Abstract
Extensive G protein-coupled receptor families in both the main and accessory olfactory systems have been implicated in axonal targeting, sensory function, and cell survival. Although sensory function seems to be mediated by G proteins, axonal guidance and cell survival may be G protein-independent processes. In the accessory olfactory system, the Go-containing neurons in the basal vomeronasal organ (VNO) project to the posterior accessory olfactory bulb (AOB), whereas more apically located VNO neurons contain Gi2 and project to the anterior AOB. Herein, we investigate the organization of the accessory olfactory system in mice with a targeted deletion in the Goα gene. The accessory olfactory system seems normal at birth; however, postnatally, the number of Go-receptor-containing VNO neurons decreases by half, and apoptotic neurons are detected. The axons of VNO neurons remain restricted to the posterior AOB. The posterior AOB is reduced in size but contains a synaptophysin-positive layer with the normal number of glomeruli. The posterior AOB has reduced mitral cell c-Fos immunoreactivity, consistent with decreased sensory activation of Go protein-coupled VNO receptor neurons. Thus, in the accessory olfactory system, receptor-coupled G proteins are required for cell survival.
The G protein-coupled receptor families of both the main and accessory olfactory systems participate in axonal targeting, sensory function, and possibly cell survival (1–6). The Golf protein downstream of main olfactory receptors is necessary for sensory function but not axonal targeting (7). The role of Golf in sensory neuron survival is unknown. The accessory olfactory system is thought to be involved in the detection of and response to pheromones (8–14). In the accessory olfactory system, the Go-containing neurons in the basal vomeronasal organ (VNO) project to the posterior accessory olfactory bulb (AOB), whereas more apically located VNO neurons contain Gi2 and project to the anterior AOB (15–17). The Go protein is concentrated both in VNO microvilli (15–17), where it is thought to play a role in sensory transduction (9, 15–17), and in axons (15–17), where it might contribute to axon guidance (16, 18, 19).
One family of VNO receptors (Gi-VN) is characterized by short extracellular segments, is expressed by cells located in the middle of the VNO epithelium, and is coexpressed with Gi2 (20–22). Recent gene targeting experiments have shown that this family of receptors participates in the projection of axons to specific glomeruli within the anterior AOB (4, 5). It is unclear whether the expression of Gi-VN receptors is required for the survival of VNO neurons (compare refs. 5 and 6). A functional role for these receptors in pheromone-induced effects is suspected but not documented. Gene-targeting studies for a second class of receptors (Go-VN, the receptors coexpressed with Go) have not been described. Herein, we have investigated the role of Goα in receptor cell survival by examining mice lacking this G protein. We find that Go-VN receptor cells require Goα for survival, thus defining a crucial role for G protein activation in the prevention of neuronal apoptosis.
Materials and Methods
The generation, screening, and biochemical characterization of mice lacking the α-subunit of Go has been described (23). In this study, this 129 Sv-ter/C57BL/6 hybrid strain was maintained by backcrossing of heterozygotes for at least five generations. In every experiment, littermate experimental and control specimens were compared. Initially, observations were obtained separately from sex-matched littermate pairs of wild-type and Go −/− mice. However, at the sexually immature ages tested in this study, no differences were noted between mice of different sexes, and data are derived from an equal number of male and female mice.
For in situ hybridization, digoxigenin-labeled riboprobes and alkaline-phosphatase-coupled anti-digoxigenin antibody were employed (24). Corresponding sense riboprobes yielded no alkaline phosphatase reaction product. The Goα probe spans the entire coding region of the rat mRNA (ref. 25, splice form A with 97% identity to the mouse sequence). The Gi2α probe is complimentary to the entire coding portion of the human cDNA (ref. 26, 91% identity to the mouse sequence). The Go-VN receptor probes are derived from mouse mRNAs (21). The G protein specificity of the hybridization signals is evident from the selective hybridization patterns in +/+ samples, and the absence of any Goα signal in the −/− mice.
