Abstract
Overexpression of the RIα subunit of cAMP-dependent protein kinase (PKA) has been demonstrated in various human cancers. PKA has been suggested as a potential target for cancer therapy. The goal of the present study was to evaluate an anti-PKA antisense oligonucleotide (mixed-backbone oligonucleotide) as a therapeutic approach to human cancer treatment. The identified oligonucleotide inhibited the growth of cell lines of human colon cancer (LS174T, DLD-1), leukemia (HL-60), breast cancer (MCF-7, MDA-MB-468), and lung cancer (A549) in a time-, concentration-, and sequence-dependent manner. In a dose-dependent manner, the oligonucleotide displayed in vivo antitumor activity in severe combined immunodeficient and nude mice bearing xenografts of human cancers of the colon (LS174T), breast (MDA-MB-468), and lung (A549). The routes of drug administration were intraperitoneal and oral. Synergistic effects were found when the antisense oligonucleotide was used in combination with the cancer chemotherapeutic agent cisplatin. The pharmacokinetics of the oligonucleotide after oral administration of 35S-labeled oligonucleotide into tumor-bearing mice indicated an accumulation and retention of the oligonucleotide in tumor tissue. This study further provides a basis for clinical studies of the antisense oligonucleotide targeted to the RIα subunit of PKA (GEM 231) as a cancer therapeutic agent used alone or in combination with conventional chemotherapy.
Keywords: antisense therapy, oral drug delivery, breast cancer, colon cancer, lung cancer
cAMP-dependent protein kinase (PKA) is involved in various cellular functions, including cell proliferation, gene induction, metabolism, secretion, and ion-channel regulation. PKA is composed of two catalytic (C) and two regulatory (R) subunits and has type-I and type-II isozymes, with different R subunits, termed RI and RII, interacting with an identical C subunit (1). Thus far, four isoforms of R subunits, RIα, RIβ, RIIα, and RIIβ, have been identified. Increased expression of the RIα subunit of PKA correlates with cell proliferation and neoplastic growth (2). Overexpression of the RIα subunit of PKA occurs in various human tumor tissues and cell lines including cancers of breast (3–5), ovary (6, 7), lung (8), and colon (9–11). Furthermore, overexpression of the RIα subunit of PKA correlates with malignancy and poor prognosis in cancer patients (5–7). In addition, the RIα subunit interacts with the cytochrome c oxidase subunit vb (12), which is involved in controlling multiple drug resistance (13, 14), and is associated with tumor sensitivity to cancer chemotherapeutic agents such as cisplatin (15). PKA also phosphorylates the epidermal growth factor receptor and decreases its tyrosine kinase activity and signal transduction both in vitro and in vivo (16). Therefore, the RIα subunit of PKA is a potential target for human cancer therapy. In the last decade, there have been increasing efforts to develop PKA-specific inhibitors as cancer therapeutic agents (2, 17).
Selective down-regulation of the RIα subunit of PKA by unmodified and phosphorothioate antisense oligonucleotides (oligos) causes growth inhibition and differentiation of various cancer cell lines and shows antitumor activity in human tumor xenografts (18, 19). Whereas the identified phosphorothioate oligodeoxynucleotide (PS-oligo) for the RIα subunit is selective, specific, and potent in inhibiting tumor growth, safety studies involving repeated administration revealed side effects in mice, thereby limiting its therapeutic utility (20). PS-oligos containing CG motifs are highly stimulatory of the immune system (20–23). After repeated doses of a PS-oligo containing CG dinucleotides to mice, a significant increase in spleen weight, a decrease in platelet counts, and an increase in serum alanine aminotransferase and aspartate aminotransferase activities were noted (20). In contrast, a modified PS-oligo with the same base composition except with CG dinucleotides being replaced by GC dinucleotides showed significantly fewer changes in the above parameters (20). Furthermore, modification of selected PS-oligos by substituting four deoxynucleosides at both the 3′ and 5′ ends with 2′-O-methylribonucleosides provided a mixed-backbone oligonucleotide (MBO). The MBO had a significantly better safety profile than did the PS-oligo (24, 25). A phase I clinical trial of the MBO (GEM 231) has been recently completed (26); phase II clinical trials are ongoing in patients with solid tumors. In the present study, we have evaluated this MBO for antitumor activity in vitro and in vivo. The class of MBOs in our previous studies is shown to be bioavailable after oral (27, 28) and colorectal (28) administration. In an early study, a 5′-end-protected unmodified oligo was found to be bioavailable after oral and rectal administration (29). Therefore, in the present study, the MBO targeted to the RIα subunit of PKA was also evaluated for its antitumor activity after oral administration.
Materials and Methods
Chemicals and Oligonucleotides.
