Abstract
The expression of major histocompatibility class II genes is necessary for proper antigen presentation and induction of an immune response. This expression is initiated by the class II transactivator, CIITA. The establishment of the active form of CIITA is controlled by a series of post-translational events, including GTP binding, ubiquitination, and dimerization. However, the role of phosphorylation is less clearly defined as are the consequences of phosphorylation on CIITA activity and the identity of the kinases involved. In this study we show that the extracellular signal-regulated kinases 1 and 2 (ERK1/2) interact directly with CIITA, targeting serine residues in the amino terminus of the protein, including serine 288. Inhibition of this phosphorylation by dominant-negative forms of ERK or by treatment of cells with the ERK inhibitor PD98059 resulted in the increase in CIITA-mediated gene expression from a class II promoter, enhanced the nuclear concentration of CIITA, and impaired its ability to bind to the nuclear export factor, CRM1. In contrast, inhibition of ERK1/2 activity had little effect on serine-to-alanine mutant forms of CIITA. These data suggest a model whereby ERK1/2-mediated phosphorylation of CIITA down-regulates CIITA activity by priming it for nuclear export, thus providing a means for cells to tightly regulate the extent of antigen presentation.
The class II transactivator CIITA2 plays a critical role in initiating an immune response by activating the expression of major histocompatibility (MHC) class II genes and associated molecules (2-6). MHC II glycoproteins are necessary for the presentation of antigenic peptides to CD4+ T lymphocytes and the subsequent initiation and propagation of CD4+ T cell-mediated immune responses and are involved in the development and maintenance of homeostasis of the CD4+ T cell population. Although constitutive expression of MHC class II genes is primarily restricted to a specific subset of antigen-presenting cells that include B cells and dendritic cells, expression is inducible in a variety of other cell types and tissues by cytokines such as interferon-γ and tumor necrosis factor-α (1). Both constitutive and inducible expression of MHC II and other related genes are contingent upon the activation of CIITA (7, 8). Loss of a functional CIITA protein results in an immunodeficiency called bare lymphocyte syndrome, which is characterized by a complete absence of MHC class II-mediated antigen presentation. Patients with this disease suffer from recurrent infections due to opportunistic infections and, ultimately, death in early childhood (9-11).
CIITA is a protein of 1130 amino acids that does not bind directly to DNA. Instead, it regulates gene expression by interacting with other transcription factors and chromatin-remodeling proteins at the W/X/Y regulatory elements in the promoter regions of class II genes (for review, see Refs. 12-17). Nuclear factors binding to CIITA include the regulatory factor X (RFX) complex (RFX5, RFXAP, RFXANK/RFX-B) (18) and nuclear factor Y (19, 20) as well as AP1, X2BP, and CREB (21-26). Extensive structure-function analyses have characterized multiple important regions of CIITA, identifying it as a member of the CATERPILLER family of genes (27). The amino terminus contains a proline/serine/threonine (PST)-rich domain, with several potential sites for post-translational modifications, and an acidic activation domain that serves to interact with factors involved in chromatin remodeling and modification as well as components of the general transcriptional machinery (21, 22, 28-32). A GTP binding domain and a series of leucine-rich repeats in the carboxyl terminus of CIITA have been shown to be involved in nuclear localization, self-association, and promoter transactivation (33-42). In addition, three separate nuclear localization signals are scattered throughout the length of the protein (15, 31, 43-45). These observations strongly implicate CIITA in regulating chromatin structure as a mechanism of activating gene transcription (46). However, little is known about the posttranslational events that modify the CIITA protein and may, thus, serve to regulate its functionality within the cell.
Phosphorylation plays a critical role in regulating the activity of a variety of different cellular proteins. The modulation of CIITA activity by phosphorylation is of particular interest, in part because the kinase or assembly of kinases that phosphorylate CIITA is still poorly understood. Moreover, phosphorylation studies of CIITA have yielded differing results on how such modifications impact its role in regulating gene expression. However, accumulating evidence points to a role for multisite phosphorylation of CIITA. Previously, we have demonstrated that CIITA localized in the nucleus is predominantly phosphorylated and have identified various serine residues as target phosphorylation sites, including serines 286, 288, and 293 (47). Mutations at these sites increase CIITA nuclear accumulation and MHC class II gene transactivation (47). Other groups have demonstrated that phosphorylation at PST sites between 253 and 321 results in an appreciable increase in CIITA transactivation potential and is prerequisite for oligomerization and aggregation at the MHC II promoter (39, 48). CIITA has been shown to be targeted by protein kinase A (PKA) on four different serines between residues 325-408, which exert down-regulatory effects on CIITA and inhibition of MHC II expression (49). To make understanding the role of phosphorylation in CIITA function even more complex, constitutively active protein kinase C (PKC) increases CIITA expression, whereas a dominant-negative mutant of PKC abrogates interferon-γ-induced MHC class II gene expression (49). These data reflect a complex and not yet fully defined array of pathways regulating CIITA activity.
Here we investigate a role for ERK1 and ERK2 mitogen-activated protein kinases (MAPKs) in the phosphorylation of CIITA. Structural analysis of ERK1 reveals 85% homology to ERK2, and it is well established that ERK1 and ERK2 (hereafter referred to as ERK1/2) share many of the same substrates (51-55). The transient formation of the ras proto-oncogene at the cell membrane eventually leads to the phosphorylation of the ERK1/2 by MAPK kinase 1 (MAPKK1 or MEK1) (52, 55). Activated monomeric or homodimeric ERK1/2 participate in signal transduction pathways involved in nearly all cellular processes by phosphorylating and regulating numerous targets in the cytoplasm and in the nucleus, including other protein kinases, transcription factors, cytoskeletal proteins, growth factor receptors, and other regulatory proteins (52). In this report we describe a novel mechanism of CIITA regulation by demonstrating a direct interaction between CIITA and ERK1/2, leading to a decrease in CIITA function.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection—COS7 monkey kidney fibroblast cells or Raw 264.7 murine macrophage cells (American Type Culture Collection) were cultured at 37 °C in 5% CO2 and grown in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal calf serum, 300 mg/liter l-glutamine, and 1% penicillin/streptomycin. All transfections were performed using FuGENE 6 (Roche Applied Science) for COS7 transfections or FuGENE 6 HD for Raw 264.7 transfections according to the instructions of the manufacturer.