Mice were anesthetized and perfused transcardially with 4% (vol/vol) paraformaldehyde in PBS. After cryopreservation, 20-μm coronal (VNO) and parasagittal (AOB) cryostat sections were collected. Rhodamine-conjugated lectins (Vector Laboratories) from Erythina cristagalli (ECL) or Bandeiraea simplicifolia (BSL) were applied directly to the slices. Primary antibodies directed against OCAM were provided by K. Mori (The Institute for Physical and Chemical Research, Wako, Japan; refs. 27 and 28), and antibodies against synaptophysin (Sigma), MAP-2 (Sigma), and c-Fos (Santa Cruz Biotechnology) were obtained commercially. For synaptophysin and OCAM, bound antibody was detected with fluorescently labeled secondary antibodies, and for c-Fos, bound antibody was detected with the avidin–biotin-complex peroxidase method (Vector Laboratories). Omission of either primary or secondary antibody abolished staining. For each antibody, the staining pattern in wild-type mice matched published patterns (10–14, 27, 28). Counterstaining with 4′,6-diamidino-2-phenylindole (0.2 μg/ml) was performed after staining for synaptophysin. Staining with terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) was performed by using a kit according to the manufacturer’s instructions (Roche Molecular Biochemicals).
Results
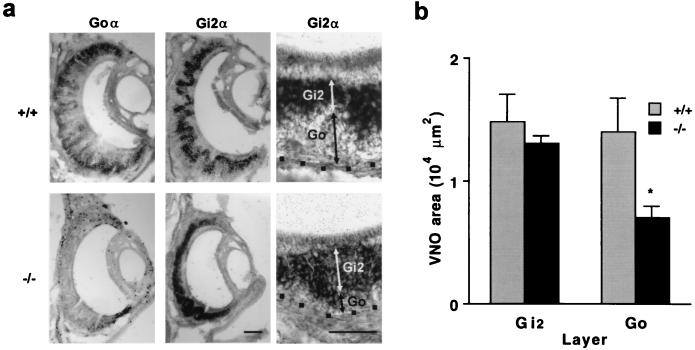
We analyzed mice with a targeted deletion in the Goα gene that abrogates expression of the specificity-determining α-subunit (23). Despite the absence of major neurodevelopmental abnormalities (23, 29), the VNO of postnatal day 15 (P15) mice lacking Go is altered in a layer-specific fashion. In situ hybridization for mRNA encoding the α-subunit of Go and Gi2 confirms the absence of Go mRNA but also shows that a greater percentage of neurons are positive for the Gi2 transcript (Fig. 1). The laminar organization of the VNO persists, with Gi2 expression in more apical layers and with basally located neurons lacking this G protein. The absolute thickness of the Gi2 layer is not altered, suggesting that there is a 50% reduction in the number of non-Gi2, Go-type neurons.
Figure 1.
Loss of Go layer cells in the VNO of Go −/− mice. (a) The mRNA for Goα or Gi2α was localized by in situ hybridization in the VNO of P15 Go +/+ and −/− mouse samples. Note that there is no expression of Goα in the knockout animals. Cells expressing Gi2α constitute a larger fraction of the epithelium in the Go −/− samples. Because the epithelium of the −/− mice consists predominantly of Gi2-positive neurons, the irregular layering of the epithelium is less prominent than in the +/+ specimens. The dots represent the base of the epithelium. (Left and Center, bar = 50 μm; Right, bar = 50 μm.) (b) The area with Gi2α mRNA was measured in the central 300-μm circumferential extent of sections through the mid portion of the VNO. The area of VNO basal to the Gi2α-expressing zone was measured as the Go zone. Note the significant reduction in the area of the Go zone in the Go −/− samples (*, P ≤ 0.05, Student’s two-tailed t test). The data are means ± SEM from 5–8 animals in each group.