The test MBO (Oligo AS, 5′-GCGUGCCTCCTCACUGGC-3′ and its mismatched control (Oligo ASM, 5′-GCAUGCATCCGCACAGGC-3′) were synthesized, purified, and analyzed as described (24, 25, 27, 30, 31). Four nucleosides at both the 3′ and 5′ ends are 2′-O-methylribonucleosides (represented by boldface letters). The remaining are deoxynucleosides; all internucleotide linkages are phosphorothioate. The underlined nucleotides of Oligo ASM are the sites of the mismatches compared with Oligo AS. The purity of the MBOs was >90% as shown by capillary gel electrophoresis and PAGE, with the remainder being n − 1 and n − 2 products. The integrity of internucleotide linkages was confirmed by 31P NMR. 35S-labeled Oligo AS was prepared as reported (31, 32), and its purity was >98% as analyzed by PAGE, with the remainder being n − 1 and n − 2 products. MEM, Earle’s balanced salt solution, RPMI medium 1640, Dulbecco–Vogt-modified Eagle’s medium/F-12 Ham’s medium (DMEM/F-12 1:1 mixture), Ham’s F-12K medium, PBS, and cisplatin were obtained from Sigma. FBS, trypsin, penicillin/streptomycin, and trypan blue stain were purchased from GIBCO/BRL. Matrigel basement membrane matrix was obtained from Becton Dickinson Labware. The anti-RIα subunit monoclonal antibody was kindly provided by Y. S. Cho-Chung (National Cancer Institute, Bethesda, MD).
Cell Culture.
The tumor cell lines, LS174T, DLD-1, HL-60, MDA-MB-468, and A549, were obtained from the American Type Culture Collection and cultured according to their instructions with slight modifications. In vitro inhibitory activities of oligos on tumor cell growth were studied by using the conditions described earlier (33–35). The cell culture media used were as follows: MEM with 0.1 mM nonessential amino acids and Earle’s balanced salt solution containing 10% FBS for LS174T cells, RPMI 1640 medium containing 10% FBS for DLD-1 cells, RPMI 1640 medium containing 20% FBS for HL-60 cells, DMEM/F-12 Ham’s medium (DMEM/F-12 1:1 mixture) containing 10% FBS for MDA-MB-468 cells, and Ham’s F-12K medium containing 10% FBS (90% Ham’s F-12K and 10% FBS) for A549 cells. All media included 1% penicillin/streptomycin. The cells were treated with oligos when they were about 50% confluent. Cells were exposed to various concentrations of oligos (0.1, 1, 5, and 10 μM) for 5 consecutive days. The medium was changed on day 3 and the same concentrations of oligos were added into the new medium. The viable cells were counted after trypan blue staining.
In Vivo Tumor Model.
Human cancer xenograft models were established by using the methods reported previously (33, 34). Female C.B-17-scid/scid mice or nude mice (5 weeks old) were purchased from Frederick Cancer Research Facility. Cultured LS174T cells were harvested, washed twice with MEM, resuspended in MEM, and injected s.c. (2 × 106 cells, total volume 0.2 ml) into the left inguinal area of the mice. Cultured MDA-MB-468 cells were harvested, washed twice with DMEM/F-12 Ham’s medium (DMEM/F-12 1:1 mixture), resuspended in DMEM/F-12 Ham’s medium:Matrigel basement membrane matrix (2:1), and injected s.c. (20 × 106 cells, total volume 0.2 ml) into the mice. The harvested A549 cells were washed with Ham’s F-12K medium, resuspended in Ham’s F-12K:Matrigel basement membrane matrix (2:1), and injected s.c. (20 × 106 cells, total volume 0.2 ml) into the mice. The animals were monitored by general clinical observation, determination of body weight, and tumor growth. Tumor growth was recorded by the measurement (with calipers) of two perpendicular diameters of the implant (33, 34). Tumor mass (in g) was estimated by calculation with the formula ½a × b2, where a is the long diameter (cm) and b is the short diameter (cm) (33, 34).
In Vivo Chemotherapy.
The chemotherapy began when the tumor mass reached 75–150 mg. The animals bearing xenografts of human cancer of the colon (LS174T), lung (A549), or breast (MDA-MB-468) were randomly divided into various treatment groups and a control group (six mice per group). Oligo AS and Oligo ASM, dissolved in physiological saline (0.9% NaCl), were administered i.p. or by gavage. The volume injected was based on the body weight (5 μl/g of body weight), and the concentrations of oligos were adjusted on the basis of the dose (0.5–10 mg/kg). The designated doses were given daily, 5 consecutive days per week. The control group received physiological saline only. For combination therapy, animals were treated with Oligo AS or Oligo ASM as above and cisplatin at an i.p. dose of 3 mg/kg in saline (0.6 mg/ml; injection volume, 5 μl/g of body weight, twice per week). The data on tumor growth were analyzed by ANOVA.
Pharmacokinetic Study.
For oral pharmacokinetic study of Oligo AS, 35S-labeled and unlabeled Oligo AS were mixed at a concentration of 5 mg/ml with a specific activity of 2 μCi/ml (1 μCi = 37 kBq). The animals bearing LS174T xenografts received Oligo AS by gavage at a dose of 10 mg/kg (three mice per group for each time point). After Oligo AS administration, each animal was placed in a metabolic cage and fed commercial diet and water ad libitum. Blood and tissue samples were collected as described (24, 25). The plasma and tissue concentrations were estimated on the basis of the total Oligo AS-derived radioactivity determined by liquid scintillation counting with methods described previously (24, 25). The pharmacokinetic parameters were estimated by using pcnonlin programs. The maximal concentration (Cmax), the time at Cmax (Tmax), the absorption half-life (T1/2A), elimination half-life (T1/2β), mean residue time, and the area under the curve were calculated (27, 30, 36).