Plasmids—Fg-tagged wild-type CIITA (FgCIITA) contains an eight-amino acid FLAG epitope upstream of the first methionine of CIITA in a pcDNA3 vector (Invitrogen) (56). Expression from this vector is controlled by a cytomegalovirus (CMV) enhancer-promoter. Full-length Xpress-tagged CIITA (XpCIITA) was created by ligating the 3.5-kilobase EcoRI FgCIITA fragment into EcoRI-digested pcDNA3 HisC. Serine-to-alanine mutations of CIITA at amino acids 286, 288, and 293 have been described previously (47). Wild-type and dominant-negative human ERK1 under the control of a CMV promoter (CMV-ERK1) and Fg-tagged ERK2 (FgERK2) expression vectors were kindly provided by Dr. Melanie Cobb (University of Texas, Southwestern Medical Center, Dallas, TX).
Luciferase Assays—COS7 or Raw 264.7 cells were split to 6-well plates 24 h before transfection. A DR-Luc reporter plasmid containing the MHC class II DR promoter fused to a luciferase reporter gene along with the indicated plasmids were transfected into cells at 60% confluence. Corresponding empty control vectors were included at identical concentrations to equilibrate the total amount of DNA transfected. Cells were harvested at 24 h post-transfection in 200 μl of reporter lysis buffer (Promega, Madison, WI). Luciferase activity was measured using the Promega luciferase assay reagent (Promega). PD98059 and U0126 (Sigma) were used at final concentrations of 2.5 μg/ml and 10 μm, respectively. Values were normalized for transfection efficiency based on protein concentrations, which was determined using BCA protein assay kit (Pierce). Each transfection was performed in triplicate. Two-sample t tests assuming unequal variances were employed to determine the significance of differences in the activity values of samples compared with control cells transfected with CIITA and the reporter plasmid. p values of less than 0.05 were considered significant.
In Vitro Translation and Kinase Assays—In vitro transcription/translation of wild-type FgCIITA was completed with TNT T7 Quick Master Mix (Promega) according to the instructions of the manufacturer. The resultant proteins were incubated at 30 °C for 90 min with 1 mm ATP and the following serine/threonine kinases: Ca2+/calmodulin-dependent protein kinase II, cyclin dependent kinase 2 (Cdc2), casein kinase II, cAMP-dependent protein kinase A (PKA), p42 mitogen-activated protein kinase (p42 ERK2) (New England Biolabs, Beverly, MA), or recombinant human active Janus kinase 2α2 (JNK2α2) (Invitrogen). The activation of calmodulin-dependent protein kinase II was prepared according to the instructions of the manufacturer. Where indicated, extracts were treated with 40 units/μl of λ-protein phosphatase (New England Biolabs) at 30 °C for 1 h.
Immunoprecipitations and Immunoblots—For co-immunoprecipitation studies, COS7 cells were split to 100-mm tissue culture plates 24 h before transfection. Cells were transfected with 1 μg of each indicated plasmid. 24-48 h post-transfection, cells were lysed with 750 μl of chilled radioimmune precipitation assay lysis buffer (150 mm NaCl, 1.0% Nonidet P-40, 1.0% deoxycholate, 0.1% SDS, 10 mm Tris (pH 7.4), 1× protease inhibitor mixture (Roche Applied Science), 2 mm EDTA) followed by centrifugation for 15 min at 14,000 × g at 4 °C. Extracts were incubated for 2 h with 2 μg of anti-Fg (M5, Sigma), anti-CRM1, or anti-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. 35 μl of M-280 magnetic Dynabeads (Dynal Biotech, Great Neck, NY) were washed and equilibrated in radioimmune precipitation assay buffer then added to the mixture and incubated for an additional 24 h. The beads were washed 6 times with 750 μl of modified radioimmune precipitation assay buffer (without 1× protease inhibitor and supplemented with 0.05% Tween 20), and pellets were each suspended in 15 μl of radioimmune precipitation assay buffer, boiled in 2× Laemmli sample buffer, separated by SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose. Immunoblots were performed using immunoprecipitated or normalized quantities of whole cell extracts detected with the indicated antibody at 1:1000 dilution and ECL detection reagents (GE Healthcare).
Immunofluorescence—The indicated plasmids were transfected into 1 × 105 COS7 cells in two-well chamber slides. At 24 h post-transfection, slides were rinsed in phosphate-buffered saline (PBS), fixed in 3:2 acetone:PBS for 3 min, then incubated for 1 h with the indicated antibody diluted 1:500 in PBS, 1% bovine serum albumin, rinsed, incubated for 1 h with fluorescein isothiocyanate-conjugated secondary Ab (1:500 dilution), then rinsed and mounted in Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Slides were observed at a 40× magnification.
RESULTS
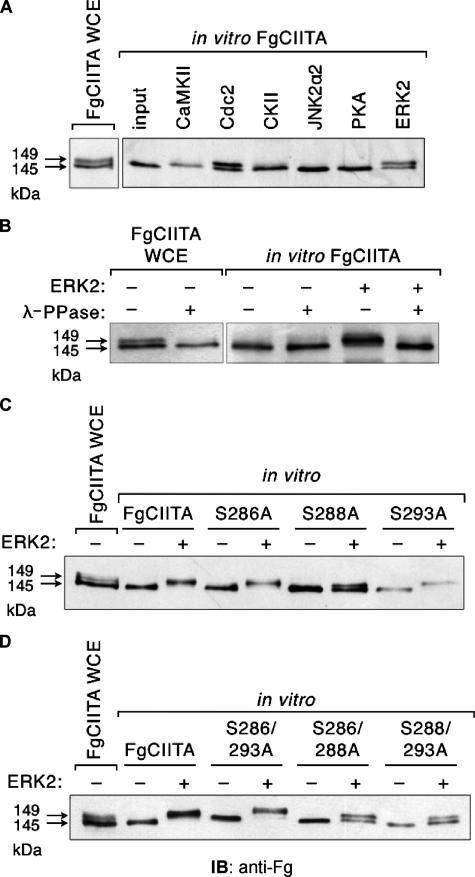
Phosphorylation of CIITA by ERK2 and Cdc2 in Vitro—Previously, we have demonstrated that CIITA in the nucleus is predominantly phosphorylated but underphosphorylated in the cytoplasm (47). Mutation of serine residues 286, 288, and 293 resulted in an increase in nuclear accumulation, suggesting that phosphorylation of these residues is not necessary for the entry of CIITA into the nucleus but, rather, after its import, unphosphorylated CIITA is targeted by a nuclear kinase (47). To understand how nuclear phosphorylation regulates CIITA function, we first sought to identify the kinase potentially involved in the phosphorylation. CIITA synthesized by in vitro transcription and translation was incubated with one of multiple different serine/threonine kinases (Fig. 1A). CIITA expressed in COS7 cells migrates as a doublet, with proteins at 145 and 149 kDa (Fig. 1A, first lane). In contrast, in vitro CIITA in the absence of kinase activity migrates only as a single band (second lane, input), correlating with the size of the lower band of the CIITA doublet seen in the whole cell extract. Although calmodulin-dependent protein kinase II, casein kinase II, PKA, and JNK2 were unable to phosphorylate CIITA in vitro, incubation with Cdc2 and ERK2 (fourth and eighth lanes, respectively) both resulted in a pattern similar to that found for CIITA obtained from whole cell extracts. This indicates that these kinases are responsible for converting a fraction of the fast-migrating species of CIITA into the slow-migrating species. Because ERK2 is well known to be capable of phosphorylating nuclear targets (52, 54, 57), subsequent experiments focused specifically on the ability of ERKs to modulate CIITA activity.
