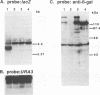
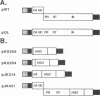
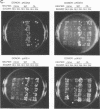
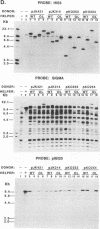
Abstract
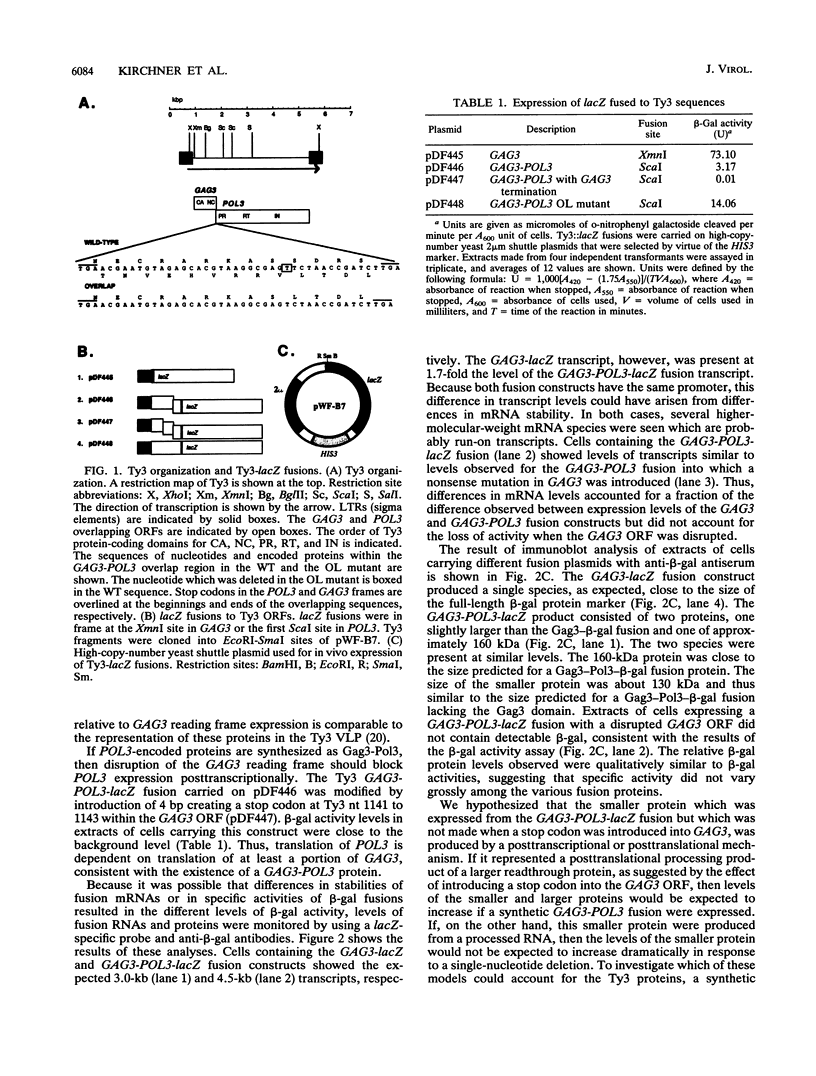
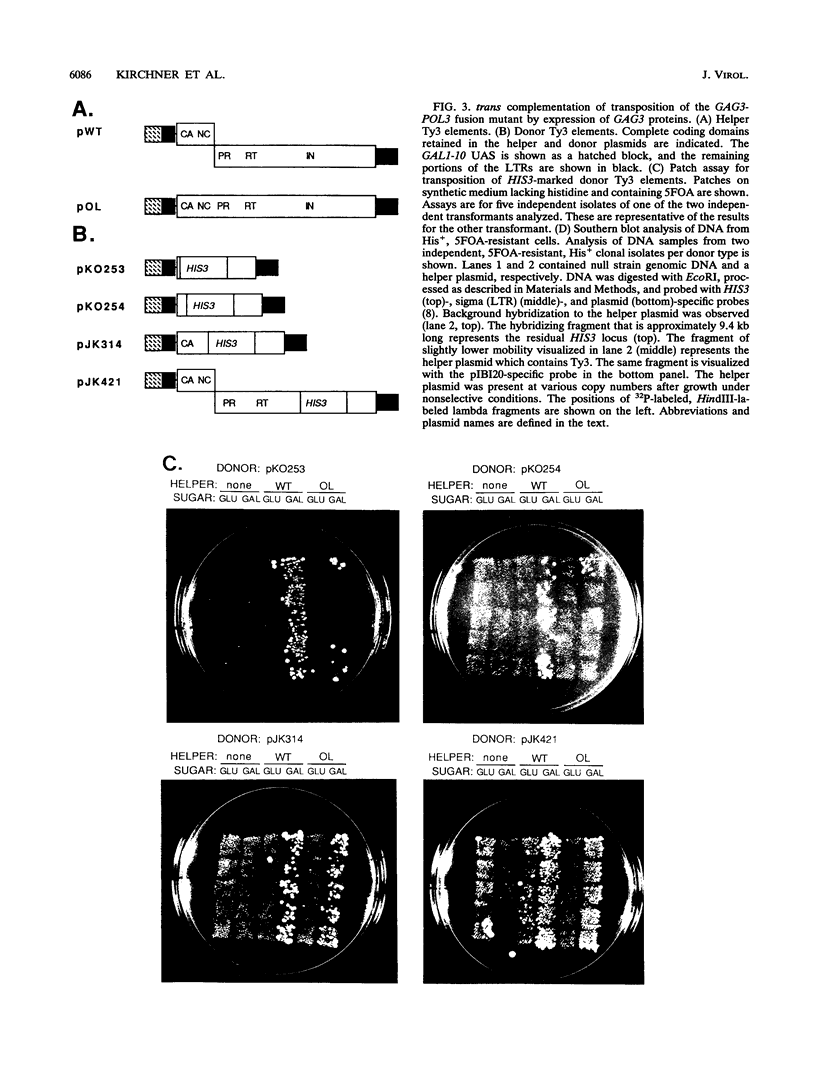
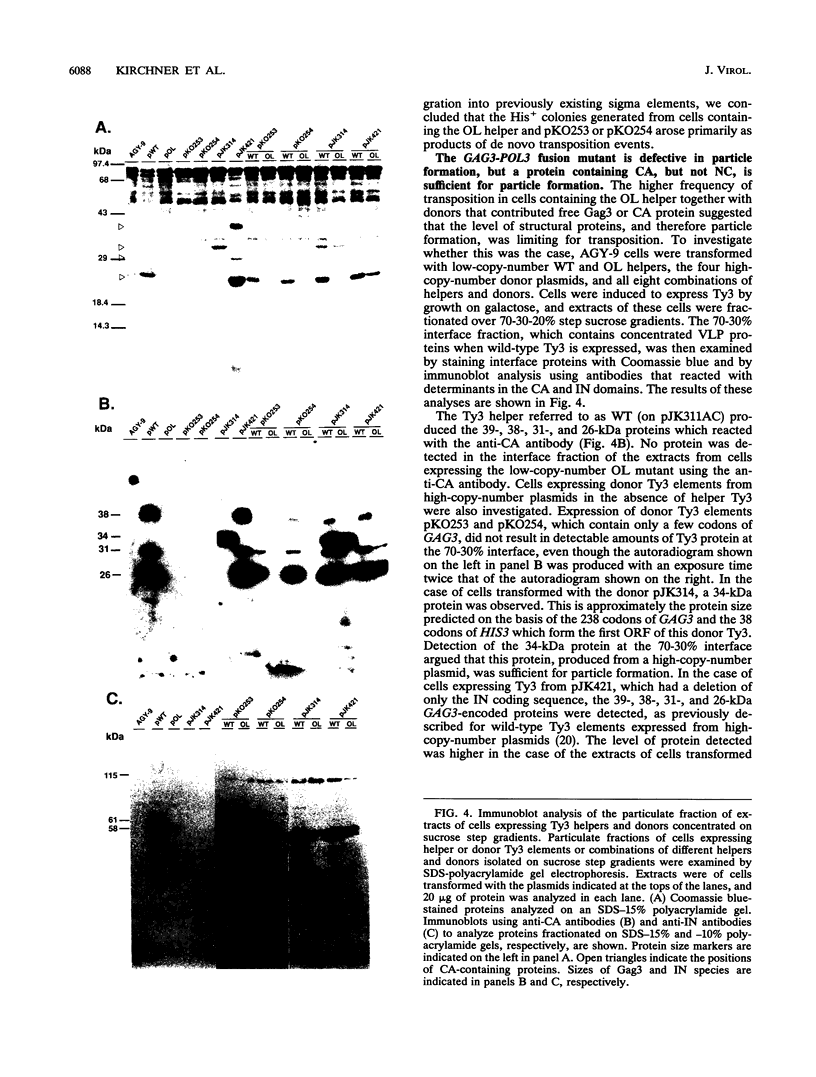
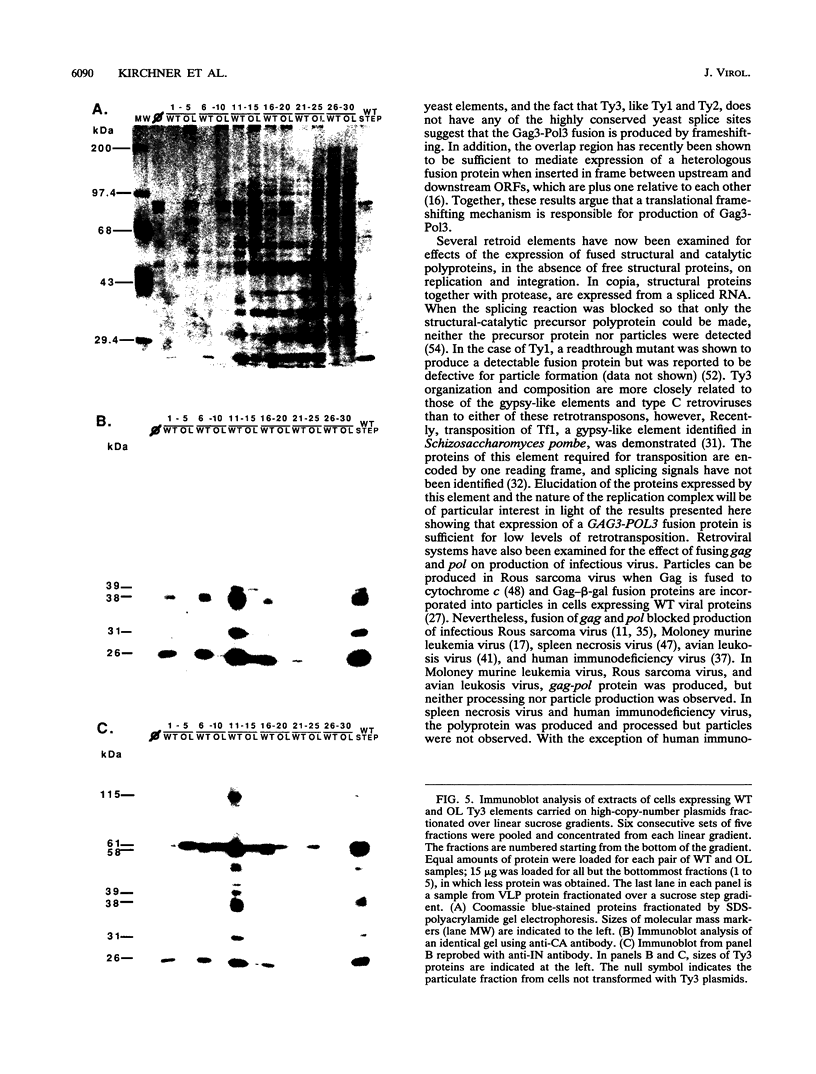
Ty3 encodes structural proteins in its upstream open reading frame (GAG3) and catalytic proteins in an overlapping open reading frame (POL3). As is the case for retroviruses, high levels of structural protein versus catalytic proteins are synthesized and we show here that catalytic proteins are derived from a GAG3-POL3 fusion polyprotein. To evaluate the relative contributions of structural and catalytic components of the Ty3 particle, we perturbed the balance of these proteins by fusing the GAG3 and POL3 frames. This fusion Ty3 was capable of complementing low levels of transposition of a donor Ty3 which contained only cis-acting sequences required for transposition. Examination of extracts of cells expressing the GAG3-POL3 fusion mutant showed that particle formation differed qualitatively and quantitatively from viruslike particle formation by wild-type Ty3. Suprisingly, expression of 238 codons of GAG3, encoding only capsid protein, complemented transposition and particle formation defects of the fusion mutant, showing that the limiting deficiency was in capsid, and not in nucleocapsid, function. In addition, protein containing the capsid domain expressed alone accumulated in the same particulate fraction as viruslike particles, showing that it was sufficient for particle formation. The activity of the Ty3 fusion mutant contrasts with the inviability of mutant retroviruses in which gag and pol frames were fused and argues that retrotransposons tolerate considerable variation in the nucleoprotein complexes that permit replication and integration.