Expression of the RIα Subunit of PKA.
The levels of PKA in cultured cells and tumor tissue were determined by Western blot analysis. In brief, cell lysates or tumor tissue homogenates containing identical amounts of total protein (50 μg or 100 μg, respectively) were fractionated by SDS/PAGE and transferred to Trans-Blot nitrocellulose membranes (Bio-Rad). The membrane was then incubated with blocking buffer (PBS containing 0.1% Tween 20 and 5% nonfat dry milk) for 1 h at room temperature, washed with the washing buffer (PBS containing 0.1% Tween 20) for 5 min twice, and then incubated with primary (anti-RIα) antibody overnight at 4°C with gentle shaking. The membrane was washed with the washing buffer for 15 min and then twice for 5 min, and next was incubated with 1:5,000 diluted goat anti-mouse IgG horseradish peroxidase-conjugated antibody (Bio-Rad) for 1 h at room temperature. After washing as described above, the protein of interest was detected by enhanced chemiluminescence reagents (ECL; Amersham Pharmacia).
Results
In Vitro Cell Growth Inhibition by Oligo AS.
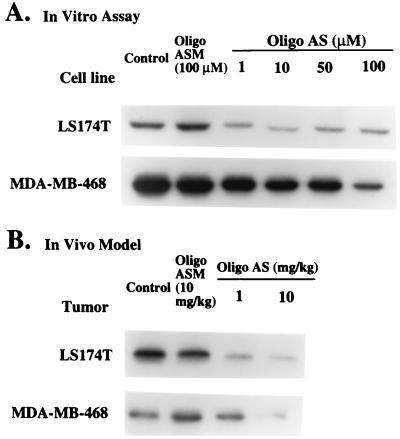
The test oligonucleotide, Oligo AS, inhibited the growth of tumor cell lines LS174T, DLD-1, HL-60, A549, and MDA-MB-468 in a dose-dependent manner. The IC50 values for the mismatched control, Oligo ASM, were 10- to 100-fold greater than that of Oligo AS, indicating the specificity of Oligo AS (Table 1). The specificity of Oligo AS was confirmed when expression of the RIα subunit of PKA was down-regulated (Fig. 1A). For LS174T cells, at the concentrations of 1 and 10 μM, Oligo AS inhibited the RIα subunit expression by about 80%, with no significant dose–response being observed at higher concentrations. However, there was a dose-dependent repression of the RIα subunit expression between 0.1 and 1 μM Oligo AS (data not shown). For MDA-MB-468 cells, a significant dose–response relationship was observed between 1 and 100 μM Oligo AS. At the highest concentration, Oligo AS inhibited the RIα subunit expression by about 75%. In contrast, under the same experimental conditions, the mismatched control, Oligo ASM, had no significant effect.
Table 1.
Effect of oligonucleotides on the growth of human cancer cell lines in culture
| Cell line | IC50, μM
|
|
|---|---|---|
| Oligo AS | Oligo ASM | |
| LS174T (colon cancer) | <0.1 | 3.7 |
| DLD-1 (colon cancer) | 0.22 | >5 |
| HL60 (leukemia) | <0.1 | >5 |
| MDA-MB-468 (breast cancer) | 1.3 | >10 |
| A549 (lung cancer) | 0.1 | 10 |
Values are means based on the data from three separate experiments. Cells were exposed to various concentrations of oligos (0.1 to 10 μM) for 5 consecutive days. The viable cell numbers were counted after trypan blue staining. The detailed cell culture conditions are described in Materials and Methods.
Figure 1.
(A) Effects of oligos on the expression of the RIα subunit of PKA in LS174T and MDA-MB-468 cells. Cultured cells were treated with Oligo AS or ASM at various concentrations for 24 h. Identical amounts of total protein (50 μg) were fractionated by SDS/PAGE and analyzed by Western blotting. For LS174T cells, at the concentrations of 1 and 10 μM, Oligo AS inhibited RIα subunit expression by about 80%, with no significant dose–response being observed at higher concentrations. For MDA-MB-468 cells, a significant dose–response relationship was observed. At the highest concentration, Oligo AS inhibited the RIα subunit expression by about 75%. In contrast, Oligo ASM had no effect on RIα subunit expression for either cell line. (B) Effects of oligos on the expression of the RIα subunit of PKA in LS174T and MDA-MB-468 tumor xenografts. Animals with LS174T xenografts were treated with oral administration of Oligo AS or ASM for 1 week. Animals with MDA-MB-468 xenografts were treated with oral administration of Oligo AS or ASM for 6 weeks. Identical amounts of total protein (100 μg) from the tumor homogenates were used in Western blot analysis. A dose-dependent inhibition of the RIα subunit expression was observed in both tumor models. At 10 mg/kg, Oligo AS inhibited RIα subunit expression by about 80%. In contrast, Oligo ASM had no effect.
In Vivo Antitumor Activity of Oligo AS Used Alone or in Combination with Cisplatin.