FIGURE 1.
ERK2 and Cdc2 directly phosphorylate CIITA in vitro. A, in vitro transcribed and translated FgCIITA was incubated alone (second lane) or with the indicated serine/threonine kinases followed by immunoblot analysis with an anti-Fg antibody. The presence of single or double bands was compared with doublets of the FgCIITA protein present in whole cell extracts prepared from COS7 cell lysates (first lane). CaMKII, Ca2+/calmodulin-dependent protein kinase II; CKII, casein kinase II. B, incubation of FgCIITA phosphorylated in vitro by ERK2 with λ-protein phosphatase eliminates the upper band of the doublet. Whole cell extracts (WCE) from FgCIITA transfected cells or in vitro prepared FgCIITA were incubated with ERK2 and λ-protein phosphatase as indicated and detected as in A. Single (C) or double point mutants (D) of CIITA at serine 286 (S286A), serine 288 (S288A), or serine 293 (S293A) were subjected to in vitro transcription and translation followed by exposure to ERK2 and immunoblot detection by anti-Fg.
To confirm that the upper and lower bands represented phosphorylated and unphosphorylated forms of CIITA, respectively, we treated the in vitro reactions with λ-protein phosphatase, a broad-acting phosphatase capable of dephosphorylating serine, threonine, and tyrosine residues. The addition of λ-protein phosphatase to CIITA derived from transiently transfected COS7 cells also results in the loss of the upper band (Fig. 1B, second lane). Similarly, phosphatase incubation of CIITA treated with ERK2 results in a single, smaller band of similar size to that found in untreated CIITA (Fig. 1B, compare the third and sixth lanes). Thus, the upper, more slowly migrating bands are consistent with the phosphorylated species of CIITA, whereas the lower bands represent the unphosphorylated form of the protein.
Serine-to-alanine mutations of amino acids 286, 288, and 293 were tested for their ability to be phosphorylated by ERK2 in vitro either individually (Fig. 1C) or as double mutants (Fig. 1D). A triple mutant of all three residues fails to express properly in cells, likely due to conformational abnormalities, and thus, could not be assessed. Mutation of serines 286 or 293 alone were still largely capable of being phosphorylated by ERK2, whereas mutation of serine 288 resulted in a more balanced ratio of upper to lower protein bands (Fig. 1C), suggesting a decrease in the ability of CIITA to be phosphorylated by ERK2. Because double serine mutants were used in previous studies and shown to have a more significant impact on CIITA activity (47), we also tested the ability of these double mutants to serve as targets of ERK2 activity. Double mutants S286A/S288A and S288A/S293A both contained a substitution of alanine for serine at residue 288, and both demonstrated a decreased susceptibility to be phosphorylated by ERK2 (Fig. 1D). It is important to note, however, that some phosphorylation of these CIITA mutants still occurred when incubated with ERK2, indicating that ERK targets one or more other serine residues on CIITA in addition to those identified here.
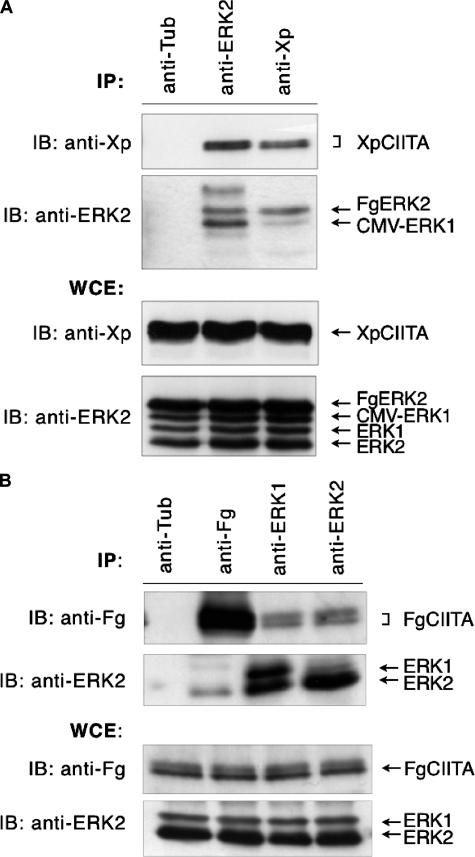
ERK1/2 Binds CIITA—Given the highly homologous nature of ERK1/2 and the fact that ERK2 can directly phosphorylate CIITA in vitro, we investigated whether the phosphorylation of CIITA in cells is concomitant with its binding of ERK. To assess whether CIITA can physically interact with ERK2 as well as ERK1, we performed co-immunoprecipitation analyses. COS7 cells were co-transfected with combinations of Xp-tagged CIITA and CMV-ERK1 or Fg-tagged ERK2. Upon ERK1/2 overexpression, both native and exogenous forms of ERK1/2 could be visualized by immunoblot analysis (Fig. 2, A and B, WCE). ERK1/2 was immunoprecipitated using anti-ERK2 antibodies, which are specific for ERK2 but can detect ERK1 to a lesser extent. The presence of the precipitated proteins was verified in an immunoblot with an anti-ERK2 antibody (Fig. 2A, IP). The pulldown of ERK1/2 co-precipitated CIITA, which was detected using an anti-Xp monoclonal antibody (second lane). However, because of the cross-reactivity of the polyclonal ERK2 antibody used in the immunoprecipitation, the pulldown of CIITA is the result of an interaction with either ERK1, ERK2, or both. In the reciprocal direction, immunoprecipitation with the antibody specific to CIITA was sufficient to pull down exogenously expressed ERK1/2 (Fig. 2A, third lane). The slight increase apparent in the amount of CIITA pulled down by the anti-ERK2 antibody as compared with that pulled down by the anti-Xp antibody is due to a slightly elevated amount of CIITA transfected and expressed in these cells, as is evident in the blot of the whole cell extracts, as well as a greater stoichiometric amount of ERK1/2 proteins in the cell than CIITA due to the additional presence of endogenous ERK1/2. As a negative control to rule out nonspecific antibody cross-reactivity, an anti-tubulin antibody protein failed to immunoprecipitate CIITA or ERK1/2 (Fig. 2A, first lane). These results indicate that CIITA is capable of interacting with overexpressed forms of ERK1/2 in transfected cells.