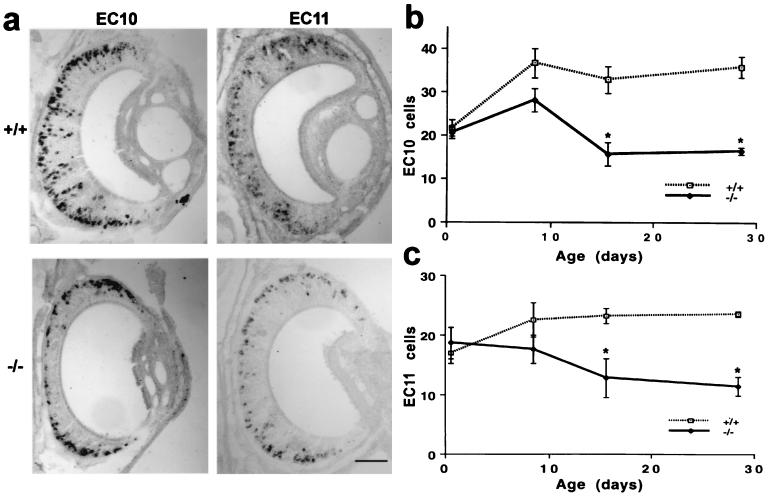
The reduction in non-Gi2 cells might be due to a selective loss of Go cells or to a more general cell loss coupled with a conversion to Gi2 expression. To examine this possibility further, we assessed the expression of two Go-VN receptor mRNAs (Fig. 2). As expected, these clones are expressed in the basal half of the VNO epithelium in wild-type mice. In Go −/− mice, the Go-VN receptor-positive cells are still found in the basal portions of the P15 VNO but are reduced in number by 50%. Coupled with the Gi2 expression data and the lack of compensatory changes in α-subunit expression in the brains of these mice (23), the data suggest that those cells expressing Go-VN receptors contain no functional G protein.
Figure 2.
Cells expressing Go-VN receptor mRNAs are reduced in number in Go −/− mice. (a) In situ hybridization for two Go-VN receptor transcripts, EC10 and EC11, is illustrated for P15 VNO samples from Go +/+ and −/− samples. Note the reduction in receptor-positive cells in the Go-deficient specimens. (Bar = 50 μm.) (b and c) The number of cells expressing EC10 or EC11 mRNA was measured in the central 200-μm circumferential extent of sections through the mid portion of the VNO. Note the significant reduction in the Go-VN-positive cells in the P15 and P28 Go −/− mice compared with age-matched +/+ mice (*, P ≤ 0.05, Student’s two-tailed t test). The data are means ± SEM from 2–8 animals in each group.
The reduction in the VNO in non-Gi2 and Go-VN cells might reflect a deficit in the generation of these cells or in their survival. The number of Go-VN cells at birth is close to normal, begins to drop by P7, and plateaus at the 50% level by P15 (Fig. 2). Thus, in utero, Go protein signaling seems to have no role in receptor selection, cell generation or cell survival.
