Summary
Background
The case-fatality for intentional self-poisoning in the rural developing world is 10–50-fold higher than that in industrialised countries, mostly because of the use of highly toxic pesticides and plants. We therefore aimed to assess whether routine treatment with multiple-dose activated charcoal, to interrupt enterovascular or enterohepatic circulations, offers benefit compared with no charcoal in such an environment.
Methods
We did an open-label, parallel group, randomised, controlled trial of six 50 g doses of activated charcoal at 4-h intervals versus no charcoal versus one 50 g dose of activated charcoal in three Sri Lankan hospitals. 4632 patients were randomised to receive no charcoal (n=1554), one dose of charcoal (n=1545), or six doses of charcoal (n=1533); outcomes were available for 4629 patients. 2338 (51%) individuals had ingested pesticides, whereas 1647 (36%) had ingested yellow oleander (Thevetia peruviana) seeds. Mortality was the primary outcome measure. Analysis was by intention to treat. The trial is registered with controlled-trials.com as ISRCTN02920054.
Findings
Mortality did not differ between the groups. 97 (6·3%) of 1531 participants in the multiple-dose group died, compared with 105 (6·8%) of 1554 in the no charcoal group (adjusted odds ratio 0·96, 95% CI 0·70–1·33). No differences were noted for patients who took particular poisons, were severely ill on admission, or who presented early.
Interpretation
We cannot recommend the routine use of multiple-dose activated charcoal in rural Asia Pacific; although further studies of early charcoal administration might be useful, effective affordable treatments are urgently needed.
Introduction
Treatment of self-poisoning involves resuscitation, antidotes, gastric decontamination, and supportive care.1,2 However, no evidence of benefit from gastric decontamination exists, resulting in widespread debate about the role of forced emesis, gastric lavage, and activated charcoal in the management of poisoned patients.3–6 Since few self-poisoning patients die in hospital in developed countries,7 decontamination seems unlikely to offer much benefit and its routine use is no longer recommended in these regions.8,9
Self-poisoning in the rural developing world, however, is quite different. Highly toxic pesticides and plants are ingested rather than pharmaceuticals and the resulting case-fatality is 10–50-fold higher than in developed countries.10 Self-poisoning causes more than 300 000 deaths each year in the Asia Pacific region alone.11,12 Effective therapies to improve treatment outcomes are urgently needed.
Activated charcoal is the only form of gastrointestinal decontamination that is still widely used.13 It is regarded as safe and volunteer studies show that early administration of one dose of activated charcoal can adsorb poison in the stomach and reduce absorption.14 Charcoal might also work long after ingestion, by interruption of enterohepatic and enterovascular cycling of poison.15 Multiple doses of activated charcoal are therefore given to some patients, irrespective of delay in presentation, to increase poison elimination.16 No evidence for clinical benefit from single-dose or multiple-dose activated charcoal regimens exists.14,17
Few patients present to hospital within 1–2 h in rural Asia; therefore, only one dose of activated charcoal is unlikely to offer benefit. By contrast, since charcoal might increase poison elimination several hours after its ingestion, several doses of activated charcoal might prove beneficial in such regions. Most Sri Lankan patients ingest either organophosphorus pesticides or yellow oleander (Thevetia peruviana) seeds, both of which are known to bind to activated charcoal.18,19 We therefore set up a trial to assess whether multiple doses of activated charcoal, compared with no charcoal, would reduce the rate of death and complications in self-poisoned patients in rural Sri Lanka. To distinguish whether any effect seen with multiple doses was a result of the first dose or all six doses, a third group of single-dose activated charcoal was added to the study.
Methods
Participants and procedures
The protocol for this randomised, controlled trial has been published.20 The trial was done in secondary referral hospitals in Anuradhapura, Polonnaruwa, and Kurunegala, Sri Lanka. We approached all patients with poison ingestion admitted to adult wards. The exclusion criteria were: age less than 14 years; treatment with charcoal during this episode; known pregnancy; ingestion of a corrosive or a hydrocarbon alone; need for medicine given orally; inability of medical staff to intubate the patient with a Glasgow coma score (GCS) less than 13; presentation more than 72 h after ingestion; and previous recruitment. All patients with poisoning presenting to hospital were admitted to the wards. The poison was identified from the patient, relatives, or referring doctor's letter, or by the clinical syndrome expressed by the patient. We have noted that such historical reporting of poison ingested is highly accurate in this cohort.19,21,22
The primary aim was to determine whether routine treatment with multiple-dose activated charcoal reduced death and complications after acute self-poisoning compared with no charcoal. We also wanted to assess whether any effect noted with multiple-dose activated charcoal was a result of the first or subsequent doses of charcoal, therefore a single-dose activated charcoal treatment was added to the trial. We examined whether any benefit was consistent across the prespecified subgroups—organophosphorus or carbamate pesticides, yellow oleander,23 and pharmaceutical drugs—and whether any effect was dependent on time from ingestion to treatment or severity on admission (asymptomatic, symptomatic with GCS 14 or 15, or symptomatic with a GCS <14).
Patients were randomised (with equal probability) to receive no charcoal, one 50 g dose of activated charcoal (Carbomix, Norit, Amersfoort, Netherlands; 2000 m2/g) dissolved in 300 mL of water, or 50 g every 4 h for six doses. Stratified block randomisation was done with the following strata: (1) substance ingested; (2) time between poisoning and recruitment (<1 h; 1–4 h; >4 h; unknown); (3) status on admission (asymptomatic, symptomatic [GCS 14 or 15], symptomatic [GCS <14]); and (4) hospital site. For each stratum, computer generated random allocation sequences with variable block sizes (3, 6, 9) were concealed within a Microsoft Access programme written for patient recruitment, randomisation, and event recording. The allocation sequence was generated independently by the study statistician and programmer. Randomisation occurred after patients' details had been entered and receipt of consent noted, and could not be manipulated by recruiting doctors. Study doctors administered the charcoal; conscious patients drank the charcoal, and unconscious patients with protected airways received it by nasogastric tube. The study was not masked.