FIGURE 2.
CIITA interacts with exogenous and endogenous ERK1/2. A, co-immunoprecipitation of CIITA·ERK1/2 complexes in COS7 cells transfected with Xp-tagged CIITA, CMV-ERK1, and Fg-tagged ERK2. After preparation of whole cell extracts, immunoprecipitations (IP) were performed using anti-tubulin, anti-ERK2, or anti-Xp antibodies, followed by anti-ERK2 or anti-Xp immunoblotting (IB) as indicated. Immunoblots of whole cell extracts (WCE) were performed as controls for the level of XpCIITA or ERK1/2 expression. B, endogenous ERK1/2 interacts with CIITA. Co-immunoprecipitation of CIITA·ERK1/2 complexes in COS7 cells transfected with Fg-tagged CIITA alone followed by immunoblots with anti-Fg or anti-ERK of immunoprecipitated complexes or whole cells extracts as above. Immunoprecipitation with the anti-tubulin antibody serves as a negative control.
To ascertain whether CIITA can bind ERK1/2 in a normal cell milieu, we prepared extracts from COS7 cells expressing only normal endogenous amounts of ERK1/2 and transfected with Fg-tagged CIITA, then immunoprecipitated these extracts using antibodies to FgCIITA, ERK1, or ERK2 (Fig. 2B). Antibodies to both ERK1 and ERK2 were able to co-immunoprecipitate FgCIITA (third and fourth lanes), confirming the cellular interaction of CIITA with endogenous ERK1/2. Using polyclonal antiserum, anti-ERK1 and anti-ERK2 antibodies also precipitated endogenous ERK2 and ERK1, respectively. Proteins of 44 and 42 kDa (third lane), which correspond to the molecular masses of ERK1 and ERK2, respectively, were immunoprecipitated in equal abundance by anti-ERK1 antibodies. As shown by the results shown in lanes 3 and 4, the presence of CIITA may be attributed to its interaction with ERK1, ERK2, or both, although the interaction is most favorable between ERK2 and CIITA. In the reverse experiment FgCIITA co-precipitated endogenous ERK2 (second lane), indicating that CIITA can associate with ERK2 even in the absence of ERK2 overexpression. These immunoprecipitation results suggest that although CIITA can bind ERK1/2 when both isoforms are overexpressed, it preferentially binds ERK2 at endogenous levels.
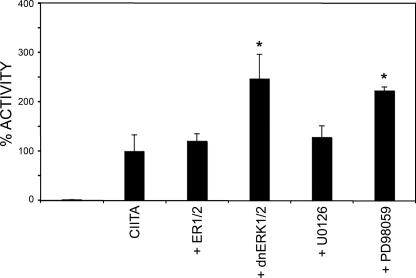
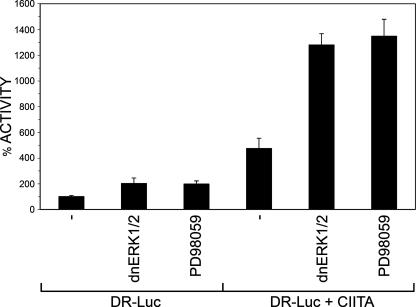
Inhibition of ERK1/2 Increases CIITA Activity—Phosphorylation plays a major role in the ability of the cell to regulate protein function. With this in mind, we wished to address the role that ERK1/2-induced phosphorylation plays in mediating CIITA activity. To do so, we expressed FgCIITA with a luciferase reporter gene under the control of the MHC class II HLA-DR promoter in COS7 cells. Before the transfection of FgCIITA, cells were transfected with wild-type or dominant-negative ERK1/2 (dnERK1/2) for 4 h to allow for sufficient expression and dimerization with endogenous ERK1/2. As shown in Fig. 3, in the presence of overexpressed ERK1/2, there was no change in expression from the CIITA-dependent promoter. This may be due to sufficiently high levels of endogenous ERK1/2 acting to maximally phosphorylate available CIITA such that the presence of additional transfected ERK1/2 is extraneous. In contrast, however, incubation with dominant-negative ERK1/2 consistently resulted in increased CIITA activity. This indicates that inhibition of ERK1/2 enhances the ability of CIITA to drive expression from an MHC class II promoter.
FIGURE 3.
CIITA activity increases in the presence of dominant-negative ERK1/2 and a MAPK inhibitor. COS7 cells were transfected with wild-type CIITA along with a luciferase reporter gene under the control of the MHC class II DR promoter. Indicated samples were also co-transfected with ERK1/2 or dominant-negative ERK1/2 or treated with U0126 and PD98059 at the time of transfection. 24 h post-transfection extracts from cells were prepared and assessed for luciferase activity. 100% activity in each cell type was defined as the level of DR-luciferase activation by wild-type CIITA. All other values were plotted as percentages of this activity. All experiments were performed in triplicate and normalized to protein concentration. *, p < .05 compared with CIITA alone.
To further address the relationship between ERK1/2 and MHC II expression, we used U0126 and PD98059, specific MEK1 inhibitors in the ERK1/2 pathway (26). It has been shown previously that PD98059 decreases ERK1/2 activity in a variety of cell lines (23, 26). To rule out nonspecific effects of PD98059, we also tested U0126. Both inhibitors are particular for the MEK1/2/ERK pathway, as they do not affect other MAPK pathways, including MEKK1-2, MKK4/JNK, or MKK6/p38 (23, 30, 58). In cells transfected with CIITA and the luciferase reporter construct, those cells treated with PD98059 exhibited more than a 2-fold increase in CIITA activity as compared with untreated cells (Fig. 3, p < 0.05). U0126-treated cells exhibited only a modest increase in CIITA activity. This is likely due to differences in the optimal concentrations required for inhibition by the two compounds. Subsequent experiments focused on ERK1/2 inhibition by treatment of cells with PD98059. These data are consistent with the model that CIITA activity increases as a result of down-regulating ERK1/2 activation either through co-transfection with a dominant-negative form of ERK or treatment of cells with an ERK inhibitor.