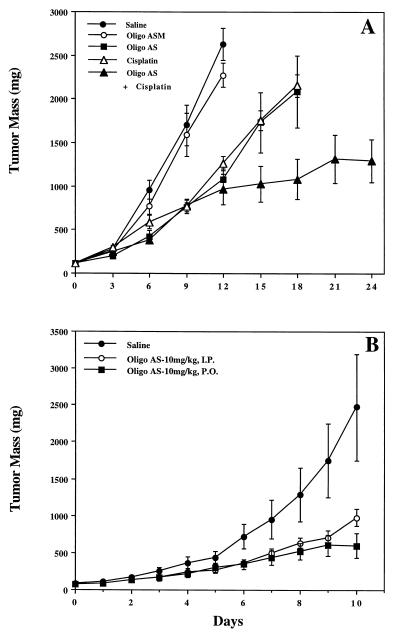
In vivo antitumor activity of Oligo AS was first evaluated in an in vivo human colon LS174T tumor model (Fig. 2). The in vivo antitumor effects of Oligo AS on the tumor growth were observed with both i.p. and gavage administration. Oligo ASM had minimal activity (Fig. 2A). Western blot analyses showed that, in mice treated with Oligo AS, expression of the RIα subunit of PKA was significantly lower compared with untreated or Oligo ASM-treated mice (Fig. 1B). These results demonstrate the sequence specificity of the antisense effects of Oligo AS. In addition, in vivo synergistic effects between Oligo AS and cisplatin were observed in the LS174T model (Fig. 2A), indicating that the anti-PKA oligo may be used as a tumor sensitizer for conventional chemotherapy. As illustrated in Fig. 2B, significant antitumor activity was seen with Oligo AS after oral administration.
Figure 2.
Effects of oligos, administered alone or in combination with cisplatin, on the growth of the LS174T xenografts in nude mice. Each point represents the mean ± SE of tumor mass from six mice. Oligo AS and Oligo ASM were administered i.p. or by gavage administration at the daily dose of 10 mg/kg 5 consecutive days per week. The control group received physiological saline only. In the combination therapy, animals were treated with Oligo AS or Oligo ASM in the same protocol as above and cisplatin at an i.p. dose of 3 mg/kg twice per week. Oligo AS has significant antitumor effects after administration alone or in combination with cisplatin (P < 0.05) (A). Similar antitumor effects were found after i.p. or gavage administration of Oligo AS at the same dose (10 mg/kg per day) (B).
Pharmacokinetics of Oligo AS After Oral Administration.
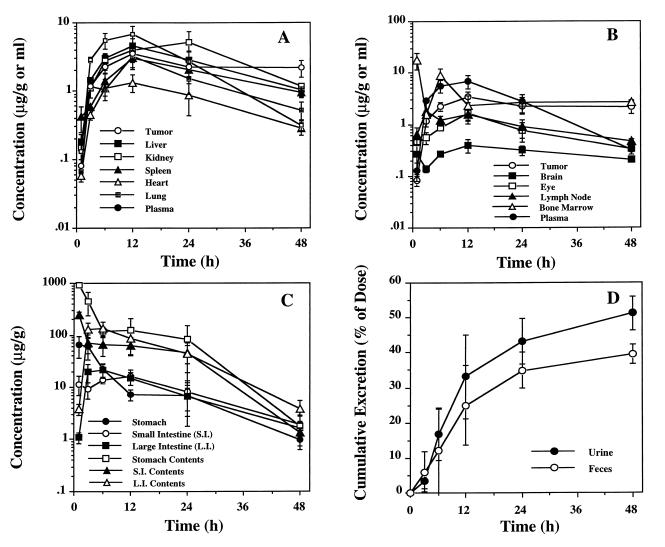
To demonstrate the pharmacological basis for the above observed in vivo antitumor activity of Oligo AS, the pharmacokinetics of Oligo AS were evaluated after oral dosing of 10 mg/kg 35S-Oligo AS to severe combined immunodeficient (SCID) mice bearing LS174T xenografts with tumor masses of 500–1,000 mg (Fig. 3). The maximum plasma concentration of Oligo AS was observed 6.8 h after oral dosing. The plasma disappearance curve could best be described by a first-order absorption one-compartment model, with an absorption half-life of 3.84 h, and an elimination half-life of 8.03 h (Table 2). The concentration–time curve in tumor tissue also could be best described by the same model, with a significantly prolonged elimination half-life (60.8 h) (Table 2), indicating a significant accumulation and retention of Oligo AS in tumor tissues. Oligo AS-derived radioactivity also was detected in other host tissues (Fig. 3 A–C) with a pattern similar to that seen in a previous study with the same type of MBO (27). By using a metabolic cage, complete urine and fecal collection was accomplished, and significant urinary excretion of Oligo AS-derived radioactivity was observed (Fig. 3D). On the basis of radioactivity quantitation, Oligo AS had good oral bioavailability (>48% in 48 h post dosing), which is similar to that previously reported with the same type of MBO (27).
Figure 3.