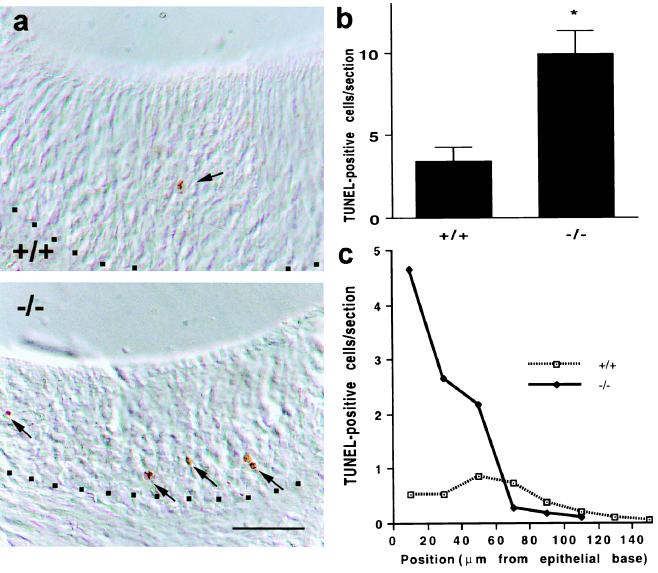
We hypothesized that, because Go-VN cells are being lost from the VNO, an increase in cell death rates in the absence of effective receptor coupling might occur. Apoptotic figures in the P15 VNO were examined by the TUNEL method (Fig. 3). In controls, rare TUNEL-positive cells are scattered through the epithelium. In the Go −/− epithelia, a marked increase in TUNEL-positive structures is noted, consistent with the idea that these cells undergo apoptosis at an increased rate in the absence of productive receptor signaling and thereby alter the overall composition of the VNO to one containing predominantly Gi2-positive cells. This signaling-dependent hypothesis concerning apoptosis suggests that cell death should be occurring selectively in the basal layers of the epithelium. Indeed, the proportion of apoptotic cells is increased selectively in this layer, and TUNEL-positive rates are not changed in apical layers (Fig. 3).
Figure 3.
Apoptosis of basal VNO cells in Go −/− mice. (a) P15 VNO sections from wild-type and Go −/− mice were stained for apoptotic figures by the TUNEL method. Positive staining is visualized as a brown reaction product in differential interference contrast microscopy images (arrows). The base of the epithelium is indicated by dots. (Bar = 50 μm.) (b) The number of TUNEL-positive structures was measured throughout the epithelium of sections through the mid portion of the P15 VNO. The number of TUNEL-positive figures is significantly greater in the Go −/− mice than in controls (*, P ≤ 0.05, Student’s two-tailed t test). The data are means ± SEM from 5–8 animals in each group. (c) The number of TUNEL-positive structures was measured relative to distance from the base of the P15 VNO epithelium as in a and b. The average number of positive cell corpses per section for each 20-μm increment from the base is illustrated. Note that the increased TUNEL staining in the Go-deficient VNO is localized to the basal portion of the epithelium. The data are from 5–8 animals in each group.
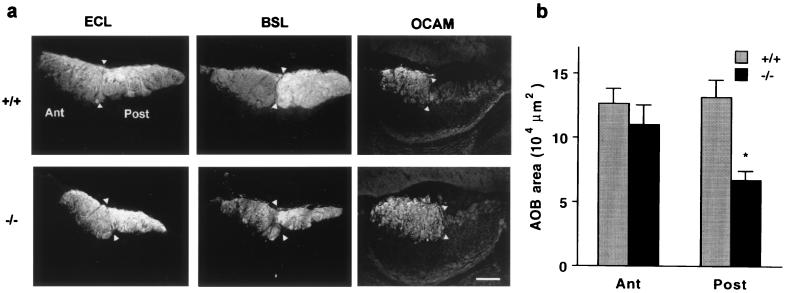
The axons of Go-VN cells project to the posterior part of the VNO, where they are segregated from the anterior AOB projections of Gi-VN cells (5, 6, 17). Because Go is highly concentrated in axonal growth cones (18), is found in VNO axons (17), and regulates neurite outgrowth (19), AOB projections might be grossly disorganized in the mice lacking Goα. Lectins provide strong staining of VNO axons in the AOB (Fig. 4). In control specimens, ECL labels the two groups of VNO axons with nearly equal intensity, whereas BSL labels Go-VN axons more intensely. There is a discrete boundary between the two territories of the AOB. In the Go −/− mice, this architecture is preserved. The anterior/posterior demarcation of AOB layers containing incoming fibers from the VNO is clearly detectable, and BSL selectively labels the posterior portion. However, the posterior territory is reduced in size by 50%, precisely matching the reduction in Go-VN cell number in the VNO. No Go-VN axons extend beyond their normal termination zone in the glomerular layer of the posterior AOB. The maintenance of the anterior/posterior, Gi2-VN/Go-VN border is also obvious in sections stained for the presence of the Gi-VN marker OCAM (Fig. 4; refs. 27 and 28). Fiber layers in the posterior AOB are OCAM-negative in specimens from both the control and knockout mice. No Gi-VN OCAM-positive fibers are detected in the posterior AOB VNO layer. Thus, the regional distribution of incoming fibers in the AOB is normal at this level of resolution, even though the number of Go-VN fibers is reduced by half.