Primary outcome was all-cause mortality during hospital admission. Secondary outcomes were identified a priori as relevant to specific poisons. For organophosphorus or carbamate pesticides,24 secondary outcomes were intubation, time ventilated, and seizures. For oleander, outcome was occurrence of cardiac dysrhythmias needing digoxin-specific antibody fragments25 or serum potassium greater than 6·0 mmol/L. Digoxin-specific antibody fragments became unavailable after 3–4 months26 and patients were then transferred for temporary pacing with the same criteria. Therefore, this secondary outcome was then modified to become need for digoxin-specific antibody fragments or transfer for pacing.
Patients were seen by study doctors within 30 min of admission. Their airway was stabilised and oxygen, atropine, fluids, and antidotes given as necessary. Gastric decontamination was only started when patients were stable. Gastric lavage or emesis, but not charcoal, was previously given in hospital clinical practice.27 In accordance with position papers about ipecac-induced forced emesis28 and gastric lavage29 published by the international associations of clinical toxicology, we initially did not give either to recruited patients. However, from patient number 1905 onwards, after much discussion within the national doctors union, all patients presenting less than 2 h after ingestion of large amounts of moderate-toxicity to high-toxicity pesticides or high doses of toxic medications (eg, tricyclic antidepressants) received gastric lavage with 300 mL of water three times at the request of the consultant physicians.
Patients remained under the care of consultant physicians using management protocols that had been agreed with the study team. The ward team made decisions about intubation and transfer of patients to intensive care20 or for cardiac pacing independently of study doctors. Patients were first managed on the medical ward; those needing intubation or inotropes were transferred to intensive care as a bed became available. All who died had a judicial post mortem.
Ethics approval was received from the Oxfordshire Clinical Research Ethics Committee and Faculty of Medicine Ethics Committee, Colombo. Written informed consent was obtained from each patient, or their relative (for patients unconscious or <16 years of age), in their own language. An independent data monitoring and ethics committee (IDMEC) was established for the trial. Interim analyses were to be supplied by the trial statistician to the IDMEC Chair as often as requested. In view of interim data, and emerging evidence from other studies, the IDMEC then informed the principal investigator if, in the committee's view, there was proof beyond reasonable doubt that the data showed that any part of the protocol under investigation became clearly indicated or contraindicated, either for all participants or for a specific subgroup of trial participants, or it was evident that no clear outcome would be obtained.
Statistical analysis
We calculated that to detect whether multiple-dose activated charcoal reduced the case fatality from 10%10 to 7%, with a significance of 5% and power of 80%, a minimum of 1400 patients was needed in each group (4200 patients overall).
Demographic factors and clinical characteristics were summarised with counts (percentages) for categorical variables, mean (SD) for normally distributed continuous variables, or median (IQR) for other continuous variables. We did an intention-to-treat analysis on all patients with available outcomes data (loss to follow-up of three [<1%] patients) analysed in the groups to which they were allocated. For the primary analysis, we reported the number and proportion of patients having an event. To establish the magnitude and direction of the treatment effect, we calculated the odds ratio (OR; plus exact 95% CI). We used logistic regression to compare the groups, estimate the OR (95% CI), and adjust for stratification factors and for geographical centre and baseline value (where appropriate).
The use of logistic regression was extended for the primary outcome to investigate the effects of potentially important prognostic factors such as previous administration of gastric lavage and forced emesis (by test of interaction), perform prespecified subgroup analyses with the test of interaction to examine whether the treatment effects were consistent across poison subgroups (suspected and confirmed), and test for trends in treatment effects for reported time from ingestion to randomisation and illness severity on admission. Poison-specific analyses were initially done for the groups identified on admission. Since the poison was sometimes identified only after randomisation (eg, patients randomised in the unknown poison group), analyses were then repeated including all patients identified as having ingested the poison.
Role of the funding source
The funding source had no role in study design, data collection, data analysis, and data interpretation; or writing of the report; or in the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patients were enrolled from March 31, 2002, in Anuradhapura and June 04, 2002, in Polonnaruwa, and from Nov 23, 2002, to Feb 03, 2003, in Kurunegala. The target sample size was reached in September, 2004. Data were then reviewed by IDMEC at its final planned analysis. Recruitment was stopped on Oct 16, 2004, as per IDMEC recommendation.
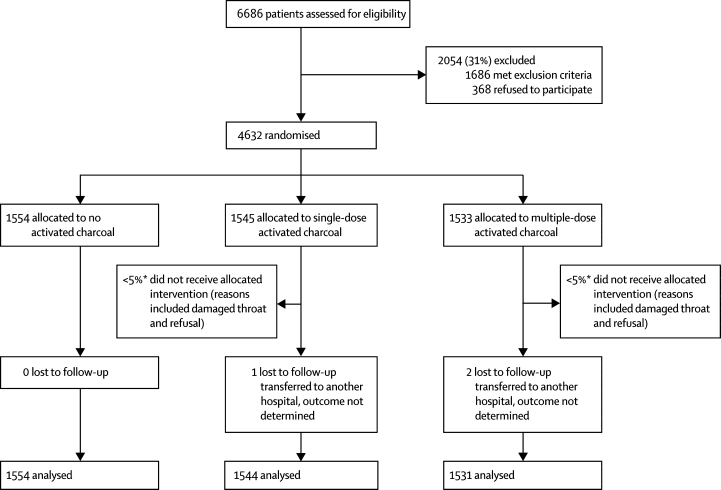
Figure 1 shows the trial profile. Table 1 shows baseline demographic and clinical characteristics in the three groups. Median time from ingestion to randomisation was similar. Although patients were eligible up to 72 h after ingestion, very few presented after 24 h—108 (2%) of 4632 presented between 24 h and 48 h and 11 (<1%) of 4632 between 48 h and 72 h. 2338 (51%) patients had ingested pesticides—1310 had ingested organophosphorus or carbamate pesticides, whereas 1647 (36%) had ingested oleander seeds. Median age was the same in each group (overall range 14–92 years).