Plasma and tissue concentration time course. Concentrations based on the quantitation of radioactivity are expressed as μg of oligonucleotide equivalents/ml or g (mean ± SD) after oral administration of 35S-labeled Oligo AS (10 mg/kg) into mice bearing LS174T xenografts (three mice for each time point). To illustrate the relative levels of Oligo AS in tumor and plasma compared with other tissues, the data on tumor and plasma were plotted in both A and B. Pharmacokinetic parameters are summarized in Table 2; data indicate a significant accumulation and retention of the MBO in tumor tissues. Oligo AS-derived radioactivities also were detected in other host tissues (A–C) with a similar pattern seen in a previous study with a same type of MBO (27). Significant urinary excretion of Oligo AS-derived radioactivity was observed (D). Based on radioactivity, the MBO had a good oral bioavailability (>48% in 48 h after dosing).
Table 2.
Plasma and tumor pharmacokinetic parameters for Oligo AS
| Tissue | Cmax, μg/ml or g | Tmax, h | T1/2A, h | T1/2β, h | AUC, (μg/ml)⋅h | MRT, h |
|---|---|---|---|---|---|---|
| Plasma | 5.92 (0.83) | 9.93 (2.20) | 3.84 (0.28) | 8.03 (1.50) | 122.07 | 17.13 |
| Tumor | 2.95 (0.45) | 16.34 (4.40) | 3.78 (0.45) | 60.78 (20.10) | 290.92 | 93.15 |
Values are means (and SE) based on the data from three mice at each time point after oral administration of 35S-labeled Oligo AS. Pharmacokinetic parameters were estimated by using a first-order absorption one-compartment model. Cmax, maximal concentration; Tmax, the time of maximal concentration; T1/2A, absorption half-life; T1/2β, elimination half-life; AUC, area under the concentration–time curve; MRT, mean residue time.
Antitumor Activities of Oligo AS After Oral Administration in the Colon, Lung, and Breast Cancer Models.
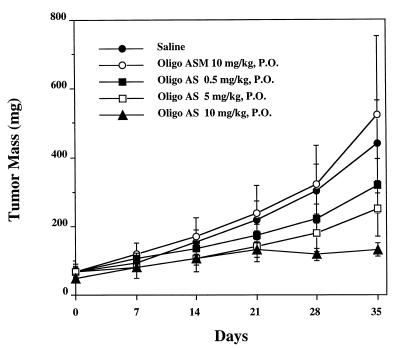
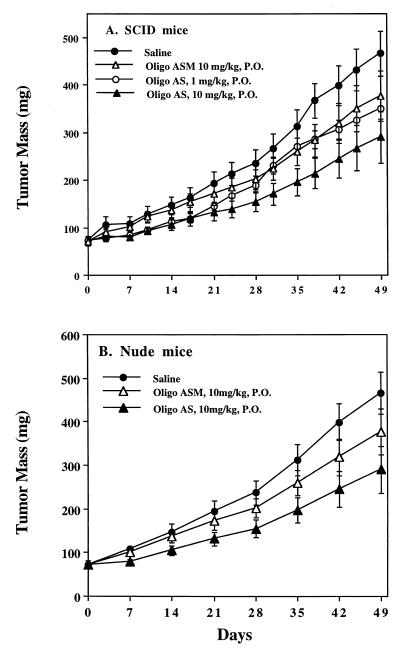
Oligo AS was administered by gavage to SCID mice bearing LS174T xenografts at the same dose as by i.p. administration (Fig. 2B). Oligo AS administered by either route showed similar antitumor activity (Fig. 2B). Oligo ASM showed no effect. The antitumor activity of Oligo AS after oral administration was further evaluated in human lung cancer (A549) xenografts (Fig. 4). Oligo AS, administered orally at 0.5, 5, and 10 mg/kg, showed dose-dependent antitumor activity. Oligo ASM had no effect, confirming the specificity of Oligo AS. Similar antitumor activity of Oligo AS was observed after oral administration in a breast cancer (MDA-MD-468) model (Fig. 5). In both nude and SCID mice, the tumor growth was delayed after treatment with Oligo AS compared with untreated mice or Oligo ASM-treated mice. Further confirmation of specificity was evident from the analysis of tumor tissues for levels of the RIα subunit of PKA, which was down-regulated in Oligo AS-treated tumors (Fig. 1B).
Figure 4.
Effects of oligos on the growth of human lung cancer A549 xenografts in C.B-17-scid/scid mice. Each point represents the mean ± SE of tumor mass from six mice. Oligo AS, administered orally at 0.5, 5, and 10 mg/kg per day at five doses per week, demonstrated dose-dependent antitumor activity. Oligo ASM has no effect, confirming the specificity of Oligo AS.
Figure 5.
Effects of oligos on the growth of human breast cancer MDA-MB-468 xenografts in C.B-17-scid/scid mice (A) and nude mice (B). Each point represents the mean ± SE of tumor mass from six mice. In both strains of mice, the tumor growth was delayed after treatment with Oligo AS compared with untreated or Oligo ASM-treated mice. Specificity was confirmed from the analysis of tumor tissues for the RIα subunits of PKA, which were down-regulated in Oligo AS-treated tumors compared with Oligo ASM-treated or untreated tumors (Fig. 1B).