Figure 4.
The posterior AOB fiber layer is reduced in Go −/− mice. (a) Parasagittal sections of the P15 AOB from control and Go knockout mice were stained with the lectins ECL or BSL or with anti-OCAM antibodies. Anterior (Ant), posterior (Post), and the border of the two AOB lobes (arrowheads) are indicated. In the Go −/− sections, the posterior half is reduced in area, but the staining characteristics are similar to those of control AOB. (Bar = 100 μm.) (b) The area of the two halves of the AOB revealed by ECL staining was measured in mid AOB sections from P15 mice. The area of the posterior AOB is significantly decreased in the Go −/− mice (*, P ≤ 0.05, Student’s two-tailed t test). The data are means ± SEM from 5–8 animals in each group.
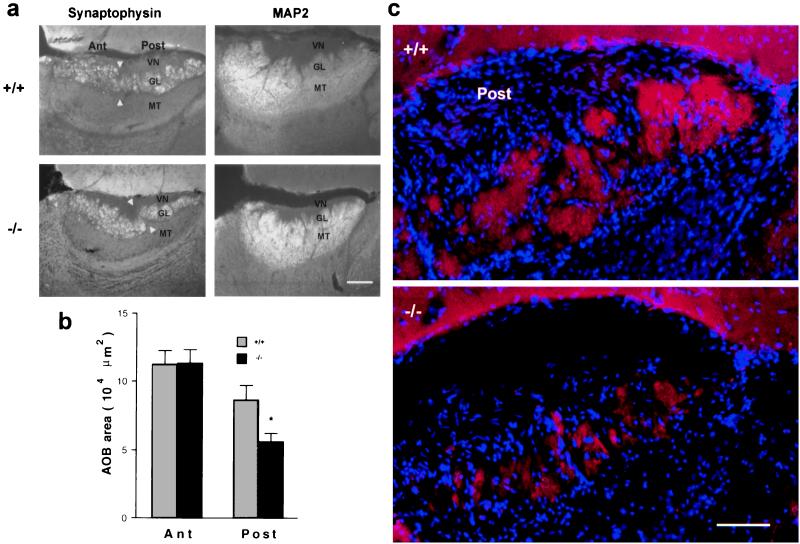
We considered whether other aspects of AOB structure are preserved in the Go knockout mice. Anti-synaptophysin antibodies selectively stain the synaptic glomerular layer where incoming VNO fibers synapse with the dendrites of underlying second-order mitral cells. The presence and position of this synaptic layer is maintained in the Go −/− mice at P15 (Fig. 5). Again, the size of this layer is reduced by nearly 50% compared with that of controls, and the changes are observed selectively in the posterior half of the glomerular layer. OCAM is known to be present at the termini of mitral cell dendrites in the posterior AOB glomerular layer as well as on the anterior Gi-VN axons (27, 28). Anti-OCAM staining of the posterior glomerular layer (Fig. 4) confirms the impressions obtained from synaptophysin immunohistology (Fig. 5). Clearly, the reduced number of Go-VN axons in Go −/− mice is capable of creating synaptophysin-immunoreactive (IR) structures in the appropriate layer of the AOB. Staining of the glomerular layer with anti-MAP2 reflects mitral cell dendritic arborizations and does not reveal any obvious secondary changes in the dendritic morphology of these cells (Fig. 5).
Figure 5.