Figure 1.
Trial profile
*The proportion of patients who did not receive their intervention is an estimate derived from a prospective study of compliance in 1649 participants.31
Table 1.
Demographic and baseline characteristics (intention-to-treat population)
| No charcoal (n=1554) | Single-dose activated charcoal (n=1545) | Multiple-dose activated charcoal (n=1533) | ||
|---|---|---|---|---|
| Women | 639 (41%) | 662 (43%) | 573 (37%) | |
| Age (years; median, IQR) | 25 (19–35) | 25 (19–35) | 25 (19–36) | |
| Toxin reported to have been ingested | ||||
| Oleander (Thevetia peruviana) seeds | 555 (36%) | 550 (36%) | 542 (35%) | |
| Organophosphorus or carbamate pesticide | 441 (28%) | 440 (28%) | 429 (28%) | |
| Organochlorine | 4 (<1%) | 3 (<1%) | 3 (<1%) | |
| Other or unknown pesticide or paraquat | 343 (22%) | 340 (22%) | 345 (23%) | |
| Medicine or unknown | 211 (14%) | 212 (14%) | 214 (14%) | |
| Clinical status on admission | ||||
| Asymptomatic | 743 (48%) | 745 (48%) | 736 (48%) | |
| Symptomatic with GCS 14 or 15 | 625 (40%) | 621 (40%) | 613 (40%) | |
| Symptomatic with GCS <14 | 186 (12%) | 179 (12%) | 184 (12%) | |
| Time between ingestion and hospital admission (h; median, IQR) | 4·2 (2·7–7·0) | 4·2 (2·7–7·1) | 4·3 (2·7–7·1) | |
| Information missing | 14 | 19 | 14 | |
| Vomited since ingestion (prehospital admission) | 809 (52%) | 745 (48%) | 767 (50%) | |
| Previous forced emesis | 832 (54%) | 859 (56%) | 812 (53%) | |
| Previous gastric lavage | 115 (7%) | 118 (8%) | 114 (7%) | |
| Systolic blood pressure (mm Hg; mean, SD) | 117·8 (17·5) | 118·0 (16·2) | 118·0 (16·1) | |
| Information missing | 97 | 111 | 116 | |
| Pulse (beats per min; mean, SD) | 90·5 (20·1) | 90·0 (19·6) | 89·3 (19·5) | |
| Information missing | 7 | 16 | 18 | |
Data are number (%), unless otherwise indicated. GCS=Glasgow coma score.
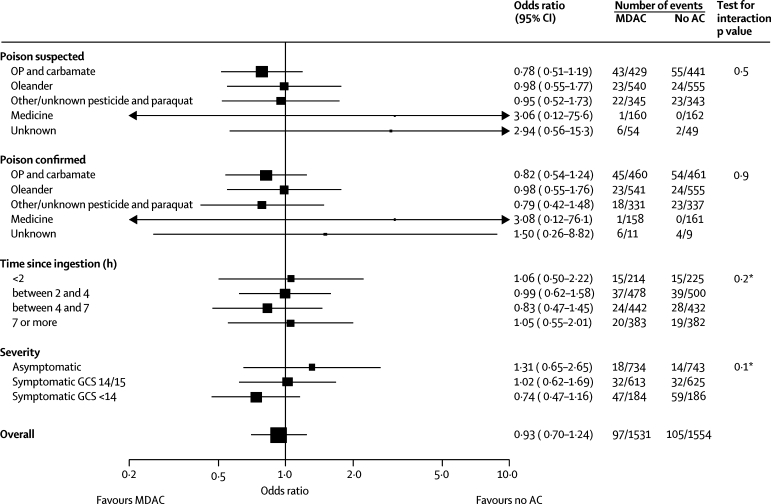
Overall mortality in the trial was 311 (6·7%) of 4629. Multiple-dose activated charcoal did not reduce mortality (table 2); however, fewer patients receiving multiple-dose activated charcoal died than those receiving no charcoal (table 2; figure 2). The trend towards increased treatment effect with increased severity on admission was not significant (p=0·1). We did not note a trend between treatment effect and time from ingestion to treatment. Reduction in mortality for patients who had ingested organophosphorus or carbamate pesticides and received multiple-dose activated charcoal was not significant (OR 0·78, 95% CI 0·51–1·19); this reduction was greater for diethyl organophosphorus poisoning than for dimethyl organophosphorus poisoning (webfigure 1).
Table 2.
Summary of outcomes overall plus comparative statistics (intention-to-treat population)
| No AC (n=1554) | SDAC (n=1544) | MDAC (n=1531) |
Odds ratio (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| MDAC vs no AC | SDAC vs no AC | MDAC vs SDAC | |||||
| Died | 105 (6·8%) | 109 (7·1%) | 97 (6·3%) | 0·93 (0·69–1·25)* | 1·05 (0·79–1·40)* | 0·89 (0·66–1·19)* | |
| 0·96 (0·70–1·33)† | 1·11 (0·82–1·52)† | 0·87 (0·64–1·18)† | |||||
| Needing intubation for ventilation | |||||||
| Baseline | 75 | 71 | 66 | ||||
| Follow-up | 76 (4·9%) | 73 (4·7%) | 73 (4·8%) | 0·97 (0·69–1·37)* | 0·97 (0·68–1·36)* | 1·01 (0·71–1·43)* | |
| 0·96 (0·67–1·37)† | 0·96 (0·67–1·37)† | 1·02 (0·71–1·45)† | |||||
| Seizures | |||||||
| Baseline | 9 | 8 | 7 | ||||
| Follow-up | 7 (0·5%) | 13 (0·8%) | 14 (0·9%) | 2·04 (0·77–5·99)* | 1·88 (0·69–5·57)* | 1·09 (0·47–2·52)* | |
| 1·92 (0·75–4·94)‡ | 1·98 (0·78–5·06)‡ | 0·97 (0·44–2·14)‡ | |||||
Data are number or number (%). AC=activated charcoal. SDAC=single-dose activated charcoal. MDAC=multiple-dose activated charcoal.