Discussion
Antisense oligonucleotides are being evaluated for their therapeutic potential for various diseases (37–42). These agents achieve their effect by targeting mRNA with which they can hybridize and specifically block protein expression (37–39); thus, antisense oligos offer the possibility for a specific and rational drug design (38–40). Several antisense oligos have entered clinical studies (26, 39–42). The antisense drug Vitravene has now been approved for the treatment of patients with cytomegalovirus-induced retinitis (40).
Pharmacokinetics of PS-oligos has been studied after i.v., i.p., and s.c. administration (24, 32, 43). Analysis of PS-oligos extracted from various tissues after administration shows that PS-oligos are degraded primarily from the 3′ end; however, degradation from the 5′ end was observed as well (44). Toxicity studies of PS-oligos in animals have demonstrated some dose-dependent side effects (39, 45, 46). In the development of new generations of antisense oligos, major efforts have been devoted to stabilizing PS-oligos in vivo by various modifications of their structure (24, 25, 27, 28, 36, 39, 47–49). Compared with PS-oligos, MBOs, which have segments of 2′-O-methyl-oligoribonucleotide phosphorothioates at both the 3′ and 5′ ends, have greater in vivo stability and better pharmacokinetic and toxicology profiles (24, 25) and better oral bioavailability (27, 28).
In the past, attempts have been made to identify selective inhibitors of regulatory subunits of PKA, but with limited success. Selective inhibition of the RIα subunit of PKA by 8-Cl-cAMP results in the growth arrest of a variety of cancer cells in vitro and in vivo (2, 19). 8-Cl-cAMP also increases the sensitivity of cytotoxic drugs. Overexpression of the mutant RIα subunit in lung carcinoma cells inhibits the in vivo metastatic property (50) and increases the sensitivity to cytotoxic drugs (13). PS-oligos targeted to the RIα subunit of PKA are effective in inhibiting tumor growth in vitro and in vivo (2, 51), but showed dose-dependent side effects, thereby limiting their therapeutic utility (20). Previous studies have indicated that PS-oligo-induced toxicities in mice and rats are primarily caused by immune stimulation and largely associated with a CG motif (20–23, 52). Recent studies further demonstrated that the immunostimulation effects of PS-oligos with the RRCGYY sequences were limited to rodents, whereas the TCG motif has immunostimulation effects in human cells (22, 23). Modification of the CG motif present in a given PS-oligo leads to a significant reduction of the side effects (20, 52). Compared with the PS-oligo, the newly designed MBO, Oligo AS, causes significantly fewer side effects in mice and rats (20, 25). In phase I clinical trials, Oligo AS (GEM 231) has shown minimal side effects (26).
The purpose of the present study was to explore the potential use of orally administered antisense oligos in the treatment of human cancer. First, the antisense MBO, Oligo AS, had a significant in vitro inhibitory effect on tumor cell growth in a sequence-specific manner. Second, after oral administration, significant antitumor effects were found in SCID and nude mice with xenografts of human cancers of the colon, breast, and lung. Third, Oligo AS showed sequence-specific effects on expression of the RIα subunit of PKA both in vitro and in vivo. Fourth, the pharmacokinetics of Oligo AS after oral administration in the LA174T model revealed that the MBO had good oral bioavailability. Furthermore, significant additive effects on tumor growth after combination treatment of Oligo AS and cisplatin were shown in the LS174T model. These results should be useful in future clinical evaluation of antisense oligos not only for the specific MBO targeted to PKA (GEM 231) but for other oligos targeted to other genes in general.
The above results, while promising, raise some intriguing questions relative to the pharmacokinetics and bioavailability properties of oligos. Oligo AS, after gavage, showed a wide tissue distribution, including the tumor. The comparative plasma and tumor areas under the curves and half-lives suggest that the absorbed Oligo AS was retained in the tumor longer than in other tissues. Although we have not analyzed the stability of Oligo AS in tissues after oral administration, the selective antitumor activity and down-regulation of the RIα subunit of PKA suggest the presence of absorbed intact Oligo AS in tissues. Further studies are needed to determine the mechanisms responsible for the uptake and accumulation of antisense oligos in tumor and host tissues.
Our results also indicate the potential use of Oligo AS as a therapeutic agent for cancer treatment alone and also in combination therapy. The major advantage of the use of antisense oligos is to avoid dose-limiting side effects associated with conventional chemotherapy if lower doses of cytotoxic agents can be used. The use of antisense oligos also may improve the therapeutic effectiveness of conventional cancer therapy such as cytotoxic agents and radiation therapy. In future studies, the molecular mechanisms for such additive or synergistic effects need to be determined. In addition, the drug resistance profile of antisense oligos also may differ from that of currently used chemotherapeutic agents (52), suggesting that a combination therapy with antisense oligos and conventional therapy may less likely induce multidrug resistance, a common problem seen with current chemotherapy.
Acknowledgments
We thank L. Nan, B. Chambless, Y. Li, L. High, W. Liu, X. Xie, M. Liao, and J. Xu for their excellent technical assistance, Dr. R. B. Diasio for helpful discussion, and Dr. D. Hill for critical review of the manuscript. This study was partially supported by National Institutes of Health Grant R01 CA 80698 (to R.Z.). Paul Zamecnik is a member of the Board of Directors of Hybridon, Inc.