Go −/− mice have smaller glomeruli in the posterior AOB. (a) P15 AOB parasagittal sections were stained with anti-synaptophysin or anti-MAP2 antibodies. The following areas of the AOB are indicated: vomeronasal nerve layer (VN), glomerular layer (GL), mitral and tufted cell layer (MT), anterior (Ant), posterior (Post), and the border of the two AOB lobes (arrowheads). In the Go −/− sections, the posterior GL is reduced in area, but the staining characteristics of different layers are normal. (Bar = 100 μm.) (b) The area of the anterior and posterior AOB-GL revealed by anti-synaptophysin staining was quantitated in mid AOB sections from P15 mice. The area of the posterior AOB-GL is significantly decreased in the Go −/− mice (*, P ≤ 0.05, Student’s two-tailed t test). The data are means ± SEM from 5–8 animals in each group. (c) Glomerular structures are visualized by double staining of P15 posterior AOB parasagittal sections with anti-synaptophysin antibody (red) and 4′,6-diamidino-2-phenylindole (blue). In the control samples, glomeruli are diffuse and large. In the Go −/− samples, the posterior GL layer has glomeruli of reduced area, but the total number is similar to that of controls. (Bar is 50 μm.)
In the main olfactory system, olfactory bulb glomeruli are fully separate from one another, and cells expressing a specific receptor project to a specific glomerulus (1, 2). For Gi-VN axons, the pattern is equally as selective, but glomeruli are less discrete (5, 6). For Go-VN axons receptor/glomerular selectivity has not been investigated, and glomeruli are even less distinct. However, genetic similarities of the Go-VN receptor family with the Gi-VN and olfactory receptor families argue that glomerular specificity is important for these receptors as well. The observations on Go-VN receptor number and glomerular layer thickness suggest that, if glomerular specificity is to be preserved in the Go −/− mice, then the size of each glomerulus should be reduced to an extent that matches the reduction in the number of cells expressing particular Go-VN receptors (i.e., 50%; Fig. 2). The number of glomeruli must remain the same if normal targeting patterns are to exist. In contrast, if the number of glomeruli were reduced by 50%, then the receptor-to-glomerulus map would be altered by necessity. Because posterior AOB glomeruli are indistinct in controls, we have not counted their number. However, there is clearly a trend toward a reduction in glomerular area and a preservation of glomerular number in the Go-deficient samples (Fig. 5). These data are consistent with a reduced number of incoming Go-VN fibers but a normal pattern of axonal targeting.
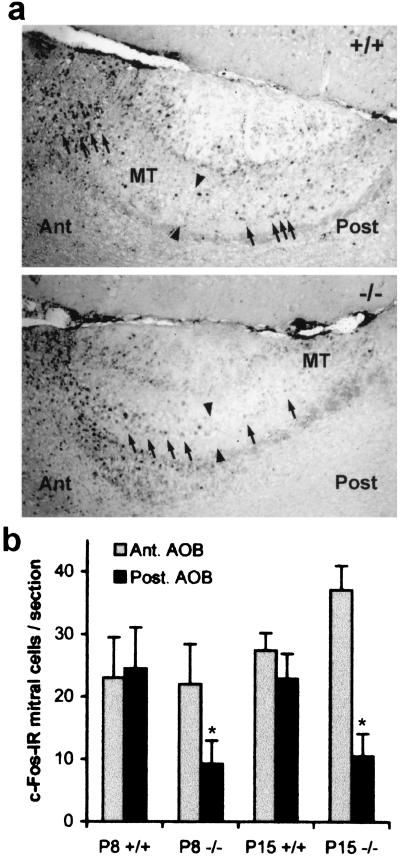
If the apoptosis of Go-VN neurons is secondary to a loss of sensory transduction, then measures of neuronal activity should be reduced before degeneration of the neurons. The expression of the immediate early gene c-fos has been correlated with sensory activity in the accessory olfactory system (10–14). c-Fos-IR mitral cells are detected throughout the AOB of juvenile +/+ mice (Fig. 6). The mice lacking Goα have a dramatic reduction of such staining in the posterior Go-VN-selective portion of the AOB. The reduction at P8 precedes the reduction of Go-VN neuron number in the VNO (Fig. 2). The decrease is similar in magnitude to the reduction in c-Fos-IR seen in the AOB of animals after VNO excision (11, 12, 14) and is consistent with Go-VN neuron insensitivity to sensory stimuli in the −/− mice.