Dominant-negative ERK1/2 Increases Nuclear Retention of CIITA—Because luciferase assays suggest that dominant-negative ERK1/2 increases CIITA trans-activation ability, we explored the possibility that this effect specifically alters CIITA distribution between the nucleus and cytoplasm. COS7 cells were transfected with a combination of XpCIITA and expression vectors for either ERK1/2 or dnERK1/2. To ensure that any observed cytoplasmic localization of proteins was not simply due to new de novo synthesis, all cells were treated with actinomycin D for 3 h before harvesting. Immunofluorescence of these transfected cells using anti-Xp antibodies showed that CIITA alone exhibits dual subcellular localization to both the cytoplasm and the nucleus (Fig. 4A), which is in agreement with previous studies (47). In the presence of overexpressed ERK1/2, CIITA showed no significant change in localization (Fig. 4B). This is consistent with the observation that transfected ERK1/2 does not alter CIITA activity in luciferase assays (Fig. 3), indicating that the presence of endogenous ERK1/2 is sufficient to carry out the phosphorylation of CIITA. In contrast, incubation with dominant-negative ERK1/2 significantly increased CIITA presence in the nucleus (Fig. 4C). Likewise, inhibition of ERK1/2 by PD98059 treatment of cells transfected with wild-type CIITA also led to enhanced nuclear concentration of the protein (Fig. 4D). We have previously shown that the serine double mutant S288A/S293A, despite showing decreased levels of phosphorylation, localizes more strongly to the nucleus than does the wild-type CIITA (Ref. 46 and Fig. 4E), demonstrating that phosphorylation of CIITA is not necessary for its nuclear import. Unlike wild-type CIITA, co-expression of the S288A/S293A mutant with ERK1/2, dnERK1/2, or treatment with PD98059 consistently resulted in little alteration of CIITA compared with the untreated form (Fig. 4, E-H); thus, only wild-type CIITA is largely susceptible to ERK1/2 or dnERK1/2 effects. In light of the current data, this suggests that the ERK1/2-mediated phosphorylation of CIITA may play a role in inducing the export of the CIITA protein from the nucleus, resulting in the loss of its ability to activate expression of MHC class II genes.
FIGURE 4.
Inhibition of endogenous ERK1/2 by dominant-negative mutants or PD98059 increases the nuclear localization of CIITA. COS7 cells were transfected with either wild-type XpCIITA (A-D) or the S288A/S293A double serine mutant (E-H) and co-transfected with ERK1/2 or dominant-negative ERK1/2 or treated with PD98059 at the time of transfection. Immunofluorescence was performed 24 h post-transfection using anti-Xp to detect the subcellular localization of CIITA. Samples were compared with the relatively even nuclear-cytoplasmic distribution of wild-type CIITA (A). DAPI, 4′,6-diamidino-2-phenylindole.
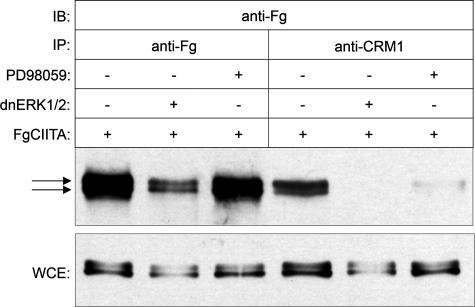
Inhibition of ERK1/2 Decreases the Ability of CIITA to Interact with the Nuclear Export Factor CRM1—If CIITA is actively exported from the nucleus, then this process is likely to be mediated by the nuclear export protein CRM1, which is known to be involved in the export of a wide variety of nuclear factors (59, 60). Previous results have demonstrated that inhibition of CRM1 by treatment with leptomycin B leads to the accumulation of wild-type CIITA in the nucleus (44). Therefore, if phosphorylation of CIITA by ERK1/2 leads to its nuclear export, we wished to determine whether the inhibition of ERK1/2 serves to prevent CIITA from interacting with CRM1, thereby explaining the increased nuclear accumulation observed in Fig. 4. To assess whether CIITA can interact with CRM1 and if such interactions are dependent on the state of CIITA phosphorylation, cells were transfected with FgCIITA alone or in the presence of dnERK1/2 or PD98059 treatment, then immunoprecipitated with anti-Fg or anti-CRM1 antibodies followed by an immunoblot to detect CIITA (Fig. 5). Anti-Fg antibodies successfully pulled down the FgCIITA protein under all conditions. As expected, both phosphorylated and unphosphorylated CIITA are shown by the presence of slower-migrating (upper) and faster-migrating (lower) bands, respectively (Fig. 5, first through third lanes). In contrast, incubation with anti-CRM1 antibodies immunoprecipitated primarily the phosphorylated form of CIITA (fourth lane) even though both forms are clearly present in the lysate controls (Fig. 5, lower panel). Co-transfection with dnERK1/2 or treatment of transfected cells with PD98059 resulted in a substantial inhibition of the amount of CIITA pulled down by CRM1 antibodies (fifth and sixth lanes). This data indicate the presence of phosphorylated and unphosphorylated CIITA in the cell, but the preferential interaction of CRM1 with the phosphorylated form. Furthermore, inhibition of ERK1/2 activity decreases the phosphorylation of CIITA and limits its ability to interact with CRM1. It should be noted that, as evidenced in these whole cell extracts, the presence of dnERK1/2 or PD98059 diminishes but does not completely abrogate CIITA phosphorylation, which is consistent with previous data indicating that other kinases, including PKA (49) and Cdc2 (Fig. 1), may also phosphorylate CIITA.
FIGURE 5.
Interaction between CIITA and CRM1 is decreased after inhibition of ERK1/2. Shown is co-immunoprecipitation (IP) of COS7 cells transfected with FgCIITA and co-transfected with dominant-negative ERK1/2 or treated with PD98059 as indicated. Extracts from cells were made 24 h post-transfection or treatment, then were subject to immunoprecipitation using anti-Fg (first through third lanes) or anti-CRM1 (fourth through sixth lanes) antibodies. FgCIITA present in the immunoprecipitated complexes was detected by immunoblotting (IB) with anti-Fg antibodies. Arrows indicate bands showing phosphorylated (upper) and unphosphorylated (lower) forms of CIITA. Immunoblots of whole cell extracts (WCE) were performed as controls for the level of FgCIITA expression.