Abbreviations
- PKA
cAMP-dependent protein kinase
- oligo
oligonucleotide
- PS-oligo
phosphorothioate oligodeoxynucleotide
- MBO
mixed-backbone oligonucleotide
- SCID
severe combined immunodeficient
- RI
regulatory subunit
References
- 1.Beebe S J, Corbin J D. In: The Enzymes: Control by Phosphorylation. Boyer P D, Kerbes E G, editors. 17, Part A. New York: Academic; 1986. pp. 43–111. [Google Scholar]
- 2.Cho-Chung Y S, Clair T. Pharmacol Ther. 1993;60:265–288. doi: 10.1016/0163-7258(93)90010-b. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury A W, Miller W R, Carter D C. Br J Cancer. 1991;63:201–204. doi: 10.1038/bjc.1991.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller W R, Watson D M A, Jack W, Chetty U, Elton R A. Breast Cancer Res Treat. 1993;26:89–94. doi: 10.1007/BF00682703. [DOI] [PubMed] [Google Scholar]
- 5.Miller W R, Hulme M J, Bartlett J M, MacCallum J, Dixon J M. Clin Cancer Res. 1997;3:2399–2404. [PubMed] [Google Scholar]
- 6.Simpson J B B, Ramage A D, Hulme M J, Burns D J, Katsaros D, Langdon S, Miller W R. Clin Cancer Res. 1996;2:201–206. [PubMed] [Google Scholar]
- 7.McDaid H M, Cairns M T, Atkinson R J, McAleer S, Harkin D P, Gilmore P, Johnston P G. Br J Cancer. 1999;79:933–939. doi: 10.1038/sj.bjc.6690149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young M R I, Montpetit M, Lozano Y, Djordjevic A, Devata S, Matthews J P, Yedavalli S, Chejfec G. Int J Cancer. 1995;61:104–109. doi: 10.1002/ijc.2910610118. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury A W, Carter D C, Miller W R, Cho-Chung Y S, Clair T. Br J Cancer. 1994;69:738–742. doi: 10.1038/bjc.1994.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bold R J, Alpard S, Ishizuka J, Townsend C M, Jr, Thompson R. Regul Pept. 1994;53:61–70. doi: 10.1016/0167-0115(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 11.Gordge P C, Hulme M J, Clegg R A, Miller W R. Eur J Cancer. 1996;32:2120–2126. doi: 10.1016/s0959-8049(96)00255-9. [DOI] [PubMed] [Google Scholar]
- 12.Yang W L, Iacono L, Tang W M, Chin K V. Biochemistry. 1998;37:14175–14180. doi: 10.1021/bi981402a. [DOI] [PubMed] [Google Scholar]
- 13.Abraham I, Chin K V, Gottesman M M, Mayo J K, Sampson K E. Exp Cell Res. 1990;189:133–141. doi: 10.1016/0014-4827(90)90265-c. [DOI] [PubMed] [Google Scholar]
- 14.Rohlff C, Glazer R T. Cell Signalling. 1995;7:431–434. doi: 10.1016/0898-6568(95)00018-k. [DOI] [PubMed] [Google Scholar]
- 15.Cvijic M E, Chin K-V. Biochem Biophys Res Commum. 1998;249:723–727. doi: 10.1006/bbrc.1998.9223. [DOI] [PubMed] [Google Scholar]
- 16.Barbier A J, Poppleton H M, Yigzaw Y, Mullenix J B, Wiepz G J, Bertics P J, Patel T B. J Biol Chem. 1999;274:14067–14073. doi: 10.1074/jbc.274.20.14067. [DOI] [PubMed] [Google Scholar]
- 17.Glazer R I, Rohlff C. Breast Cancer Res Treat. 1994;31:263–271. doi: 10.1007/BF00666159. [DOI] [PubMed] [Google Scholar]
- 18.Yokozaki H, Budillon A, Tortora G, Meissner S, Beaucage S L, Miki K, Cho-Chung Y S. Cancer Res. 1993;53:868–887. [PubMed] [Google Scholar]
- 19.Cho-Chung Y S, Nesterova M, Kondrashin A, Noguchi K, Srivastava R, Pepe S. Antisense Nucleic Acid Drug Dev. 1997;7:217–223. doi: 10.1089/oli.1.1997.7.217. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Zhao Q. Curr Opin Chem Biol. 1998;2:519–528. doi: 10.1016/s1367-5931(98)80129-4. [DOI] [PubMed] [Google Scholar]
- 21.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Nishioka Y, Reich C F, Pisetsky D S, Lipsky P E. J Clin Invest. 1996;98:1119–1129. doi: 10.1172/JCI118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann G, Weiner G J, Krieg A M. Proc Natl Acad Sci USA. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, Channavajjala L, Saxinger C, Zhang R. Proc Natl Acad Sci USA. 1997;94:2620–2625. doi: 10.1073/pnas.94.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal S, Zhao Q. Antisense Nucleic Acid Drug Dev. 1998;8:135–139. doi: 10.1089/oli.1.1998.8.135. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Ness E, Marshell J, Martin R, Dvorchik B, Rizvi N, Marquis J, Dahut W, Hawkins M J. Proc Am Soc Clin Oncol. 1999;18:159a. (abstr.). [PubMed] [Google Scholar]