Exact CIs.
Logistic regression adjusted for stratification factors: severity status on admission (categories asymptomatic=1 [reference category]; symptomatic with Glasgow coma score [GCS] 14/15=2; and symptomatic with GCS <14=3); toxin stated to have been ingested (categories oleander [Thevetia peruviana]=1 [reference category]; organophosphorus or carbamate pesticides=2; organochlorine=3; other or unknown pesticide or paraquat=4; medicine or unknown=5); reported time between poisoning and randomisation (continuous: linear and quadratic terms), hospital site (categories Anuradhapura=1 [reference category]; Kurunegala=2; Polonnaruwa=3); and whether intubated at baseline (categories: no=0 [reference category]; and yes=1).
As † plus term for presence of seizures at baseline (categories: no=0 [reference category]; and yes=1].
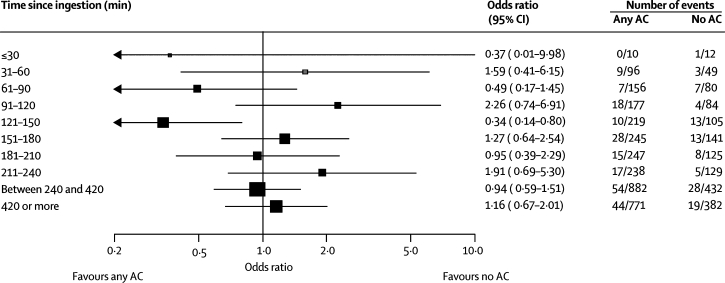
Figure 2.
Forest plot of mortality for multiple-dose activated charcoal versus no activated charcoal
MDAC=multiple-dose activated charcoal. AC=activated charcoal. OP=organophosphorus. GCS=Glasgow comma score. *Test for trend.
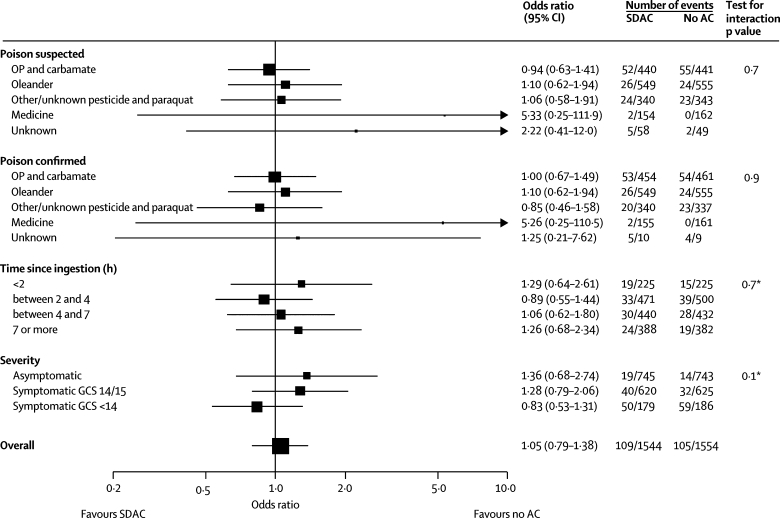
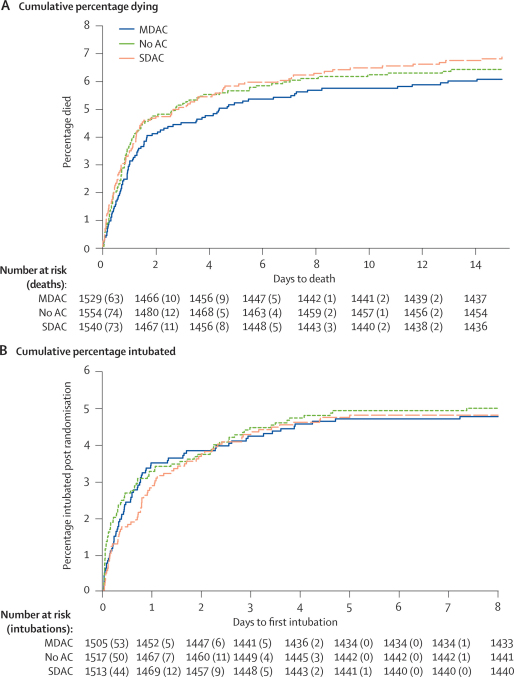
Comparison of single-dose activated charcoal versus no charcoal (figure 3) and multiple-dose activated charcoal versus single-dose activated charcoal did not provide any evidence that charcoal affected mortality (webfigures 2–4). We did not note a consistent trend between treatment effect and either severity on admission or time from ingestion to treatment. We did not find any evidence of a beneficial effect of charcoal on time to death (figure 4) and most of the deaths occurred within the first 24 h in all poison groups. Multivariate logistic regression showed no effect (no evidence of an interaction) of our change in gastric lavage policy 12 months into the trial (in which all patients presenting within 2 h of a serious ingestion received lavage; data not shown).
Figure 3.
Forest plot of mortality for single-dose activated charcoal versus no activated charcoal
SDAC=single-dose activated charcoal. AC=activated charcoal. OP=organophosphorus. GCS=Glasgow comma score. *Test for trend.
Figure 4.
Cumulative percentage—for days to death (A) and to first intubation (B)
(A) For the purpose of survival analysis, the clock was started at randomisation and stopped either at death or discharge (assumed to be 35 days if discharged alive sooner than 35 days). (B) For the purpose of survival analysis, the clock was started either at randomisation or, in the case of those who were intubated at randomisation, when the patient was first extubated. The clock stopped either at the first postrandomisation intubation or at death or discharge (assumed to be 8 days if discharged without intubation after less than 8 days). MDAC=multiple-dose activated charcoal. AC=activated charcoal. SDAC=single-dose activated charcoal.