Inhibition of ERK1/2 Enhances Endogenous CIITA-mediated Gene Expression in a Macrophage Cell Line—Although these experiments were conducted in COS7 cells, it was important to establish whether inhibition of ERK1/2 affected endogenous CIITA. Using Raw 264.7 murine macrophages, which express endogenous CIITA, we examined the effect of ERK1/2 inhibitors on the expression of a luciferase reporter gene from the class II DR promoter (Fig. 6). Luciferase activity from Raw cells transfected only with the DR-luciferase reporter construct was defined as 100% activity (Fig. 6, first lane). Co-transfection of cells with dnERK1/2 or treatment with PD98059 resulted in a 2-fold increase in CIITA-mediated gene expression (second and third lanes), consistent with the enhanced expression seen in COS7 cells similarly treated (Fig. 3). Because this activation may also be due to the release from ERK1/2-mediated repression of CIITA gene expression (62), we wished to determine whether co-transfection with CIITA would further enhance luciferase activity and the subsequent impact of the ERK1/2 inhibitors. As expected, co-transfection of Raw cells with CIITA resulted in a 4-fold increase in luciferase activity (Fig. 6, fourth lane). Furthermore, inhibition of ERK1/2 by application of dnERK1/2 or PD98059 caused a 13-fold increase in CIITA activity. This synergistic effect is consistent with the observation that ERK1/2 down-regulates CIITA-mediated class II gene expression by repressing expression of the CIITA gene and by phosphorylating the CIITA protein to induce its association with CRM1 and subsequent export from the nucleus.
FIGURE 6.
Inhibition of ERK1/2 increases endogenous CIITA activity and synergistically activates CIITA in a macrophage cell line. Raw 264.7 macrophage cells were transfected with the luciferase reporter under the control of the MHC class II DR promoter. Indicated cells were co-transfected with dnERK1/2 (lanes 2, 5), exogenous FgCIITA (lanes 4-6), or were treated with PD98059 (lanes 3, 6). Extracts were prepared at 24 h post-transfection and assayed for luciferase activity. The level of DR-luciferase activation by endogenous CIITA alone (first lane) was defined as 100% activity, and values of the remaining samples were plotted as percentages relative to this level. All experiments were performed in triplicate and normalized to protein concentration.
DISCUSSION
Since its isolation over a decade ago, wide-ranging studies on CIITA have revealed its critical role as a transcription factor responsible for initiating the expression of MHC class II genes and the subsequent induction of the immune response. More recent evidence has shown that the activity of CIITA is tightly regulated at multiple levels, including expression, GTP binding, dimerization, nuclear translocation, and phosphorylation (42, 61-65), whereas the intracellular signaling pathways involved in CIITA regulation, namely phosphorylation, are only starting to emerge. Although several kinases have been identified as mediators of CIITA expression and function, their respective roles differ, reflecting a complex regulatory mechanism alternatively capable of activating or inhibiting CIITA function. One mode by which kinases regulate CIITA is by controlling expression of the CIITA gene. Interferon-γ activates PKC, thereby causing interferon regulatory factor-1 and CREB to activate CIITA expression through its type IV promoter, resulting in MHC class II gene expression (50, 53, 66). Likewise, chemical inhibition of PKC blocks CIITA gene expression (67). In contrast to PKC, the MAPK pathway kinase ERK1/2 down-regulates the expression of CIITA by decreasing histone acetylation at the CIITA promoter in antigen-presenting cells (62).
Phosphorylation also appears to play a role in modulating CIITA function by directly affecting the CIITA protein itself as a post-translational modification. PKA targets residues at the carboxyl terminus and down-regulates CIITA activity, accounting for the mechanism by which prostaglandins inhibit MHC II expression in monocytes (49). Similarly, our previous results have indicated that phosphorylation at serine residues in the amino terminus inhibits CIITA activity (47), whereas other studies have demonstrated that phosphorylation can induce CIITA oligomerization and activation (39, 48).
Here, we present evidence that ERK1/2 directly interacts with and phosphorylates CIITA. These data support a model whereby CIITA phosphorylation by ERK1/2 in the nucleus results in a loss of CIITA function and permits association with CRM1 and the subsequent export of CIITA from the nucleus. In vitro kinase assays indicate that both Cdc2 and ERK2 can phosphorylate CIITA. Cdc2 plays a key role in the control of eukaryotic cell cycle, whereas members of the MAPK family, including ERK1/2, are major signaling enzymes by which cells transduce extracellular stimuli to regulate intricate intracellular processes. The observation that these two kinases can phosphorylate CIITA suggests that pathways activating the immune response and those that are predominantly associated with cell proliferation are cross-linked to control MHC II expression. In support of the idea that ERK1/2 can phosphorylate CIITA, lysates of cells transfected with a tagged form of CIITA demonstrate the ability of the CIITA protein to interact with both exogenous and endogenous ERK1/2. This interaction with CIITA is evident for both ERK1 and ERK2, consistent with the high homology of these proteins as well as their overlapping function in the cell.
Inhibition of ERK1/2 either through the use of dominant-negative mutant forms of ERK1/2 or a chemical inhibitor of ERK leads to an increase in the ability of CIITA to drive reporter gene expression from an MHC class II promoter. The extent of this increase is moderate, being 2-3-fold above the level of activation by wild-type CIITA alone. This degree of activation is consistent with what has been reported for serine-to-alanine mutations at amino acids 286, 288, and 293 of CIITA, which demonstrate a similar increase in activity compared with wild-type CIITA (47). This suggests that those sites that are mutated are among the residues targeted for phosphorylation by ERK1/2. This is confirmed by the in vitro phosphorylation data showing that the double serine mutants containing serine 288 undergo incomplete phosphorylation compared with wild-type CIITA when incubated with ERK.
If ERK1/2-mediated phosphorylation of CIITA results in a loss of CIITA activity, then one explanation for how this may occur is that after its interaction with ERK, phosphorylated CIITA is primed to be shunted out of the nucleus and redistributed within the cell. Normally, CIITA is found in both the cytoplasm and the nucleus, although CIITA can be retained within the nucleus by treatment of cells with leptomycin B, an inhibitor of nuclear export (43), indicating that the CIITA protein is actively exported from the nucleus. In the current study, the presence of dominant-negative ERK1/2 or PD98059 causes CIITA to become concentrated in the nucleus. Furthermore, inhibition of CIITA phosphorylation by any of these methods decreases the ability of CIITA to interact with CRM1, the mediator of nuclear export. In addition, elevating levels of ERK1/2 in the cells did not exhibit any down-regulatory effects on class II expression nor did it alter the subcellular localization of CIITA. One explanation for this observation may be a “ceiling effect,” which indicates a certain maximal level of phosphorylated CIITA, and thus, transfecting cells with additional ERK1/2 may have no effect on target CIITA sites that are already fully saturated by endogenous ERK1/2.
Previous studies have indicated that ERK1/2 negatively regulates CIITA by blocking expression of the CIITA gene (67). The results here indicate that ERK1/2 also represses activity of existing CIITA protein by phosphorylating CIITA and priming it for export out of the nucleus. This is confirmed by the synergistic effect seen on class II gene expression after inhibition of ERK1/2 in macrophage cells expressing endogenous CIITA and supplemented with exogenous CIITA. Combined with the previous results, this suggests that ERK represses CIITA at both the gene and protein levels.