- 27.Agrawal S, Zhang X, Zhao H, Lu Z, Yan J, Cai H, Diasio R B, Habus I, Jiang Z, Iyer R P, et al. Biochem Pharmacol. 1995;50:571–576. doi: 10.1016/0006-2952(95)00160-2. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal S, Zhang R. In: Antisense Research and Application. Crooke S T, editor. Berlin: Springer; 1998. pp. 525–543. [Google Scholar]
- 29.Vlassov V V, Karamyshev V N, Yakubov L A. FEBS Lett. 1993;327:271–274. doi: 10.1016/0014-5793(93)81002-h. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Lu Z, Zhao H, Zhang X, Diasio R B, Habus I, Jiang Z, Iyer R P, Yu D, Agrawal S. Biochem Pharmacol. 1995;50:545–556. doi: 10.1016/0006-2952(95)00159-w. [DOI] [PubMed] [Google Scholar]
- 31.Padmapriya A P, Tang J Y, Agrawal S. Antisense Res Dev. 1994;4:185–199. doi: 10.1089/ard.1994.4.185. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal S, Temsamani J, Tang J-Y. Proc Natl Acad Sci USA. 1991;88:7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Q, Lindsey J R, Zhang R. Int J Oncol. 1997;10:953–960. doi: 10.3892/ijo.10.5.953. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Cai Q, Lindsey J R, Li Y, Chambless B, Naguib F N M. Int J Oncol. 1997;10:1147–1156. doi: 10.3892/ijo.10.6.1147. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Zhang R. Int J Oncol. 1998;12:793–804. [PubMed] [Google Scholar]
- 36.Zhang R, Iyer P, Yu D, Zhang X, Lu Z, Zhao H, Agrawal S. J Pharmacol Exp Ther. 1996;278:971–979. [PubMed] [Google Scholar]
- 37.Zamecnik P C. In: Antisense Therapeutics. Agrawal S, editor. Totowa, NJ: Humana; 1996. pp. 1–11. [Google Scholar]
- 38.Smith J B, Wickstrom E. J Natl Cancer Inst. 1998;90:1146–1154. doi: 10.1093/jnci/90.15.1146. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal S. Trends Biotechnol. 1996;14:376–387. doi: 10.1016/0167-7799(96)10053-6. [DOI] [PubMed] [Google Scholar]
- 40.Crooke S T. Antisense Nucleic Acid Drug Dev. 1998;8:vii–viii. doi: 10.1089/oli.1.1998.8.vii. [DOI] [PubMed] [Google Scholar]
- 41.Crooke S T, Grillone L R, Tendolkar A, Garrett A, Fratkin M J, Leeds J, Barr W H. Clin Pharmacol Ther. 1994;56:641–646. doi: 10.1038/clpt.1994.189. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Yan J, Shahinian H, Amin G, Lu Z, Liu T, Saag M S, Jiang Z, Temsamani J, Martin R R, et al. Clin Pharmacol Ther. 1995;58:44–53. doi: 10.1016/0009-9236(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 43.Sands H, Gorey-Feret L J, Cocuzza A J, Hobbs F W, Chidester D, Trainor G L. Mol Pharmacol. 1994;45:932–943. [PubMed] [Google Scholar]
- 44.Temsamani J, Roskey A, Chaix C, Agrawal S. Antisense Nucleic Acid Drug Dev. 1997;7:159–165. doi: 10.1089/oli.1.1997.7.159. [DOI] [PubMed] [Google Scholar]
- 45.Galbraith W M, Hobson W C, Giclas P C, Schechter P, Agrawal S. Antisense Res Dev. 1994;4:201–206. doi: 10.1089/ard.1994.4.201. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal S, Rustagi P K, Shaw D. Toxicol Lett. 1996;82/83:431–434. doi: 10.1016/0378-4274(95)03573-7. [DOI] [PubMed] [Google Scholar]
- 47.Crooke S T. Antisense Nucleic Acid Drug Dev. 1998;8:115–122. doi: 10.1089/oli.1.1998.8.115. [DOI] [PubMed] [Google Scholar]
- 48.Crooke S T. Adv Pharmacol. 1997;40:1–49. doi: 10.1016/s1054-3589(08)60136-2. [DOI] [PubMed] [Google Scholar]
- 49.Crooke S T, Graham M J, Zuckerman J E, Brooks D, Conklin B S, Cummins L L, Greig M J, Guinosso C J, Kornbrust D, Manoharan M, et al. J Pharmacol Exp Ther. 1996;277:923–937. [PubMed] [Google Scholar]
- 50.Young M R I, Young M E, Lozano Y, Bagash J M. Invasion Metastasis. 1992;12:253–263. [PubMed] [Google Scholar]
- 51.Srivastava R K, Srivastava A R, Park Y G, Agrawal S, Cho-Chung Y S. Breast Cancer Res Treat. 1998;49:97–107. doi: 10.1023/a:1005905723550. [DOI] [PubMed] [Google Scholar]
- 52.Barker R H, Jr, Metelev V, Rapaport E, Zamecnik P. Proc Natl Acad Sci USA. 1996;93:514–518. doi: 10.1073/pnas.93.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]