Neither multiple-dose nor single-dose activated charcoal reduced the need for intubation, the rate of seizures, or (in oleander poisoning) cardiac dysrhythmias (tables 2 and 3). A similar proportion of patients needed intubation in each group; no difference was recorded in the time to first intubation (figure 4). Median duration of ventilation (excluding deaths) was similar with multiple-dose activated charcoal (83·8 [IQR 35·0–173·0] h) and no charcoal (88·5 [38·5–203·1] h), but longer with single-dose activated charcoal (112·0 [36·6–234·9] h). Analyses were repeated, as stated in the protocol, after reclassification of cases in which the poison was identified after randomisation. Reallocation of these cases did not tangibly affect the findings for any analysis (figures 2 and 3; webfigures 1–4).
Table 3.
Summary of poison-specific outcomes plus comparative statistics (intention-to-treat population)
| No AC | SDAC | MDAC |
Odds ratio (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| MDAC vs no AC | SDAC vs no AC | MDAC vs SDAC | |||||
| Number | 441 | 440 | 429 | ||||
| Patients with reported organophosphorus or carbamate poisoning needing intubation for ventilation | |||||||
| Baseline | 63 | 51 | 48 | ||||
| Follow-up | 53 (12·0%) | 48 (10·9%) | 50 (11·7%) | 0·97 (0·63–1·49)* | 0·90 (0·58–1·39)* | 1·08 (0·69–1·68)* | |
| 0·90 (0·58–1·41)† | 0·83 (0·54–1·29)† | 1·12 (0·72–1·74)† | |||||
| Seizures | |||||||
| Baseline | 7 | 2 | 2 | ||||
| Follow-up | 2 (0·5%) | 7 (1·6%) | 6 (1·4%) | 3·11 (0·55–31·67)* | 3·55 (0·67–35·15)* | 0·88 (0·24–3·08)* | |
| 3·15 (0·61–16·18)‡ | 3·44 (0·70–16·81)‡ | 0·89 (0·29–2·71)‡ | |||||
| Number | 555 | 549 | 540 | ||||
| Patients with reported oleander (Thevetia peruviana) poisoning given cardiac pacing or antitoxin | |||||||
| Baseline | 2 | 2 | 1 | ||||
| Follow-up | 101 (18·2%) | 101 (18·4%) | 85 (15·7%) | 0·84 (0·60–1·17)* | 1·01 (0·74–1·40)* | 0·83 (0·60–1·15)* | |
| 0·85 (0·61–1·18)§ | 1·02 (0·74–1·41)§ | 0·82 (0·59–1·14)§ | |||||
Data are number or number (%). AC=activated charcoal. SDAC=single-dose activated charcoal. MDAC=multiple-dose activated charcoal.
Exact CIs.
Logistic regression adjusted for stratification factors: severity status on admission (categories asymptomatic=1 [reference category]; symptomatic with Glasgow coma score [GCS] 14/15=2; and symptomatic with GCS <14=3); reported time between poisoning and randomisation (continuous: linear and quadratic terms); hospital site (categories: Anuradhapura=1 [reference category]; Kurunegala=2; and Polonnaruwa=3); and whether intubated at baseline (categories: no=0 [reference category]; and yes=1).
As † plus term for presence of seizures at baseline (categories: no=0 [reference category]; and yes=1).
As † plus term for whether patient received cardiac pacing at baseline (categories: no=0 [reference category]; and yes=1).
Although routine forced emesis and gastric lavage are no longer recommended internationally for self-poisoned patients, most had received mechanical forced emesis (2503 [54·0%] of 4632) or gastric lavage (347 [7·5%] of 4632), or both (64 [1·4%] of 4632), in a peripheral hospital before transfer. We used logistic regression analysis to assess whether previous gastric decontamination at the referring hospital affected the results of this study for the primary outcome—ie, death. We saw no evidence of an interaction with either forced emesis or gastric lavage for each of the treatment comparisons (data not shown).
439 patients were admitted within 2 h of poison ingestion and allocated to either multiple-dose (n=214) or single-dose activated charcoal (n=225; figures 2 and 3). The demographic and clinical baseline characteristics of early and late arrivals (defined as before or after 2 h, respectively) were very similar with respect to age, blood pressure, sex, severity of symptoms, intubation, and seizures. Oleander poisoning featured more commonly in late arrivals (1497 [38%] of 3919) than in early arrivals (141 [21%] of 664). Most patients who presented late were transfers from other hospitals in which they had received forced emesis or gastric lavage. Comparison of these 439 patients with 225 who were admitted within 2 h of poison ingestion and allocated to no charcoal (figure 5) did not show evidence of benefit on death of early charcoal administration (34 of 439 vs 15 of 225; OR 1·18 [exact 95% CI 0·61–2·38]; test of interaction p=0·5). Additionally, there was no evidence of an interaction between early charcoal administration and any of the secondary outcomes.
Figure 5.
Forest plot of effect of time to recruitment on mortality for multiple-dose or single-dose activated charcoal versus no activated charcoal, with detailed breakdown of less than 4 h
AC=activated charcoal.
Administration of charcoal seemed safe. Despite 2957 patients ingesting poisons (1647 oleander and 1310 organophosphorus or carbamate pesticides) that are treated with atropine, which would reduce bowel motility, only two were referred for surgical review for acute abdomen. None of the patients who died in the study had substantial quantities of charcoal in their lungs at judicial post-mortem examination.
The number of patients with absent bowel sounds on abdominal auscultation was small—17 (1·1%) of 1531 receiving multiple-dose activated charcoal, seven (0·5%) of 1544 receiving single-dose activated charcoal, and 17 (1·1%) of 1554 receiving no charcoal. A small non-significant increase in the occurrence of seizures was seen in patients receiving either regimen of charcoal compared with no charcoal (table 2).
Discussion
This randomised, controlled trial showed no benefit from routine administration of multiple-dose activated charcoal in Sri Lankan district hospitals. Most patients had ingested yellow oleander seeds or pesticides. Both poisons have major effects that are delayed for several hours, most deaths from oleander seeds occurred after 12 h (data not shown), and the median time to intubation and death after admission for all poisoned patients was 12–24 h (figure 4), potentially giving multiple-dose activated charcoal time to work. Absence of benefit was seen irrespective of the poison ingested or time to presentation. A non-significant trend towards benefit with charcoal was seen in the most ill patients at admission.