The results presented here support the following model at work in the cell for tightly controlling CIITA protein function and down-regulating its ability to induce expression of MHC class II genes. CIITA is synthesized and enters the nucleus in an unphosphorylated state, activating the expression of target MHC class II genes. ERK1/2 subsequently phosphorylates CIITA at residues including serine 288, resulting in the loss of CIITA transactivation potential by enabling it to interact with CRM1. CRM1 then exports CIITA from the nucleus. Prevention of phosphorylation maintains CIITA in an active state, enhancing class II expression and blocking the ability of CIITA to bind to CRM1, thus increasing its nuclear concentration. Although ERK-mediated phosphorylation of CIITA leads to its down-regulation, it is clear that phosphorylation by other kinases also plays a critical role in regulating CIITA function, and it will be important for future studies to identify the balance of factors that control the timing of phosphorylation and the interplay of the different cellular pathways involved in modulating CIITA-induced class II gene expression.
Acknowledgments
We thank Z. Kratovac, X. Li, E. Lin, and M. Santspree for assistance with experiments, A. Pham for excellent technical assistance, D. King for assistance with statistical analysis, and L. Olson and S. Calvin for critical discussion of results.
This work was supported by National Science Foundation Grant MCB0515853 and a grant from the Sara Lee Schupf Foundation (to D. E. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CIITA, class II transactivator; ERK, extracellular signal-regulated kinase; PKA and PKC, protein kinase A and C, respectively; JNK, Janus kinase; Cdc, cyclin-dependent kinase; MAPK, mitogen-activated protein kinase; MHC, major histocompatibility; CREB, cyclic AMP response element-binding protein; CMV, cytomegalovirus; dn, dominant negative.
References
- 1.Grilli, M., Chiu, J. J., and Lenardo, M. J. (1993) Int. Rev. Cytol. 143 1-62 [DOI] [PubMed] [Google Scholar]
- 2.Chang, C. H., and Flavell, R. A. (1995) J. Exp. Med. 181 765-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin, K. C., Mao, C., Skinner, C., Riley, J. L., Wright, K. L., Moreno, C. S., Stark, G. R., Boss, J. M., and Ting, J. P. (1994) Immunity 1 687-697 [DOI] [PubMed] [Google Scholar]
- 4.Nagarajan, U. M., Bushey, A., and Boss, J. M. (2002) J. Immunol. 169 5078-5088 [DOI] [PubMed] [Google Scholar]
- 5.Steimle, V., Siegrist, C. A., Mottet, A., Lisowska-Grospierre, B., and Mach, B. (1994) Science 265 106-109 [DOI] [PubMed] [Google Scholar]
- 6.Taxman, D. J., Cressman, D. E., and Ting, J. P. (2000) J. Immunol. 165 1410-1416 [DOI] [PubMed] [Google Scholar]
- 7.Drozina, G., Kohoutek, J., Nishiya, T., and Peterlin, B. M. (2006) J. Biol. Chem. 281 39963-39970 [DOI] [PubMed] [Google Scholar]
- 8.Krawczyk, M., and Reith, W. (2006) Tissue Antigens 67 183-197 [DOI] [PubMed] [Google Scholar]
- 9.DeSandro, A. M., Nagarajan, U. M., and Boss, J. M. (2000) Mol. Cell. Biol. 20 6587-6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reith, W., and Mach, B. (2001) Annu. Rev. Immunol. 19 331-373 [DOI] [PubMed] [Google Scholar]
- 11.Scholl, T., Mahanta, S. K., and Strominger, J. L. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 6330-6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boss, J. M., and Jensen, P. E. (2003) Curr. Opin. Immunol. 15 105-111 [DOI] [PubMed] [Google Scholar]
- 13.Harton, J. A., and Ting, J. P. (2000) Mol. Cell. Biol. 20 6185-6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linhoff, M. W., Harton, J. A., Cressman, D. E., Martin, B. K., and Ting, J. P. (2001) Mol. Cell. Biol. 21 3001-3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nekrep, N., Geyer, M., Jabrane-Ferrat, N., and Peterlin, B. M. (2001) Mol. Cell. Biol. 21 5566-5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisk, T. J., Roys, S., and Chang, C. H. (2001) Mol. Cell. Biol. 21 4919-4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Elsen, P. J., Holling, T. M., Kuipers, H. F., and van der Stoep, N. (2004) Curr. Opin. Immunol. 16 67-75 [DOI] [PubMed] [Google Scholar]
- 18.Jabrane-Ferrat, N., Nekrep, N., Tosi, G., Esserman, L., and Peterlin, B. M. (2003) Int. Immunol. 15 467-475 [DOI] [PubMed] [Google Scholar]
- 19.Boss, J. M. (1999) Microbes Infect. 1 847-853 [DOI] [PubMed] [Google Scholar]
- 20.Mach, B., Steimle, V., Martinez-Soria, E., and Reith, W. (1996) Annu. Rev. Immunol. 14 301-331 [DOI] [PubMed] [Google Scholar]
- 21.Fontes, J. D., Kanazawa, S., Jean, D., and Peterlin, B. M. (1999) Mol. Cell. Biol. 19 941-947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretsovali, A., Agalioti, T., Spilianakis, C., Tzortzakaki, E., Merika, M., and Papamatheakis, J. (1998) Mol. Cell. Biol. 18 6777-6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochamy, J., Rogers, E. M., and Boss, J. M. (2007) Mol. Immunol. 44 837-847 [DOI] [PubMed] [Google Scholar]
- 24.Moreno, C. S., Beresford, G. W., Louis-Plence, P., Morris, A. C., and Boss, J. M. (1999) Immunity 10 143-151 [DOI] [PubMed] [Google Scholar]
- 25.Moreno, C. S., Emery, P., West, J. E., Durand, B., Reith, W., Mach, B., and Boss, J. M. (1995) J. Immunol. 155 4313-4321 [PubMed] [Google Scholar]
- 26.Zhu, X. S., Linhoff, M. W., Li, G., Chin, K. C., Maity, S. N., and Ting, J. P. (2000) Mol. Cell. Biol. 20 6051-6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harton, J. A., Linhoff, M. W., Zhang, J., and Ting, J. P. (2002) J. Immunol. 169 4088-4093 [DOI] [PubMed] [Google Scholar]