Full text
PDF






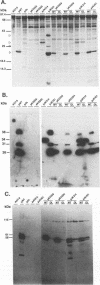
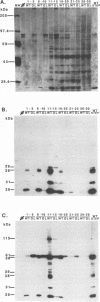
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belcourt M. F., Farabaugh P. J. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990 Jul 27;62(2):339–352. doi: 10.1016/0092-8674(90)90371-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. P., Rhee S., Craven R. C., Hunter E., Wills J. W. Amino acids encoded downstream of gag are not required by Rous sarcoma virus protease during gag-mediated assembly. J Virol. 1991 Jan;65(1):272–280. doi: 10.1128/jvi.65.1.272-280.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S. C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989 May 19;57(4):537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns N. R., Saibil H. R., White N. S., Pardon J. F., Timmins P. A., Richardson S. M., Richards B. M., Adams S. E., Kingsman S. M., Kingsman A. J. Symmetry, flexibility and permeability in the structure of yeast retrotransposon virus-like particles. EMBO J. 1992 Mar;11(3):1155–1164. doi: 10.1002/j.1460-2075.1992.tb05156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker D. L., Sandmeyer S. B. Transfer RNA genes are genomic targets for de Novo transposition of the yeast retrotransposon Ty3. Genetics. 1990 Dec;126(4):837–850. doi: 10.1093/genetics/126.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare J. J., Belcourt M., Farabaugh P. J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Bilanchone V. W., Haywood L. J., Dildine S. L., Sandmeyer S. B. A yeast sigma composite element, TY3, has properties of a retrotransposon. J Biol Chem. 1988 Jan 25;263(3):1413–1423. [PubMed] [Google Scholar]
- Craven R. C., Bennett R. P., Wills J. W. Role of the avian retroviral protease in the activation of reverse transcriptase during virion assembly. J Virol. 1991 Nov;65(11):6205–6217. doi: 10.1128/jvi.65.11.6205-6217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Elder R. T., Loh E. Y., Davis R. W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein K. M., Goff S. P. Expression of the gag-pol fusion protein of Moloney murine leukemia virus without gag protein does not induce virion formation or proteolytic processing. J Virol. 1988 Jun;62(6):2179–2182. doi: 10.1128/jvi.62.6.2179-2182.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. The gene and the primary structure of ornithine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1987 Jul 25;262(21):10127–10133. [PubMed] [Google Scholar]
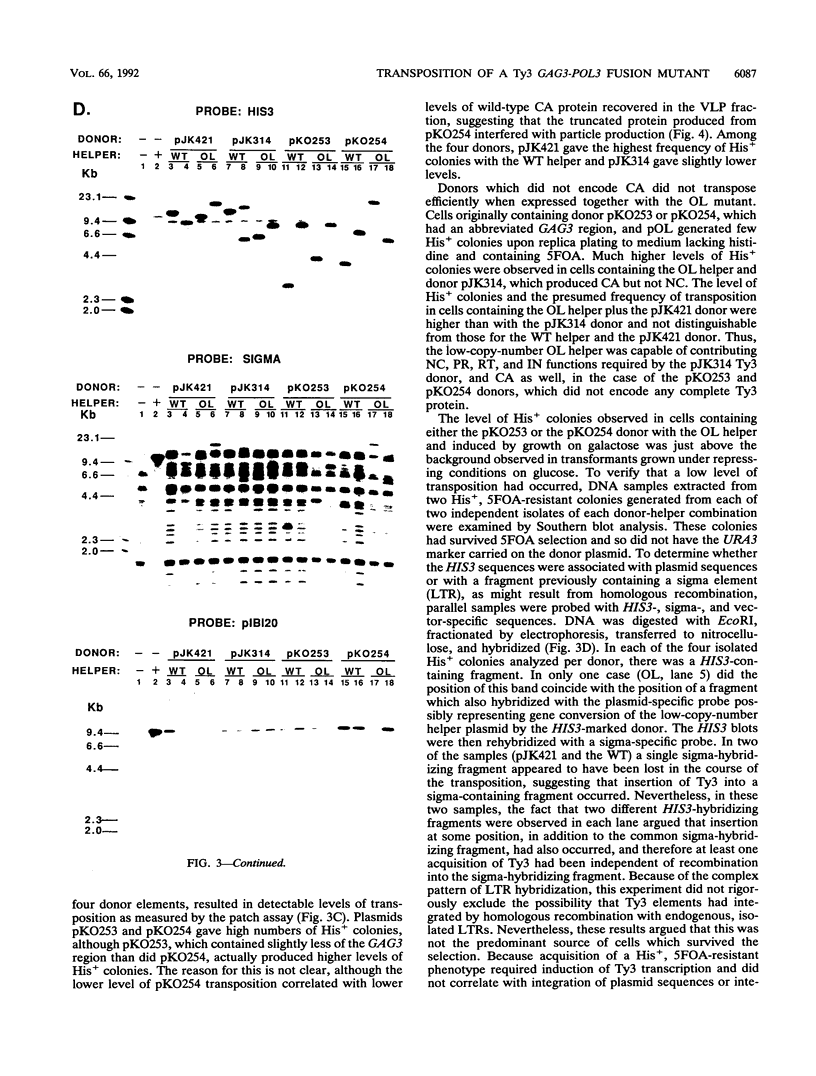
- Hansen L. J., Chalker D. L., Orlinsky K. J., Sandmeyer S. B. Ty3 GAG3 and POL3 genes encode the components of intracellular particles. J Virol. 1992 Mar;66(3):1414–1424. doi: 10.1128/jvi.66.3.1414-1424.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. J., Sandmeyer S. B. Characterization of a transpositionally active Ty3 element and identification of the Ty3 integrase protein. J Virol. 1990 Jun;64(6):2599–2607. doi: 10.1128/jvi.64.6.2599-2607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Oroszlan S. The where, what and how of ribosomal frameshifting in retroviral protein synthesis. Trends Biochem Sci. 1990 May;15(5):186–190. doi: 10.1016/0968-0004(90)90159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. Translational suppression in gene expression in retroviruses and retrotransposons. Curr Top Microbiol Immunol. 1990;157:93–124. doi: 10.1007/978-3-642-75218-6_4. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Blaug G., Hansen M., Barklis E. Assembly of gag-beta-galactosidase proteins into retrovirus particles. J Virol. 1990 May;64(5):2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin H. L., Boeke J. D. Demonstration of retrotransposition of the Tf1 element in fission yeast. EMBO J. 1992 Mar;11(3):1145–1153. doi: 10.1002/j.1460-2075.1992.tb05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., Weaver D. C., Boeke J. D. Two related families of retrotransposons from Schizosaccharomyces pombe. Mol Cell Biol. 1990 Dec;10(12):6791–6798. doi: 10.1128/mcb.10.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J., Fulton S. M., Dobson M. J., Wilson W., Kingsman S. M., Kingsman A. J. A retrovirus-like strategy for expression of a fusion protein encoded by yeast transposon Ty1. Nature. 1985 Jan 17;313(5999):243–246. doi: 10.1038/313243a0. [DOI] [PubMed] [Google Scholar]
- Oertle S., Bowles N., Spahr P. F. Complementation studies with Rous sarcoma virus gag and gag-pol polyprotein mutants. J Virol. 1992 Jun;66(6):3873–3878. doi: 10.1128/jvi.66.6.3873-3878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Morrow C. D. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J Virol. 1991 Sep;65(9):5111–5117. doi: 10.1128/jvi.65.9.5111-5117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Stewart L., Vogt V. M. trans-acting viral protease is necessary and sufficient for activation of avian leukosis virus reverse transcriptase. J Virol. 1991 Nov;65(11):6218–6231. doi: 10.1128/jvi.65.11.6218-6231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Stetler G. L., Thorner J. The yeast repeated element sigma contains a hormone-inducible promoter. Mol Cell Biol. 1987 Feb;7(2):749–759. doi: 10.1128/mcb.7.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow S. L., Coffin J. M. Truncated gag-related proteins are produced by large deletion mutants of Rous sarcoma virus and form virus particles. J Virol. 1985 Jul;55(1):79–85. doi: 10.1128/jvi.55.1.79-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. A., Talbot K. J., Panganiban A. T. Spleen necrosis virus gag polyprotein is necessary for particle assembly and release but not for proteolytic processing. J Virol. 1990 Jun;64(6):2642–2652. doi: 10.1128/jvi.64.6.2642-2652.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon R. A., Jr, Erdie C. R., Oliver M. G., Wills J. W. Incorporation of chimeric gag protein into retroviral particles. J Virol. 1990 Sep;64(9):4169–4179. doi: 10.1128/jvi.64.9.4169-4179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Dollard C., Malone E. A., Clare J., Kapakos J. G., Farabaugh P., Minehart P. L. Three genes are required for trans-activation of Ty transcription in yeast. Genetics. 1987 Apr;115(4):649–656. doi: 10.1093/genetics/115.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Boeke J. D. Host genes that influence transposition in yeast: the abundance of a rare tRNA regulates Ty1 transposition frequency. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8360–8364. doi: 10.1073/pnas.87.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Honma H., Zushi M., Kondo S., Togashi S., Miyake T., Shiba T. Virus-like particle formation of Drosophila copia through autocatalytic processing. EMBO J. 1990 Feb;9(2):535–541. doi: 10.1002/j.1460-2075.1990.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]