In 2003, de Silva and co-workers30 published the results of a single-blind, randomised, placebo-controlled trial investigating the effect of multiple-dose activated charcoal in yellow oleander poisoning. They reported a case fatality with multiple-dose activated charcoal of five (2·5%) of 201 patients versus 16 (8%) of 200 with single-dose activated charcoal (p=0·025, relative risk [RR] 0·31, 95% CI 0·12–0·83). The corresponding comparison in our study showed deaths in 23 (4·3%) of 540 patients given multiple-dose activated charcoal versus 26 (4·7%) of 549 given single-dose activated charcoal; a result showing a small non-significant benefit in favour of multiple-dose activated charcoal (0·90, 0·52–1·56). A longer regimen was given in de Silva and co-workers' study30—50 g every 6 h for 12 doses during 72 h—than in our study—50 g every 4 h for six doses during 20–24 h. However, 87% of oleander-induced deaths occurred within 24 h of admission, indicating that the continuation of charcoal therapy after 24 h could not account for the major difference in effectiveness of multiple-dose activated charcoal between studies.
We do not think that absence of benefit in our study was caused by poor compliance. Although we could not objectively measure it, we did estimate compliance in 1103 patients receiving charcoal in two study hospitals.31 Overall, patients ingested 80% of their first dose; and thereafter compliance decreased for further doses until 60% of the sixth dose was ingested.31 Compliance was not formally measured in de Silva and colleagues' study,30 but they reported that “none refused to take it”. This finding contrasts with our absolute refusal rates of 2% for the first dose, increasing to 12% by the sixth dose.31 However, such differences are unlikely to have caused the effect we report. Nor does it seem likely that the difference was caused by the charcoal used—Carbomix is used widely worldwide and has a surface area of 2000 m2/g compared with Haycarb (Hayes, Colombo, Sri Lanka; 1600 m2/g), which de Silva and colleagues' used.30 Overall, the combined evidence does not suggest a major effect of charcoal administration in oleander poisoning.
No benefit was noted from single-dose activated charcoal (or from the first dose of multiple-dose activated charcoal). Our study was not specifically designed to look at patients presenting within 2 h, in whom single-dose activated charcoal is proposed to have its major effect on reduction of poison absorption.14 The median time to admission in our trial was longer than 2 h; only 664 patients presented within 2 h. However, many patients had ingested oleander seeds, which are slowly absorbed over many hours19 and single-dose activated charcoal was worse than no charcoal in these patients (figure 3).
The absence of effect of single-dose activated charcoal in our study contrasts with studies of volunteers receiving pharmaceutical drugs that reduced absorption when single-dose activated charcoal was given early14 and also contrasts with a pharmacokinetic study that showed increased clearance in patients with moderate oleander poisoning.19 However, subtoxic doses of pharmaceutical drugs were given to the volunteers and these doses were probably far less than those that produced clinical outcomes in our study. Furthermore, charcoal is most likely to be effective with highly toxic poisons that cause clinical effects at small doses.14 Sri Lanka has banned the most toxic WHO class I pesticides, leaving only a few moderately toxic pesticides. The pharmacokinetic study that showed differences in oleander poisoning19 did not include patients with major clinical outcomes occurring within 24 h, suggesting that higher doses of poison were ingested by patients in our randomised controlled trial.
Mortality in the control no charcoal group was lower than predicted for two main reasons. First, frequent careful monitoring by the study team, its use of protocols, and administration of gastric lavage27 during resuscitation to only a few patients resulted in a substantial reduction in deaths for the patients recruited to the trial (data not shown). Second, because of poor outcome after paraquat ingestion, hospital doctors were not in equipoise for patients with paraquat poisoning and wanted to give Fuller's earth or single-dose activated charcoal. Therefore few paraquat-poisoned patients were recruited, lowering overall mortality.
The fewer than predicted number of events reduced the power of our trial. However, at the third planned interim analysis, when the prespecified recruitment target had been reached, the IDMEC considered the likelihood of finding a clinically meaningful benefit from giving charcoal if the trial was extended over and beyond the original target sample size. After assessment of the accumulating evidence from this trial, the IDMEC recommended that the trial terminate recruitment.
Another limitation was the absence of masking. We believed that masking was difficult because of the impossibility to conceal from a reviewing doctor whether a patient had received any charcoal. An absence of masking might have allowed for performance bias for the secondary outcomes. To counter this potential bias, the medical team made decisions about intubation and transfer of patients independently of the study doctors. This pragmatic response to an issue of internal validity was regarded as acceptable since the primary outcome—death during the admission—was unambiguous.
The poisons ingested by our study patients were typical of rural Asia. Unlike western countries, where patients generally ingest pharmaceuticals with varying mechanisms of action, most patients in our study took two types of poisons—anticholinesterase pesticides (28·3%) and cardenolides (35·6%). The large number of patients ingesting these two types of poisons suggests that the results are especially applicable to them.
We believe our study is relevant to rural Asia because it shows that routine multiple-dose activated charcoal administration is unlikely to be beneficial even in locations with high case-fatalities in which an intervention might be expected to offer greatest benefit. Our study shows that multiple-dose activated charcoal is not indicated for oleander poisoning or for organophosphorus and carbamate pesticides—all common and highly toxic poisons across the region.
We were not able to assess the effect of early administration of single-dose activated charcoal on outcome since few patients presented within 2 h. The OR 95% CI stretched from 0·61 to 2·38, suggesting little likelihood of benefit. However, our results might be useful in establishing a cluster randomised controlled trial in rural hospitals with the aim of recruiting large numbers of patients presenting within 2 h, especially those with a GCS of less than 14 on admission.