- 28.Mahanta, S. K., Scholl, T., Yang, F. C., and Strominger, J. L. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 6324-6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudhasani, R., and Fontes, J. D. (2002) Mol. Cell. Biol. 22 5019-5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley, J. L., Westerheide, S. D., Price, J. A., Brown, J. A., and Boss, J. M. (1995) Immunity 2 533-543 [DOI] [PubMed] [Google Scholar]
- 31.Spilianakis, C., Papamatheakis, J., and Kretsovali, A. (2000) Mol. Cell. Biol. 20 8489-8498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zika, E., Fauquier, L., Vandel, L., and Ting, J. P. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 16321-16326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown, J. A., Rogers, E. M., and Boss, J. M. (1998) Nucleic Acids Res. 26 4128-4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camacho-Carvajal, M. M., Klingler, S., Schnappauf, F., Hake, S. B., and Steimle, V. (2004) Int. Immunol. 16 65-75 [DOI] [PubMed] [Google Scholar]
- 35.Chin, K. C., Li, G. G., and Ting, J. P. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 2501-2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hake, S. B., Masternak, K., Kammerbauer, C., Janzen, C., Reith, W., and Steimle, V. (2000) Mol. Cell. Biol. 20 7716-7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harton, J. A., Cressman, D. E., Chin, K. C., Der, C. J., and Ting, J. P. (1999) Science 285 1402-1405 [DOI] [PubMed] [Google Scholar]
- 38.Raval, A., Weissman, J. D., Howcroft, T. K., and Singer, D. S. (2003) J. Immunol. 170 922-930 [DOI] [PubMed] [Google Scholar]
- 39.Sisk, T. J., Nickerson, K., Kwok, R. P., and Chang, C. H. (2003) Int. Immunol. 15 1195-1205 [DOI] [PubMed] [Google Scholar]
- 40.Towey, M., and Kelly, A. P. (2002) Mol. Immunol. 38 627-634 [DOI] [PubMed] [Google Scholar]
- 41.Zika, E., Greer, S. F., Zhu, X. S., and Ting, J. P. (2003) Mol. Cell. Biol. 23 3091-3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bewry, N. N., Bolick, S. C., Wright, K. L., and Harton, J. A. (2007) J. Biol. Chem. 282 26178-26184 [DOI] [PubMed] [Google Scholar]
- 43.Cressman, D. E., Chin, K. C., Taxman, D. J., and Ting, J. P. (1999) Immunity 10 163-171 [DOI] [PubMed] [Google Scholar]
- 44.Cressman, D. E., O'Connor, W. J., Greer, S. F., Zhu, X. S., and Ting, J. P. (2001) J. Immunol. 167 3626-3634 [DOI] [PubMed] [Google Scholar]
- 45.Kretsovali, A., Spilianakis, C., Dimakopoulos, A., Makatounakis, T., and Papamatheakis, J. (2001) J. Biol. Chem. 276 32191-32197 [DOI] [PubMed] [Google Scholar]
- 46.Zika, E., and Ting, J. P. (2005) Curr. Opin. Immunol. 17 58-64 [DOI] [PubMed] [Google Scholar]
- 47.Greer, S. F., Harton, J. A., Linhoff, M. W., Janczak, C. A., Ting, J. P., and Cressman, D. E. (2004) J. Immunol. 173 376-383 [DOI] [PubMed] [Google Scholar]
- 48.Tosi, G., Jabrane-Ferrat, N., and Peterlin, B. M. (2002) EMBO J. 21 5467-5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, G., Harton, J. A., Zhu, X., and Ting, J. P. (2001) Mol. Cell. Biol. 21 4626-4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giroux, M., Schmidt, M., and Descoteaux, A. (2003) J. Immunol. 171 4187-4194 [DOI] [PubMed] [Google Scholar]
- 51.Boulton, T. G., and Cobb, M. H. (1991) Cell Regul. 2 357-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulton, T. G., Nye, S. H., Robbins, D. J., Ip, N. Y., Radziejewska, E., Morgenbesser, S. D., DePinho, R. A., Panayotatos, N., Cobb, M. H., and Yancopoulos, G. D. (1991) Cell 65 663-675 [DOI] [PubMed] [Google Scholar]
- 53.Cobb, M. H., Boulton, T. G., and Robbins, D. J. (1991) Cell Regul. 2 965-978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cobb, M. H., Robbins, D. J., and Boulton, T. G. (1991) Curr. Opin. Cell Biol. 3 1025-1032 [DOI] [PubMed] [Google Scholar]
- 55.Robbins, D. J., Zhen, E., Owaki, H., Vanderbilt, C. A., Ebert, D., Geppert, T. D., and Cobb, M. H. (1993) J. Biol. Chem. 268 5097-5106 [PubMed] [Google Scholar]
- 56.Chin, K. C., Li, G., and Ting, J. P. (1997) J. Immunol. 159 2789-2794 [PubMed] [Google Scholar]
- 57.Roux, P. P., and Blenis, J. (2004) Microbiol. Mol. Biol. Rev. 68 320-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, H., and Glimcher, L. H. (1995) Immunity 2 545-553 [DOI] [PubMed] [Google Scholar]
- 59.Kutay, U., and Guttinger, S. (2005) Trends Cell Biol. 15 121-124 [DOI] [PubMed] [Google Scholar]
- 60.Weis, K. (2003) Cell 112 441-451 [DOI] [PubMed] [Google Scholar]
- 61.Al-Kandari, W., Koneni, R., Navalgund, V., Aleksandrova, A., Jambunathan, S., and Fontes, J. D. (2007) J. Mol. Biol. 369 1175-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao, Y., Xu, Q., Kwon, M. J., Matta, R., Liu, Y., Hong, S. C., and Chang, C. H. (2006) J. Immunol. 177 70-76 [DOI] [PubMed] [Google Scholar]
- 63.Kwon, M. J., Yao, Y., Walter, M. J., Holtzman, M. J., and Chang, C. H. (2007) Mol. Immunol. 44 2841-2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, M., Flynt, F. L., Hong, M., Chen, H., Gilbert, C. A., Briley, N. T., Bolick, S. C., Wright, K. L., and Piskurich, J. F. (2007) Mol. Immunol. 44 2923-2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, W., Cha, E. N., Lee, C., Park, C. Y., and Schindler, C. (2007) J. Immunol. 179 463-471 [DOI] [PubMed] [Google Scholar]
- 66.Kwon, M. J., Soh, J. W., and Chang, C. H. (2006) J. Immunol. 177 950-956 [DOI] [PubMed] [Google Scholar]
- 67.Nikodemova, M., Watters, J. J., Jackson, S. J., Yang, S. K., and Duncan, I. D. (2007) J. Biol. Chem. 282 15208-15216 [DOI] [PubMed] [Google Scholar]