Figure 6.
Reduced c-Fos-IR in the mitral cells of the posterior AOB in Go −/− mice. (a) P15 AOB parasagittal sections were stained with anti-c-Fos. The following features are labeled: mitral and tufted cell layer (MT), anterior (Ant), posterior (Post), and the border of the two AOB lobes (arrowheads). In the Go −/− sections, the posterior MT layer has fewer c-Fos-IR nuclei (arrows) than the +/+ sample. The width of the (Post) label corresponds to 70 μm. (b) The number of c-Fos-IR mitral cell nuclei was quantitated in mid AOB sections from P8 and P15 mice for the anterior and posterior portions. The number of c-Fos-IR cells in the posterior AOB is decreased significantly in the Go −/− mice compared with the number in +/+ control samples (*, P [ltequ} 0.05, Student’s two-tailed t test). The data are means ± SEM from 5–8 animals in each group.
Discussion
The major finding of the present study is that the absence of Go reduces the number of Go-VN receptor cells in the accessory olfactory system. These cells are formed and express Go-VN receptors normally in the absence of G protein coupling but then undergo apoptotic cell death at an increased rate. Lack of c-Fos expression in this posterior division of the AOB suggests that the Go-VN cells are unresponsive to sensory stimuli. Apparently, activation of a G protein cascade in these cells is required to prevent programmed cell death. Previously, the necessity of G protein-coupled receptor function for cell survival in olfactory systems has been unclear (2, 5, 6). Even in those studies documenting a loss of receptor gene-driven lacZ expression in the absence of receptor (2, 6), altered axonal targeting with altered trophic effects distinct from G protein transduction might explain the results. The data here show that some level of G protein stimulation is required for the survival of these cells. This effect might be cell-autonomous or be related to an interplay of inactive Go-VN cells with other structures, such as AOB synaptic target cells. Because the Gi-VN cells survive in the −/− mice, the initiating event in Go-VN neuronal death is likely to be reduced Go-dependent signaling in the Go-VN neurons.
G protein-coupled receptors are required for axonal targeting to specific glomeruli in the main olfactory system and in Gi2-VN division of the accessory olfactory system (5, 6). Previous studies of Golf had indicated that receptor expression, but not G protein coupling, is required for axonal targeting to specific glomeruli (7). Our data indicate that projection to the posterior half of the AOB does not depend on Go protein activity. It remains possible that other G proteins partially compensate for the absence of Goα. The present data do not indicate whether glomerular specificity is disrupted by Goα gene deletion. The formation of a normal number of glomeruli is more consistent with preserved glomerular specificity than with a failure of axonal targeting. The relative preservation of axonal targeting suggests that Go-VN cell apoptosis is not secondary to altered axonal projections. Future generation of crosses between Go −/− mice and Go-VN receptor lacZ knock-in mice should extend this analysis to the glomerular level.
The dependence of cell survival on G protein signaling may be a general phenomenon in cells that are specialized to transduce one particular signal. For example, receptor cell loss in the absence of signal transduction components occurs in the visual system as well as in the olfactory system (30–32). In contrast to these specialized cells, most central nervous system neurons express Go in concert with a multitude of receptor signaling pathways and are not sensitive to such trophic effects.