Intentional self-poisoning continues to kill many people throughout rural Asia. We have little evidence on which to base medical therapy of pesticide poisoning 12 and effective treatments for oleander poisoning are often unaffordable.26 Clinical trials to identify effective therapy and new therapeutic interventions with which to prevent these unnecessary deaths are urgently needed.
Acknowledgments
Acknowledgments
We thank Palitha Abeykoon and Kan Tun (WHO), Lakshman Karalliedde, D G S Alahakoon, and W M T B Wijekoon, and the Directors, medical and nursing staff of the study hospitals for their help and support, the IDMEC, Robin Ferner, and Doug Altman for advice, Geoff Isbister, Simon Thomas, Lewis Nelson, and Nick Bateman for critical review, Ly-Mee Yu and Nicola Alder for statistical support, Shukry Zawahir, and Chathura Palagasinghe for help with the final patient audit; and the Ox-Col study doctors for their work in the face of many pressures. ME is a Wellcome Trust Career Development Fellow; this work was funded by grant 063560 from the Wellcome Trust's Tropical Interest Group to ME. The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant 071669.
Contributors
ME designed and set up the randomised controlled trial, and wrote the first draft of the report. EJ helped design the study and did the statistical analysis. NB helped design and set up the study. LS and FM ran the trial centres, and together with ME extracted and checked patient data for analysis. WD, AH, SA, KJ, and SJ had clinical responsibility for the patients in the study. MHRS and DAW are the senior coordinators of the Ox-Col Collaboration. All authors had a role in improving the study design and in reviewing and editing the final version of the report.
Conflict of interest statement
We declare that we have no conflict of interest.
Ox-Col poisoning study collaborators
Darren Roberts, Asanka Perera, Manjula Rajapakshe, K Reginald, Sapumal Haggalla, Samantha Wijesundara, Jaya Ratnayake, S M T Bandara, Subashini Kumarasinghe, Manjula Weerakoon, Ayanthi Karunaratne, Manonath Marasinghe, Ruwan Kumara, Sumedha Kumara, Nilan Suranga, Jamal Dean, Dharshana Fernando, Sagara Kumara, Koshitha Gunarathne, R M Senanayake, Najeeb Khan, Kalum Dhammika, Anuradhi Weerasinghe, M S F Zanoona, Samanmali Edirisinghe, Medhangi Karunaratne, Sampath Attapattu, Upul Hendalage, Indika Wanasinghe, Lal Bogahawattage, R D S M Peiris, S M Dayarathne, Gayan Costa, Chandana de Silva, Prabath Abeyrathna, Bandula Senadeera, Gayan Gunarathne, Kusal Wijayaweera, M Senthilkumaran, Y Ruthra, K Sutharshan, Dimuth de Silva, Anjana Amarasinghe, Janaka Balasooriya, Damithe Pitahawatte, Asangha Dissanayaka, Aravinda Perera, Nalinda Deshapriya, Suranga Gurusinghe, Ruwan Seneviratne, Saman Chandana; Mubashi Mohamed, Koshala Abeysundera, Nasmiyar Mubarak, Lumbini de Silva, Daniel, Sandima Gunatilake, Indika Weerasinghe, Thushara Diunugala, Sriyantha Adikari, Suwini Karunaratne, Prabath Piyasena, Senarath Angammana, Deepal Inguruwatte, Samithe Egodage, Mathisha Dissanayake, Waruna Wijeyasiriwardene, Shammi Rajapakshe, Sidath Yawasinghe, Samanthi Bandara, Sumith Kumara, Thushita Kumara, Nilumdima Wijekoon.
Independent data monitoring and ethics committee
Mike Clarke (Director, UK Cochrane Centre, Oxford; Chair); Keith Hawton (Department of Psychiatry, Oxford); Julian Higgins (MRC Biostatistics Unit, Cambridge University; Statistician); Saroj Jayasinghe (Department of Clinical Medicine, Colombo); Nimal Senanayake (Department of Clinical Medicine, Peradeniya); Kris Weerasuriya (WHO/SEARO, New Delhi).
Web Extra Material
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for multiple-dose activated charcoal vs no activated charcoal)
MDAC=multiple-dose activated charcoal. AC=activated charcoal. OP=organophosphorus.
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for single-dose activated charcoal vs no activated charcoal)
SDAC=single-dose activated charcoal. AC=activated charcoal. OP=organophosphorus.
Forest plot of mortality for multiple-dose activated charcoal versus single-dose activated charcoal
MDAC=multiple-dose activated charcoal. SDAC=single-dose activated charcoal. OP=organophosphorus. GCS=Glasgow coma score. *Test for trend.
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for multiple-dose activated charcoal versus single-dose activated charcoal)
MDAC=multiple-dose activated charcoal. SDAC=single-dose activated charcoal. OP=organophosphorus.
