- Traber MG, Sies H (1996) Vitamin E and humans: demand and delivery. Annu Rev Nutr 16 321–347 [DOI] [PubMed] [Google Scholar]
- Trebst A, Depka B, Holländer-Czytko H (2002) A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett 516 156–160 [DOI] [PubMed] [Google Scholar]
- Tsegaye Y, Shintani DK, DellaPenna D (2002) Overexpression of the enzyme p-hydroxyphenylpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol Biochem 40 913–920 [Google Scholar]
- Tuquet C, Newman DW (1980) Aging and regreening in soybean cotyledons. I. Ultrastructural changes in plastids and plastoglobuli. Cytobios 29 43–59 [PubMed] [Google Scholar]
- van Wijk R, Kobayashi M, van Wijk EP (2006) Anatomic characterization of human ultra-weak photon emission with a moveable photomultiplier and CCD imaging. J Photochem Photobiol B 83 69–76 [DOI] [PubMed] [Google Scholar]
- Vavilin DV, Ducruet J-M (1998) The origin of 115-130°C thermoluminescence bands in chlorophyll containing material. Photochem Photobiol 68 191–198 [Google Scholar]
- Vidi P-A, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dörmann P, Kessler F, Bréhélin C (2006) Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J Biol Chem 281 11225–11234 [DOI] [PubMed] [Google Scholar]
- Vieira Dos Santos C, Cuiné S, Rouhier N, Rey P (2005) The Arabidopsis plastidic methionine sulfoxide reductase B proteins: sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A and induction by photooxidative stress. Plant Physiol 138 909–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Gombos Z, Murata N (1990) Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 347 200–203 [DOI] [PubMed] [Google Scholar]
- Walker D (1987) The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis. Research Institute of Photosynthesis, University of Sheffield, Sheffield, UK
- Wise RR (1995) Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45 79–97 [DOI] [PubMed] [Google Scholar]
- Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Kanno A, Kameya T (1996) Cool temperature-induced chlorosis in rice plants. Plant Physiol 112 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Niki E, Nogushi N (2003) Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem Phys Lipids 123 63–75 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.