Acknowledgments
We thank Catherine Dulac for providing Go-VN receptor cDNAs and for helpful discussions and Eva J. Neer for providing mice with a targeted mutation in the Goα gene. This work was supported by a grant to S.M.S. from the National Institutes of Health. S.M.S. is an Investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Abbreviations
- AOB
accessory olfactory bulb
- VNO
vomeronasal organ
- Go-VN
Go protein-coupled VNO receptors
- Gi-VN
Gi2 protein-coupled VNO receptors
- ECL
Erythina cristagalli
- BSL
Bandeiraea simplicifolia
- Pn
postnatal day n
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
- IR
immunoreactive
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Nemes A, Mendelsohn M, Axel R. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 3.Krautwurst D, Yau K W, Reed R R. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Ivic L, Otaki J M, Hashimoto M, Mikoshiba K, Firestein S. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez I, Feinstein P, Mombaerts P. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 6.Belluscio L, Koentges G, Axel R, Dulac C. Cell. 1999;97:209–220. doi: 10.1016/s0092-8674(00)80731-x. [DOI] [PubMed] [Google Scholar]
- 7.Belluscio L, Gold G H, Nemes A, Axel R. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 8.Halpern M. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- 9.Kreiger J, Schmitt A, Lobel D, Gudermann T, Schultz G, Breer H, Boekhoff L. J Biol Chem. 1999;274:4655–4662. doi: 10.1074/jbc.274.8.4655. [DOI] [PubMed] [Google Scholar]
- 10.Kelliher K R, Liu Y C, Baum M J, Sachs B D. Neuroscience. 1999;92:1025–1033. doi: 10.1016/s0306-4522(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 11.Dudley C A, Moss R L. Neuroscience. 1999;91:1549–1556. doi: 10.1016/s0306-4522(98)00711-8. [DOI] [PubMed] [Google Scholar]
- 12.Inamura K, Kashiwayanagi M, Kurihara K. Eur J Neurosci. 1999;11:2254–2260. doi: 10.1046/j.1460-9568.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Halem H A, Cherry J A, Baum M J. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- 14.Fernandez-Fewell G D, Meredith M. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinohara H, Asano T, Kato K. J Neurosci. 1992;12:1275–1279. doi: 10.1523/JNEUROSCI.12-04-01275.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berghard A, Buck L B. J Neurosci. 1996;16:909–918. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia C, Halpern M. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- 18.Strittmatter S M, Valenzuela D, Kennedy T E, Neer E J, Fishman M C. Nature (London) 1990;344:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 19.Strittmatter S M, Fishman M C, Zhu X P. J Neurosci. 1994;14:2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulac C, Axel R. Cell. 1995;20:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 21.Herrada G, Dulac C. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- 22.Matsunami H, Buck L B. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer E J, Fishman M C. Proc Natl Acad USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goshima Y, Nakamura F, Strittmatter P, Strittmatter S M. Nature (London) 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 25.Jones D T, Reed R R. J Biol Chem. 1987;15:14241–14249. [PubMed] [Google Scholar]
- 26.Wong Y H, Federman A, Pace A M, Zachary I, Evans T, Pouyssegur J, Bourne H R. Nature (London) 1991;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- 27.Campenhausen H, Yoshihara Y, Mori K. NeuroReport. 1997;8:2607–2612. doi: 10.1097/00001756-199707280-00037. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara Y, Kawasaki M, Tamada A, Fujita H, Hayashi H, Kagamiyama H, Mori K. J Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang M, Gold M S, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphries M M, Rancourt D, Farrar G J, Kenna P, Hazel M, Bush R A, Sieving P A, Sheils D M, McNally N, Creighton P, et al. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 31.Lem J, Krasnoperova N V, Calvert P D, Kosaras B, Cameron D A, Nicolo M, Makino C L, Sidman R L. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biel M, Seeliger M, Pfeifer A, Kohler K, Gerstner A, Ludwig A, Jaissle G, Fauser S, Zrenner E, Hofmann F. Proc Natl Acad Sci USA. 1999;96:7553–7557. doi: 10.1073/pnas.96.13.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]