References
- 1.Shannon MW, Haddad LM. The emergency management of poisoning. In: Haddad LM, Shannon MW, Winchester JF, editors. Clinical management of poisoning and drug overdose. 3rd edn. WBSaunders; Philadelphia: 1998. pp. 2–31. [Google Scholar]
- 2.Keyes DC, Dart RC. Initial diagnosis and treatment of the poisoned patient. In: Dart RC, Caravati EM, McGuigan MA, editors. Medical toxicology. 3rd edn. Lippincott Williams and Wilkins; Philadelphia: 2004. pp. 21–31. [Google Scholar]
- 3.Proudfoot AT. Abandon gastric lavage in the accident and emergency department? Arch Emerg Med. 1984;2:65–71. doi: 10.1136/emj.1.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale JA, Proudfoot AT. How useful is activated charcoal? BMJ. 1993;306:78–79. doi: 10.1136/bmj.306.6870.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krenzelok EP, Vale JA. Position statements: gut decontamination. J Toxicol Clin Toxicol. 1997;35:695–697. [PubMed] [Google Scholar]
- 6.Henry JA, Hoffman JR. Continuing controversy on gut decontamination. Lancet. 1998;352:420–421. doi: 10.1016/S0140-6736(05)79183-2. [DOI] [PubMed] [Google Scholar]
- 7.Kapur N, Turnbull P, Hawton K. Self-poisoning suicides in England: a multicentre study. Q J Med. 2005;98:589–597. doi: 10.1093/qjmed/hci089. [DOI] [PubMed] [Google Scholar]
- 8.Bateman DN. Gastric decontamination - a view for the millennium. J Accident Emerg Med. 1998;16:84–86. doi: 10.1136/emj.16.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond GR. The role of activated charcoal and gastric emptying in gastrointestinal decontamination: a state-of-the-art review. Ann Emerg Med. 2002;39:273–286. doi: 10.1067/mem.2002.122058. [DOI] [PubMed] [Google Scholar]
- 10.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 11.WHO . World Health Report 2002. Reducing risks, promoting healthy life. World Health Organisation; Geneva: 2002. [Google Scholar]
- 12.Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. Where is the evidence for the management of pesticide poisoning—is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol. 2004;42:113–116. doi: 10.1081/clt-120028756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardagh M, Flood D, Tait C. Limiting the use of gastrointestinal decontamination does not worsen the outcome from deliberate self-poisoning. N Z Med J. 2001;114:423–425. [PubMed] [Google Scholar]
- 14.Chyka PA, Seger D, Krenzelok EP, Vale JA, American Academy of Clinical Toxicology and European Association of Poison Centres and Clinical Toxicologists Position paper: single-dose activated charcoal. J Toxicol Clin Toxicol. 2005;43:61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- 15.Levy G. Gastrointestinal clearance of drugs with activated charcoal. N Engl J Med. 1982;307:676–678. doi: 10.1056/NEJM198209093071109. [DOI] [PubMed] [Google Scholar]
- 16.Bradberry SM, Vale JA. Poisons: initial assessment and management. Clin Med. 2003;3:107–110. doi: 10.7861/clinmedicine.3-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Academy of Clinical Toxicology and European Association of Poison Centres and Clinical Toxicologists Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol. 1999;37:731–751. doi: 10.1081/clt-100102451. [DOI] [PubMed] [Google Scholar]
- 18.Guven H, Tuncok Y, Gidener S. In vitro adsorption of dichlorvos and parathion by activated charcoal. J Toxicol Clin Toxicol. 1994;32:157–163. doi: 10.3109/15563659409000445. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DM, Southcott E, Potter JM, Roberts MS, Eddleston M, Buckley NA. Pharmacokinetics of digoxin cross-reacting substances in patients with acute yellow oleander (Thevetia peruviana) poisoning, including the effect of activated charcoal. Ther Drug Monitor. 2006;28:784–792. doi: 10.1097/FTD.0b013e31802bfd69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddleston M, Juszczak E, Buckley NA. Study protocol: a randomised controlled trial of multiple and single dose activated charcoal for acute self-poisoning. BMC Emerg Med. 2007;7:2. doi: 10.1186/1471-227X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eddleston M, Eyer P, Worek F. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 22.Roberts DM, Seneviratne R, Mohamed F, Patel R, Abeysinghe M, Hittarage A. Deliberate self-poisoning with the chlorphenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) Ann Emerg Med. 2005;46:275–284. doi: 10.1016/j.annemergmed.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddleston M, Ariaratnam CA, Meyer PW. Epidemic of self-poisoning with seeds of the yellow oleander tree (Thevetia peruviana) in northern Sri Lanka. Trop Med Int Health. 1999;4:266–273. doi: 10.1046/j.1365-3156.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 24.Lotti M. Clinical toxicology of anticholinesterase agents in humans. In: Krieger RI, Doull J, editors. Handbook of pesticide toxicology. Volume 2. Agents. 2nd edn. Academic Press; San Diego: 2001. pp. 1043–1085. [Google Scholar]
- 25.Eddleston M, Rajapakse S, Rajakanthan K. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet. 2000;355:967–972. doi: 10.1016/s0140-6736(00)90014-x. [DOI] [PubMed] [Google Scholar]
- 26.Eddleston M, Senarathna L, Mohamed F. Deaths due to absence of an affordable antitoxin for plant poisoning. Lancet. 2003;362:1041–1044. doi: 10.1016/s0140-6736(03)14415-7. [DOI] [PubMed] [Google Scholar]
- 27.Eddleston M, Haggalla S, Reginald K. The hazards of gastric lavage for intentional self-poisoning in a resource poor location. Clin Toxicol. 2007;45:136–143. doi: 10.1080/15563650601006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists Position statement: ipecac syrup. J Toxicol Clin Toxicol. 1997;35:699–709. doi: 10.3109/15563659709162567. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists Position statement: gastric lavage. J Toxicol Clin Toxicol. 1997;35:711–719. doi: 10.3109/15563659709162568. [DOI] [PubMed] [Google Scholar]
- 30.de Silva HA, Fonseka MMD, Pathmeswaran A. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361:1935–1938. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed F, Senarathna L, Azher S, Sheriff MHR, Buckley NA, Eddleston M. Compliance for single and multiple dose regimens of superactivated charcoal: a prospective study of patients in a clinical trial. Clin Toxicol. 2007;45:132–135. doi: 10.1080/15563650600981145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for multiple-dose activated charcoal vs no activated charcoal)
MDAC=multiple-dose activated charcoal. AC=activated charcoal. OP=organophosphorus.
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for single-dose activated charcoal vs no activated charcoal)
SDAC=single-dose activated charcoal. AC=activated charcoal. OP=organophosphorus.
Forest plot of mortality for multiple-dose activated charcoal versus single-dose activated charcoal
MDAC=multiple-dose activated charcoal. SDAC=single-dose activated charcoal. OP=organophosphorus. GCS=Glasgow coma score. *Test for trend.
Subgroup analysis of diethyl organophosphorus versus dimethyl organophosphorus poisoning (mortality for multiple-dose activated charcoal versus single-dose activated charcoal)
MDAC=multiple-dose activated charcoal. SDAC=single-dose activated charcoal. OP=organophosphorus.